Icariside II Exerts Anti-Type 2 Diabetic Effect by Targeting PPARα/γ: Involvement of ROS/NF-κB/IRS1 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Oral Glucose Tolerance Test and Insulin Tolerance Test
2.3. Microarray Processing and Data Analysis
2.4. Immunohistochemistry
2.5. Cell Culture and Drug Treatment
2.6. Determination of Cell Viability
2.7. ELISA Assay
2.8. DHE Staining and Mito-Sox Staining
2.9. Oil Red O Staining
2.10. RNA Interference
2.11. Protein Quantification and Western Blot Analysis
2.12. Statistical Analysis
3. Results
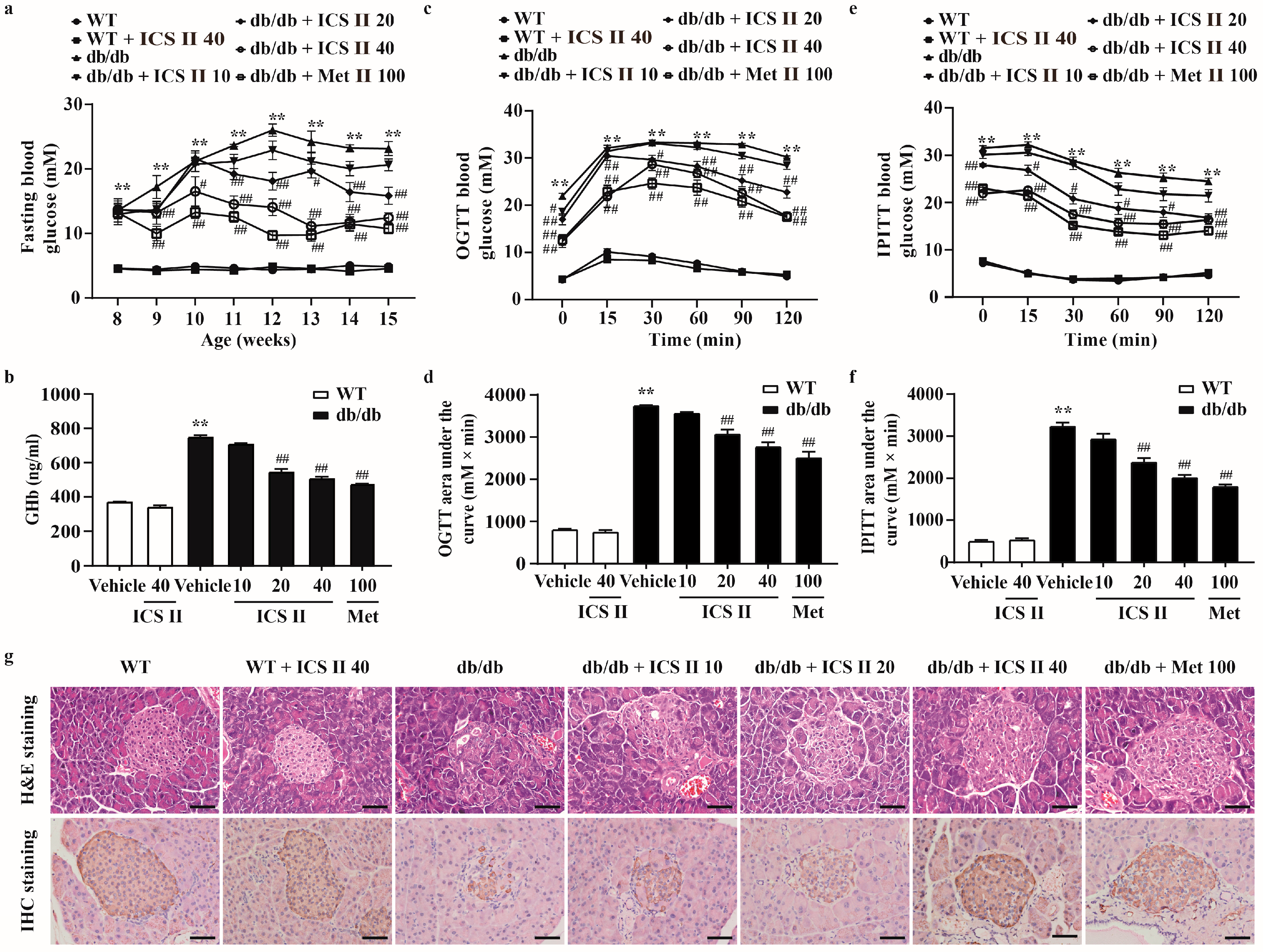
3.1. ICS II Prevented Hyperglycemia and Restored the Structural Integrity of the Pancreas in db/db Mice
3.2. ICS II Mitigated Hepatic Steatosis and Dyslipidemia in db/db Mice
3.3. Microarray Data Analysis
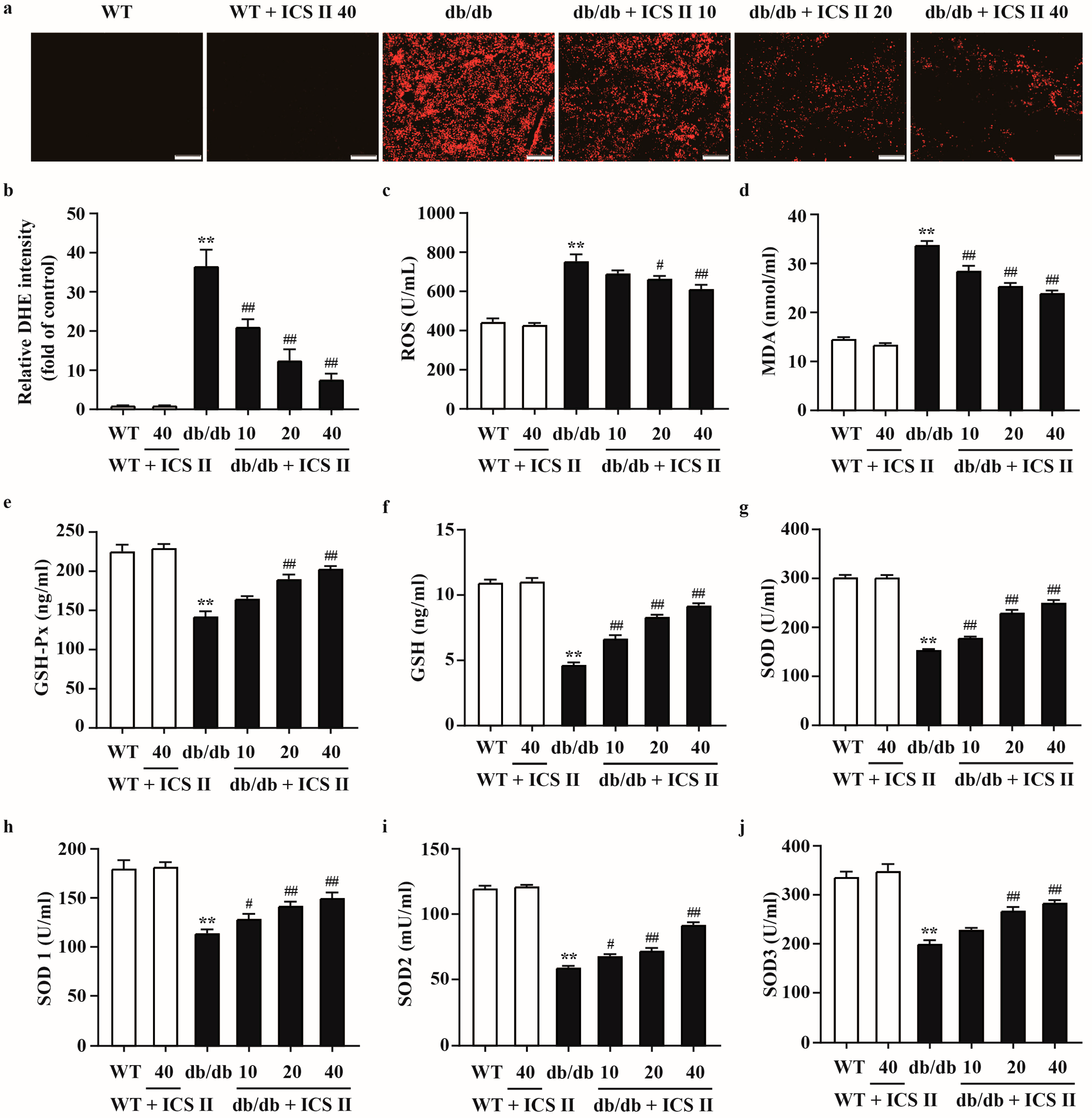
3.4. ICS II Attenuated Oxidative Injury in db/db Mice
3.5. ICS II Elevated the Protein Expressions of PPARα/γ and Inhibited NF-κB Signaling Pathway in db/db Mice
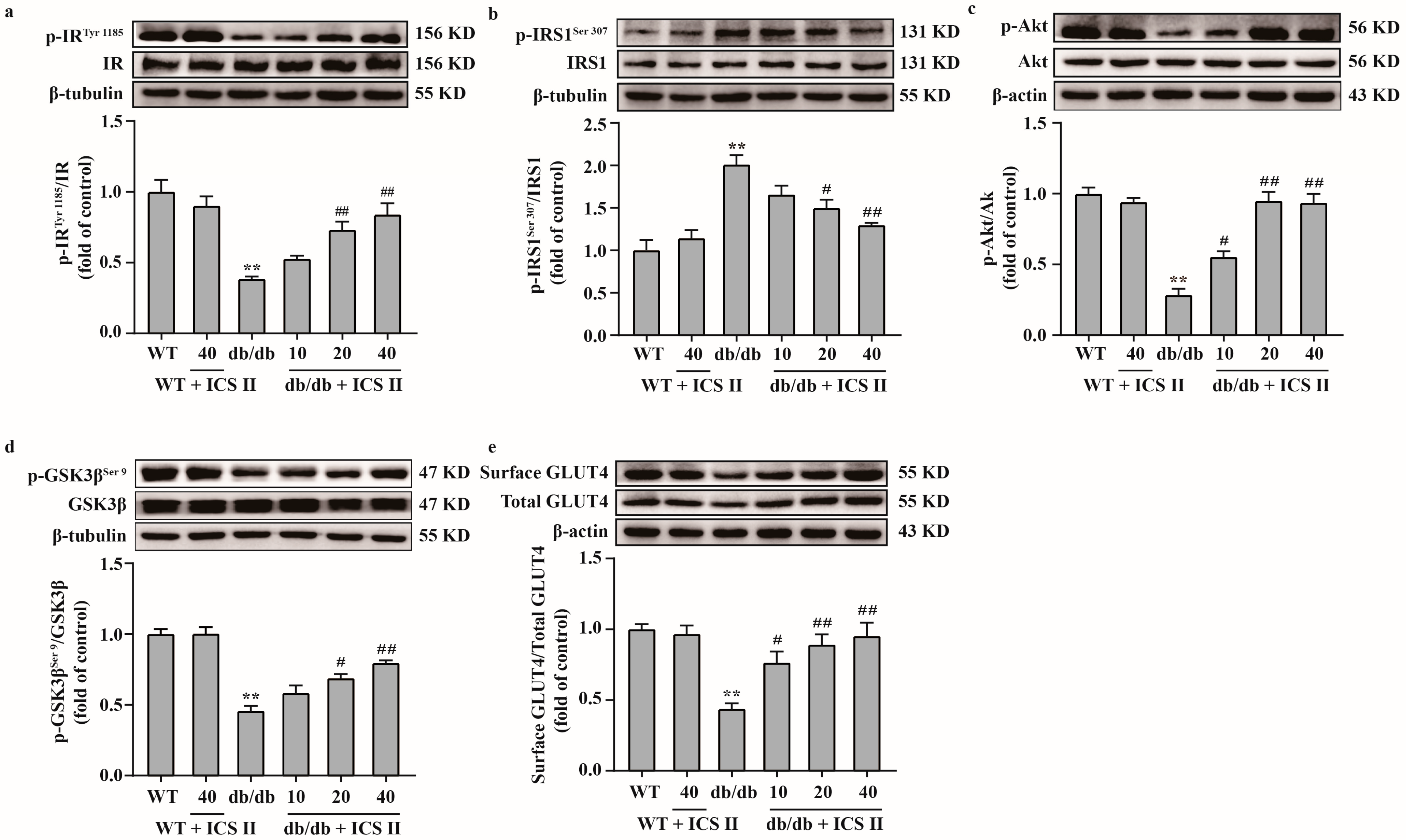
3.6. ICS II Attenuated Insulin Resistance by Regulating the IRS1/Akt Signaling Transduction Pathway in db/db Mice
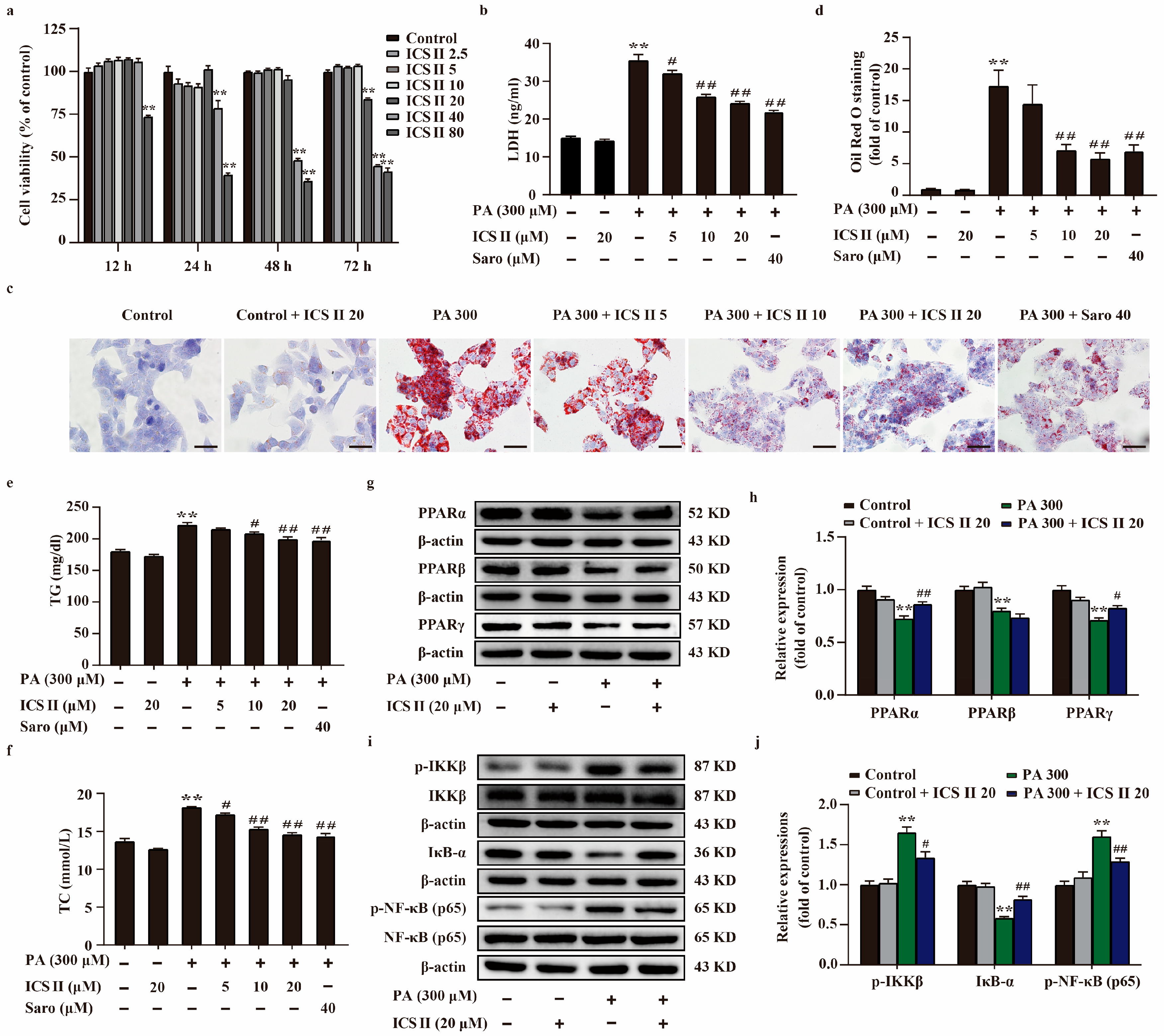
3.7. ICS II Protected against PA-Induced Injury in HepG2 Cells and MIN6 Cells via Upregulating the Protein Expressions of PPARα/γ, and Inhibited NF-κB Signaling Pathway
3.8. ICS II Mitigated Oxidative Stress in PA-Induced HepG2 Cells
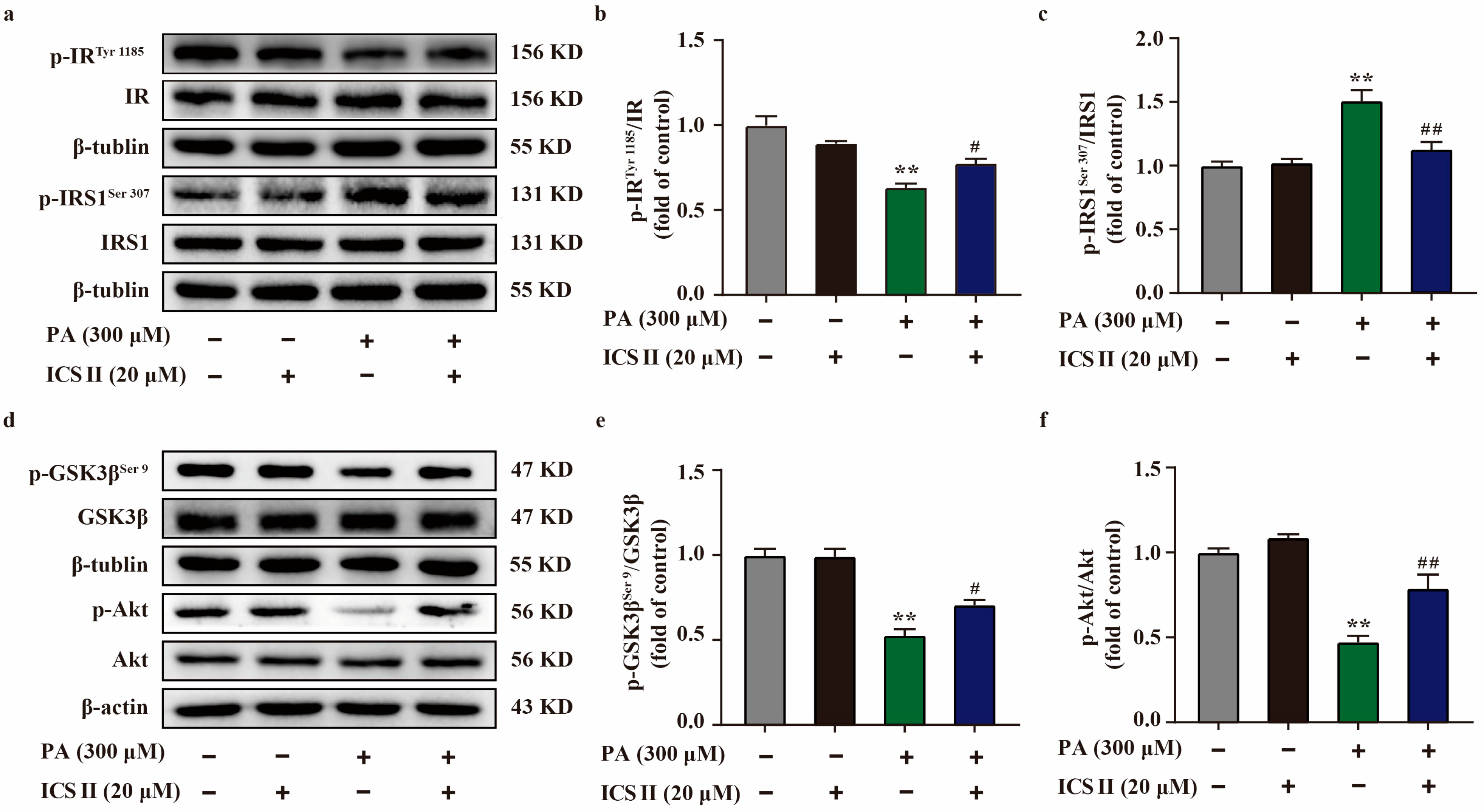
3.9. ICS II Attenuated Insulin Resistance in PA-Induced HepG2 Cells by IRS1/Akt Signaling Transduction Pathway
3.10. Effect of ICS II on PA-Induced Injury in PPARα/γ-KO HepG2 Cells and MIN6 Cells
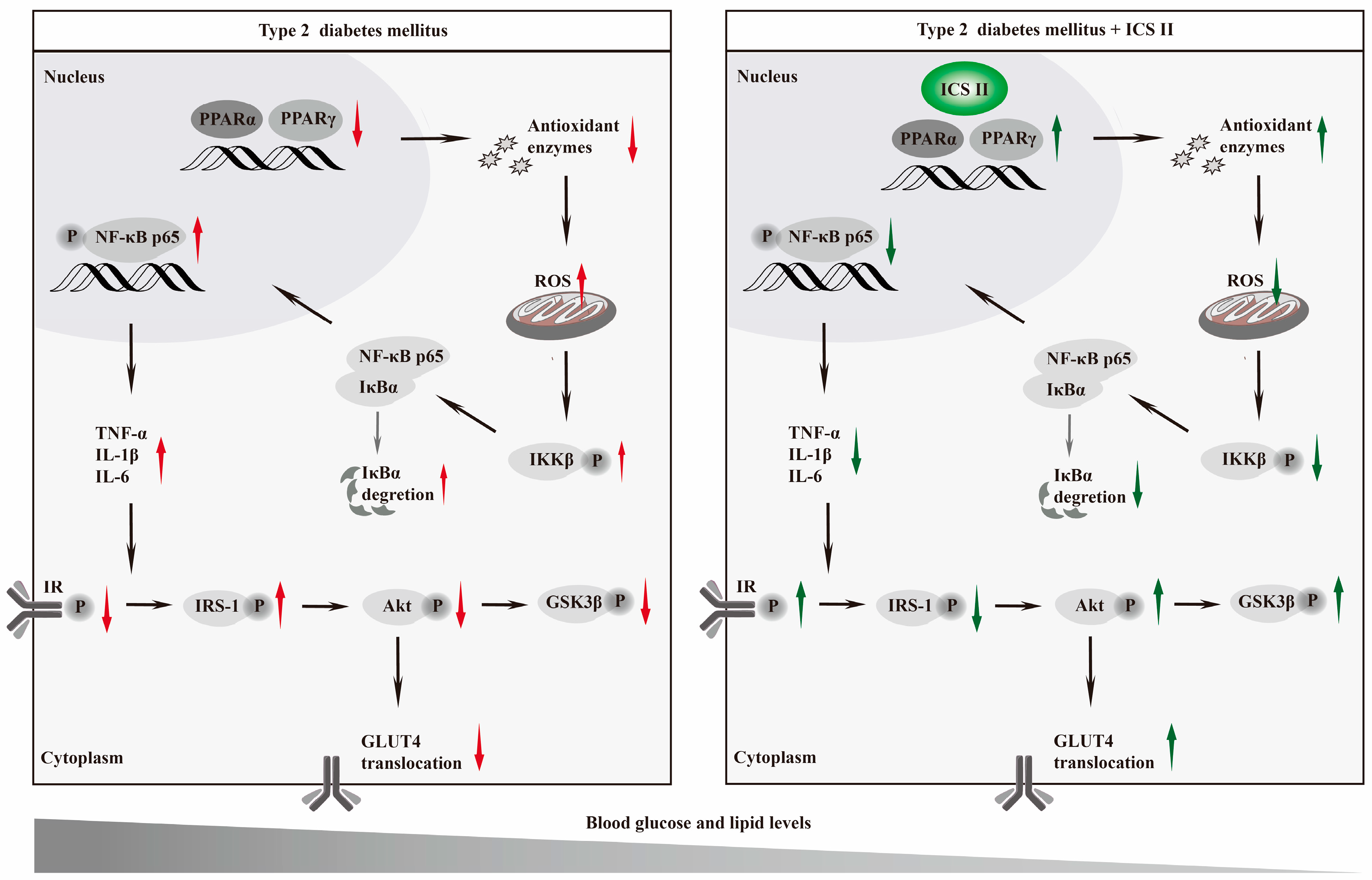
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, S.; Davies, M.J.; Heller, S.; Speight, J.; Snoek, F.J.; Khunti, K. Diabetes structured self-management education programmes: A narrative review and current innovations. Lancet Diabetes Endocrinol. 2018, 6, 130–142. [Google Scholar] [CrossRef]
- Ali, M.K.; Pearson-Stuttard, J.; Selvin, E.; Gregg, E.W. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia 2022, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, E.; Goldberg, T.; Marzella, N. Management of type 2 diabetes: Consensus of diabetes organizations. Drugs Context 2020, 9, 212607. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Abad-Jimenez, Z.; Maranon, A.M.; Iannantuoni, F.; Escribano-Lopez, I.; Lopez-Domenech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Alu, S.N.; Los, E.A.; Ford, G.A.; Stone, W.L. Oxidative Stress in Type 2 Diabetes: The Case for Future Pediatric Redoxomics Studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal therapy with regulated Nrf2/NF-kappaB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Lin, B.Q.; Zhang, C.F.; Cui, L.L.; Ruan, S.X.; Yang, Z.L.; Li, F.; Ji, D. Timosaponin B-II Ameliorates Palmitate-Induced Insulin Resistance and Inflammation via IRS-1/PI3K/Akt and IKK/NF-κB Pathways. Am. J. Chin. Med. 2016, 44, 755–769. [Google Scholar] [CrossRef]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Venteclef, N.; Jakobsson, T.; Steffensen, K.R.; Treuter, E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metab. 2011, 22, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARalpha/PPARgamma coactivator-1alpha pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef]
- Rigano, D.; Sirignano, C.; Taglialatela-Scafati, O. The potential of natural products for targeting PPARalpha. Acta Pharm. Sin. B 2017, 7, 427–438. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef]
- Bortolini, M.; Wright, M.B.; Bopst, M.; Balas, B. Examining the safety of PPAR agonists-current trends and future prospects. Expert Opin. Drug Saf. 2013, 12, 65–79. [Google Scholar] [CrossRef]
- Feng, L.; Luo, H.; Xu, Z.; Yang, Z.; Du, G.; Zhang, Y.; Yu, L.; Hu, K.; Zhu, W.; Tong, Q.; et al. Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-gamma and PPAR-alpha agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice. Diabetologia 2016, 59, 1276–1286. [Google Scholar] [CrossRef]
- Sun, Y.S.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Icariside II inhibits tumorigenesis via inhibiting AKT/Cyclin E/CDK2 pathway and activating mitochondria-dependent pathway. Pharmacol. Res. 2020, 152, 104616. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Long, L.; Wang, K.; Zhou, J.; Zeng, L.; He, L.; Gong, Q. Icariside II, a Broad-Spectrum Anti-cancer Agent, Reverses Beta-Amyloid-Induced Cognitive Impairment through Reducing Inflammation and Apoptosis in Rats. Front. Pharmacol. 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, Y.; Gao, J.M.; Lv, C.; Lang, L.H.; Shi, J.S.; Yu, C.Y.; Gong, Q.H. Icariside II inhibits lipopolysaccharide-induced inflammation and amyloid production in rat astrocytes by regulating IKK/IkappaB/NF-kappaB/BACE1 signaling pathway. Acta Pharmacol. Sin. 2020, 41, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Deng, Y.; Yin, C.; Liu, Y.; Zhang, W.; Shi, J.; Gong, Q. Icariside II, a novel phosphodiesterase 5 inhibitor, protects against H2O2-induced PC12 cells death by inhibiting mitochondria-mediated autophagy. J. Cell. Mol. Med. 2017, 21, 375–386. [Google Scholar] [CrossRef]
- Feng, L.; Gao, J.; Liu, Y.; Shi, J.; Gong, Q. Icariside II alleviates oxygen-glucose deprivation and reoxygenation-induced PC12 cell oxidative injury by activating Nrf2/SIRT3 signaling pathway. Biomed. Pharmacother. 2018, 103, 9–17. [Google Scholar] [CrossRef]
- Yin, C.; Deng, Y.; Liu, Y.; Gao, J.; Yan, L.; Gong, Q. Icariside II Ameliorates Cognitive Impairments Induced by Chronic Cerebral Hypoperfusion by Inhibiting the Amyloidogenic Pathway: Involvement of BDNF/TrkB/CREB Signaling and Up-Regulation of PPARalpha and PPARgamma in Rats. Front. Pharmacol. 2018, 9, 1211. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D.; Yin, C.; Liu, B.; Shi, J.; Gong, Q. Icariside II protects against cerebral ischemia-reperfusion injury in rats via nuclear factor-kappaB inhibition and peroxisome proliferator-activated receptor up-regulation. Neurochem. Int. 2016, 96, 56–61. [Google Scholar] [CrossRef]
- Shi, Y.L.; Zhang, Y.P.; Luo, H.; Xu, F.; Gao, J.M.; Shi, J.S.; Gong, Q.H. Trilobatin, a Natural Food Additive, Exerts Anti-Type 2 Diabetes Effect Mediated by Nrf2/ARE and IRS-1/GLUT2 Signaling Pathways. Front. Pharmacol. 2022, 13, 828473. [Google Scholar] [CrossRef]
- Bril, F.; Cusi, K. Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Call to Action. Diabetes Care 2017, 40, 419–430. [Google Scholar] [CrossRef]
- Johnson, J.D. On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes. Diabetologia 2021, 64, 2138–2146. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, Z.; Deng, L.; Ren, Q.; Cai, Z.; Wang, B.; Li, Z.; Wang, G. HWL-088, a new and highly effective FFA1/PPARdelta dual agonist, attenuates nonalcoholic steatohepatitis by regulating lipid metabolism, inflammation and fibrosis. J. Pharm. Pharmacol. 2020, 72, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. beta-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014, 37, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; Garcia-Ocana, A.; Stewart, A.F. Diabetes mellitus--advances and challenges in human beta-cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yang, X.; Wu, D.; Xing, S.; Bian, F.; Li, W.; Chi, J.; Bai, X.; Wu, G.; Chen, X.; et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3beta pathway. Br. J. Pharmacol. 2015, 172, 3284–3301. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, N.; Yang, X.; Hou, T.; Fu, W.; Yuan, F.; Wang, L.; Wen, H.; Tian, Y.; et al. WDFY2 Potentiates Hepatic Insulin Sensitivity and Controls Endosomal Localization of the Insulin Receptor and IRS1/2. Diabetes 2020, 69, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-R.; Alam, M.B.; Lee, S.-H. Terpenoid-Rich Extract of Dillenia indica L. Bark Displays Antidiabetic Action in Insulin-Resistant C2C12 Cells and STZ-Induced Diabetic Mice by Attenuation of Oxidative Stress. Antioxidants 2022, 11, 1227. [Google Scholar] [CrossRef]
- Hancock, M.L.; Meyer, R.C.; Mistry, M.; Khetani, R.S.; Wagschal, A.; Shin, T.; Ho Sui, S.J.; Naar, A.M.; Flanagan, J.G. Insulin Receptor Associates with Promoters Genome-wide and Regulates Gene Expression. Cell 2019, 177, 722–736. [Google Scholar] [CrossRef]
- Chakraborty, A.; Koldobskiy, M.A.; Bello, N.T.; Maxwell, M.; Potter, J.J.; Juluri, K.R.; Maag, D.; Kim, S.; Huang, A.S.; Dailey, M.J.; et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 2010, 143, 897–910. [Google Scholar] [CrossRef]
- Zhu, Y.; Jing, L.; Li, X.; Zheng, D.; Zhou, G.; Zhang, Y.; Sang, Y.; Shi, Z.; Sun, Z.; Zhou, X. Decabromodiphenyl ether disturbs hepatic glycolipid metabolism by regulating the PI3K/AKT/GLUT4 and mTOR/PPARgamma/RXRalpha pathway in mice and L02 cells. Sci. Total Environ. 2021, 763, 142936. [Google Scholar] [CrossRef]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Alrashood, S.T.; Alharbi, S.A.; Ignacimuthu, S. Friedelin exhibits antidiabetic effect in diabetic rats via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2021, 268, 113659. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of PPARgamma and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta 2013, 1830, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zheng, M.; Zhang, R.; Zhang, H.; Liu, Y.; Li, W.; Sun, X.; Yu, Q.; Tipoe, G.L.; Xiao, J. S-Allylmercaptocysteine improves alcoholic liver disease partly through a direct modulation of insulin receptor signaling. Acta Pharm. Sin. B 2021, 11, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Cui, S.C.; Zheng, T.N.; Ma, H.J.; Xie, Z.F.; Jiang, H.W.; Li, Y.F.; Zhu, K.X.; Huang, C.G.; Li, J.; et al. Sarsasapogenin improves adipose tissue inflammation and ameliorates insulin resistance in high-fat diet-fed C57BL/6J mice. Acta Pharmacol. Sin. 2021, 42, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Cardoso, S. Diabetes-Alzheimer’s Disease Link: Targeting Mitochondrial Dysfunction and Redox Imbalance. Antioxid. Redox Signal. 2021, 34, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cocheme, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Clayton, J.A. NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar]





| Gene | gRNA Sequences |
|---|---|
| PPARα | CACCGCATCTGTCCTCTCTCCCCAC AAAC GTGGGGAGAGAGGACAGATG |
| PPARγ | CACCGCAACTTCGGAATCAGCTCTG AAACCAGAGCTGATTCCGAAGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Y.; Chen, N.; Feng, L.; Gao, J.; Zeng, N.; He, Z.; Gong, Q. Icariside II Exerts Anti-Type 2 Diabetic Effect by Targeting PPARα/γ: Involvement of ROS/NF-κB/IRS1 Signaling Pathway. Antioxidants 2022, 11, 1705. https://doi.org/10.3390/antiox11091705
Li Y, Li Y, Chen N, Feng L, Gao J, Zeng N, He Z, Gong Q. Icariside II Exerts Anti-Type 2 Diabetic Effect by Targeting PPARα/γ: Involvement of ROS/NF-κB/IRS1 Signaling Pathway. Antioxidants. 2022; 11(9):1705. https://doi.org/10.3390/antiox11091705
Chicago/Turabian StyleLi, Yiqi, Yeli Li, Nana Chen, Linying Feng, Jianmei Gao, Nan Zeng, Zhixu He, and Qihai Gong. 2022. "Icariside II Exerts Anti-Type 2 Diabetic Effect by Targeting PPARα/γ: Involvement of ROS/NF-κB/IRS1 Signaling Pathway" Antioxidants 11, no. 9: 1705. https://doi.org/10.3390/antiox11091705
APA StyleLi, Y., Li, Y., Chen, N., Feng, L., Gao, J., Zeng, N., He, Z., & Gong, Q. (2022). Icariside II Exerts Anti-Type 2 Diabetic Effect by Targeting PPARα/γ: Involvement of ROS/NF-κB/IRS1 Signaling Pathway. Antioxidants, 11(9), 1705. https://doi.org/10.3390/antiox11091705







