Abstract
Essential oils are widely used in aromatherapy, medicine, and food industries due to a wide spectrum of bioactivity. Their antioxidant properties can be considered as markers of therapeutic effect and quality. Constant–current coulometry with electrogenerated titrants has been successfully applied for these purposes for the first time. Fifteen types of essential oils from various plant materials have been studied. Their composition has been identified by gas chromatography with mass-spectrometric detection (GC-MS). The reactivity of individual antioxidants of essential oils towards electrogenerated titrants (bromine and ferricyanide ions) has been estimated. Total antioxidant parameters, in particular total antioxidant capacity (TAC) and ferric reducing power (FRP) based on the reactions of essential oil antioxidants with electrogenerated bromine and ferricyanide ions, respectively, have been evaluated. Positive correlations (r = 0.7051–0.9558) with common antioxidant tests (antioxidant activity by reaction with 2,2-diphenyl-1-picrylhydrazyl (DPPH•) and total phenolic content by the Folin–Ciocalteu method) have been obtained. Coulometric approaches overcome the limitations of spectrophotometry and are applicable to a wider range of essential oils.
1. Introduction
Plant materials are widely studied objects in different branches of chemistry. One of the intensively developed directions in this field is the investigation of the antioxidant properties and their impact on human health. Various types of plant material-based samples (extracts, decoctions, infusions, tinctures, oleoresins and essential oils) are in focus to date. Among them, essential oils are of practical interest due to their wide spectrum of biological activity and application area. In nature, essential oils are involved in the plant defense system and signaling processes. For example, plant aroma compounds participate in the protection of plants from microorganisms, insects, and herbivores, in the attraction of the pollinating insects and fruit-distributing animals, in water regulation, and allelopathic interactions [1].
Since ancient times, essential oils have been used in aromatherapy [2]. Antibacterial, anti-inflammatory, antioxidant and antiviral properties of essential oils are successfully used in the auxiliary therapy of a wide range of human diseases [3]. On the other hand, these properties make possible application of the essential oils as additives in the food industry to increase the shelf life of final products [4,5].
Essential oils are highly concentrated extracts from flowers, leaves, stems, fruits, and roots obtained by hydrodistillation, steam or dry distillation, as well as mechanically (for citrus fruits) [1]. The major constituents of essential oils determining their aromas and bioactivity are saturated and unsaturated hydrocarbons, alcohols, aldehydes, esters and ethers, ketones, phenols, and terpenes [6,7]. Many of them possess antioxidant properties allowing the usage of total antioxidant parameters for the essential oils’ characterization.
Antioxidant properties of essential oils are usually evaluated by spectrophotometric approaches [8] characterized by simplicity and cost-efficiency. Typical antioxidant parameters estimated are the antioxidant activity by reaction with 2,2-diphenyl-1-picrylhydrazyl (DPPH•) [9,10,11,12,13] or peroxyl radicals obtained from 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+•) [11,12,13,14]. The total phenolics assay performed by the Folin–Ciocalteu method [15,16], ferric reducing-antioxidant power (FRAP) and β-carotene bleaching assay are used more rarely [9,13,14]. Furthermore, extracts obtained from corresponding plant materials are often studied instead of essential oil. Most published works are focused on the essential oils from one type of plant material, while the screening studies are almost out of consideration.
Further development in the field could be focused on the development of electrochemical approaches for the evaluation of essential oils’ antioxidant parameters as far as reactions of antioxidants proceed with the electron transfer that partially mimicks their action in living systems. Moreover, a wide range of electrochemical methods has been developed for the estimation of total antioxidant parameters of plant materials and their extracts [17,18,19,20,21,22,23,24]. Electrooxidation of the plant antioxidants at the electrode surface under conditions of chronocoulometry [17] and voltammetric monitoring of the reactions with DPPH• [18] and galvinoxyl [19] radicals immobilized at the electrode surface as well as homogeneous reactions with electrochemically generated superoxide anion radicals [22,23,24] that have been successfully realized for the plant extracts, medicinal plant tinctures, infusions and decoctions. Unfortunately, essential oils almost do not get attention as samples of interest in electroanalytical estimation of total antioxidant parameters nowadays. Just one voltammetric approach based on the reaction of antioxidants with superoxide anion radicals has been reported with an example of the Mentha species [24]. The method is based on the decrease of oxygen electroreduction currents corresponding to the formation of superoxide anion radicals in the presence of essential oils. Differential pulse voltammetry has been used for reaction monitoring.
Summarizing that mentioned above, development of simple, reliable and cost-effective electrochemical approaches for the evaluation of total antioxidant parameters of essential oils is of practical interest. Constant–current coulometry with electrogenerated titrants has been shown to be a powerful tool for the estimation of total antioxidant parameters of different types of foodstuffs, biological fluids, spice extracts, and phytopharmaceuticals [25]. Halogens (iodine, bromine, and chlorine) and hypohalogenite ions are used as coulometric titrants. Halogens are generated from halogenides in acidic medium, while hypohalogenites in basic medium [25,26]. Electrogenerated halogens differ by oxidative strength, which is increased in the order iodine < bromine < chlorine. Bromine acting as an oxidant of average strength is the most suitable for evaluating the antioxidant properties of various samples. Iodine is too weak an oxidant and does not react with many antioxidants [25]. On the contrary, chlorine is a very strong oxidant leading to deep transformation of the antioxidants as well as to the side reactions that is reflected in the irreproducible stoichiometric coefficients of the titration [27]. Hypohalogenite are used in organic analysis for the determination of sulfanilamide preparations, acid hydrazides and amides, amino acids, and sulfur-containing pharmaceuticals which are stable in basic media [26].
The application of a coulometric approach to the analysis of essential oils will expand its analytical capabilities.
The aim of the present study is to show applicability of constant–current coulometry with electrogenerated titrants for the essential oils analysis. This type of reagent is favorable vs. radicals or other typical reagents due to the following reasons:
- No need to prepare a standard solution of the reagents i.e., the standardization stage is excluded;
- Titration is an absolute that excludes usage of calibration plots (in fact, an electron acts as a titrant);
- No effect of sample dilution;
- Coverage of almost all types of antioxidants based on titrants’ reactivity;
- Ease of calculation and the possibility of using different standard antioxidants;
- High sensitivity, reliability, and reproducibility of the measurements;
- Possibility of automation for routine applications.
Coulometric titrants (electrogenerated bromine and ferricyanide ions) have been used as reactive species. Their reactions with individual antioxidants of essential oils have been studied. The data obtained allow evaluation of the total parameters of essential oils in particular total antioxidant capacity (TAC) and ferric reducing power (FRP). Fifteen types of essential oils from various plant materials have been investigated and their components identification has been performed by GC-MS. The correlation analysis of total antioxidant parameters with antioxidant activity towards DPPH• and total phenolic contents has been carried out.
2. Materials and Methods
2.1. Reagents and Samples under Investigation
Thymol of 99.5% purity (Sigma, Steinheim, Germany), 98% carvacrol, 99% eugenol, 98% syringaldehyde (Aldrich, Steinheim, Germany), 99% vanillin and 97% phythol (Sigma-Aldrich, Steinheim, Germany), 99% benzyl alcohol, 96% limonene, 98% α-pinene, 98% β-pinene, 98% D-carvone, 75% camphene, as well as α-fenchene, myrcene, 99.5% L-menthol, and 97% L-borneol (Acros Organics, Geel, Belgium) were used as standard antioxidants. Their 10 mM stock solutions were prepared by dissolving an accurately weighed portion in 5.0 mL of ethanol (rectificate). Less concentrated solutions were obtained with an exact dilution prior to measurements. Other reagents were of chemical purity and used as received.
The commercially available essential oils of various plant materials (Table 1) of different trademarks were studied. Their ten-fold dilution with ethanol was used exactly before the measurements.

Table 1.
Essential oils under investigation.
2.2. Constant–Current Coulometry
Coulometric measurements were carried out on the coulometric analyzer “Exper-006” (Econix-Expert, Moscow, Russia) supplied with the electrochemical cell consisting of four electrodes: a working electrode (bare platinum wire with 0.5 cm2 surface area), an auxiliary electrode (platinum wire separated from the anodic compartment of the cell with the semipermeable membrane), and two indicator electrodes (platinum needle electrodes) for the detection of the titration end point. A polarizing potential of 200 mV was applied between the indicator electrodes. The surface of the platinum electrodes was cleaned with nitric acid followed by rinsing with distilled water.
Constant–current generation of coulometric titrants was performed at the current density of 5 mA cm−2 providing their 100% current yield. 0.2 M KBr in 0.1 M sulfuric acid and 0.1 M K4Fe(CN)6 in 2 M sodium hydroxide were used as precursors and supporting electrolytes for the generation of bromine and ferricyanide ions, respectively.
Coulometric titration was performed in a 50 mL cell containing 20.0 mL of the precursor in the supporting electrolyte. The generating circuit was switched on until a 40 µA indicator current was achieved. Then, an aliquot portion (10–100 μL) of the antioxidant standard solution or 10-fold diluted essential oil was added to the cell, and the timer was started simultaneously. The titration end-point was detected by achieving the initial value of the indicator current. The timer was stopped, and the generating circuit was turned off. Faraday’s law of electrolysis was used for the calculation of the number of electrons participating in the reaction of individual antioxidants with titrants.
The total antioxidant parameters, in particular the total antioxidant capacity and ferric reducing power, were evaluated for the essential oils as the quantity of electricity spent for the titration of the sample and recalculated per 1 mL of the essential oil.
2.3. GC-MS Identification of Essential Oil Components
Essential oils were dissolved in diethyl ether in 1:10 (v/v) ratio [28] and analyzed by GC-MS using an Agilent Technologies 7890B GC System equipped with a HP-5MS capillary column ((5%-phenyl)-methylpolysiloxane phase, 30 m, 0.25 mm i.d. and 0.25 μm film thickness) and mass-selective detector Agilent Technologies 5977A MSD (Agilent Technologies, Santa Clara, CA, USA). In addition, 1 µL of the diluted sample was injected in the 1:50 split and splitless mode. Acetylation, methylation and silylation procedures were performed using standard approaches [29,30,31]. The samples obtained were measured in splitless mode. GC analysis was performed under the following conditions: injector and detector temperatures were set at 280 °C. The column temperature was initially 50 °C for 2 min, then gradually increased to 240 °C at 3 °C min−1, and finally increased to 280 °C at 20 °C min−1 and kept at 280 °C for 5 min. Helium with a flow rate of 1 mL min−1 was used as a carrier gas. An electron ionization system with an ionization energy of 70 eV was used. Mass spectra were recorded with total ion currents over m/z 20−550 Da. The components identification was performed by comparison of the retention times and retention indices with reported ones [32,33] as well as by matching the mass-spectra obtained with data from NIST 14 (National Institute of Standards and Technologies, Mass Spectra Libraries, Palmer, MA, USA) [34]. The NIST MS Search 2.2 software (National Institute of Standards and Technologies, Mass Spectra Libraries, Palmer, MA, USA) was used for these purposes.
2.4. Antioxidant Activity Assay
DPPH• stable radical (Aldrich, Steinheim, Germany) was used as a reagent to evaluate the antioxidant activity of essential oils. The standard procedure was applied [35]. Briefly, 4 μL of 10-fold diluted ethanol essential oil were thoroughly mixed with 3 mL of 0.10 mM DPPH• methanol solution and incubated in the dark for 20 min. Then, the absorption was read at 515 nm vs. methanol with the addition of 4 μL of the sample using spectrophotometer PE-5300 (NPO Ecros, Saint Petersburg, Russia). Control DPPH• absorption was measured vs. methanol. Antioxidant activity of the essential oils was expressed as a relative decrease of the DPPH• absorption due to the reaction with the antioxidants of the sample.
2.5. Total Phenolics Assay
The Folin–Ciocalteu colorimetric method [36] with some modifications was used for the total phenolics quantification. In addition, 0.5 mL of the sample (10,000-fold diluted clove and thyme essential oils and 1000-fold diluted cinnamon and nutmeg essential oils) or standard solution of eugenol (5.00, 9.98, 25.0, 50.1, 75.4 and 100.5 mg L−1) were placed in a 5.0 mL volumetric flask. Then, 2.5 mL of diluted Folin–Ciocalteu reagent (1:10 (v/v)) (Aldrich, Steinheim, Germany) were added and thoroughly mixed. After 4 min, 2.5 mL of 7.5% Na2CO3 solution were added, mixed and incubated for 1 h. The absorbance of the solution was measured at 765 nm in a 0.5 cm cuvette. The blank solution contained all the reagents excluding essential oil, which was replaced with 0.5 mL of ethanol. Total phenolic content was expressed in eugenol equivalents per 1 mL of essential oil.
2.6. Statistics and Correlation Analysis
Five parallel measurements were performed for constant–current coulometry (three replications for chromatography and spectrophotometry). A significance level of 5% was used for the statistical treatment of the experimental data. The results were presented as an average value and a coverage interval. Relative standard deviation (RSD) was used for random error characterization.
Correlation analysis was performed using OriginPro 8.1 software (OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. Essential Oils Characterization by GC-MS
The essential oils under consideration have been studied by GC-MS for the components identification. The number of constituents varied in a range of 30–79 depending on the type of essential oil (Table 2).

Table 2.
Essential oils composition according to GC-MS data (RI—retention index).
The major components of the essential oils are terpenes and their derivatives as well as phenolic volatile compounds (eugenol, carvacrol, thymol and methoxyphenolics). The most widely distributed terpenes are α-pinene, camphene, β-pinene, myrcene, tricyclene, α-thujene, m-cymene, limonene, eucalyptol, trans-β-ocimene, γ-terpinene, linalool, α-terpineol, linalyl acetate, α-copaene, β-elemene, trans-β-caryophyllene, caryophyllene oxide I, α-humulene, α- and γ-muurolene. Other terpenoids are more specific to the type of essential oil. The chromatographic data obtained agree well with those reported in [1,37,38,39] for essential oils of various plants.
Such composition of essential oils suggests the possibility of using constant–current coulometry with electrogenerated titrants to assess their antioxidant properties. Coulometric titration of essential oils antioxidants has been performed for confirmation.
3.2. Reactions of Individual Antioxidants with Electrogenerated Titrants
The reactivity of individual antioxidants of essential oils toward coulometric titrants (electrogenerated bromine and ferricyanide ions) has been studied. These two reagents are very different in properties.
Oxidation of bromide ions at a platinum electrode in acidic medium results in the formation of tribromide anion, molecular bromine and short-living bromine radicals adsorbed at the electrode surface [25]. Therefore, electrogenerated bromine acts as an oxidizing agent of medium strength, and also participates in reactions of electrophilic addition to multiple bonds and electrophilic substitution in aromatic systems. This behavior of the titrant makes it possible to cover a wide range of antioxidants of various nature and action mechanisms, in particular, phenolic compounds, terpenoids and their derivatives, as well as unsaturated hydrocarbons.
Electrogenerated ferricyanide ions show relatively weak oxidative properties. On the other hand, it acts as a one-electron oxidant that prevents possible accompanying steps that complicate the reaction.
3.2.1. Reactivity of Essential Oils Antioxidants towards Electrogenerated Bromine
Reactions with electrogenerated bromine proceed rapidly and quantitatively for most of the antioxidants studied (phenolics, terpenes, and their derivatives). The number of electrons participating in the reactions calculated using Faraday’s law is consistent with the number of reactive functional groups in their structure. The only exclusions are benzyl alcohol, L-menthol and L-borneol (Figure 1) that do not interact with the titrant due to the absence of reactive functional groups in their structure.
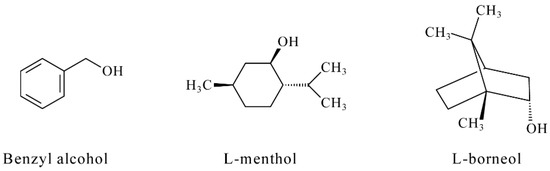
Figure 1.
Structure of benzyl alcohol, L-menthol and L-borneol.
Eugenol, carvacrol and thymol (Figure 2) interact with electrogenerated bromine with the participation of 4, 5 and 4 electrons, respectively. Eugenol is oxidized to o-quinone with the participation of two electrons as well as the electrophilic addition of bromine to double bond occurs. As for the isopropylmethylphenols (carvacrol and thymol), both oxidation and bromination reactions take place, leading to the formation of several products, in particular thymoquinone and mono- and dibromo derivatives [40,41].
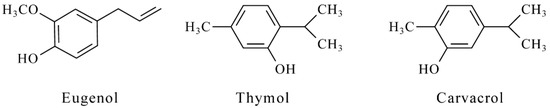
Figure 2.
Structure of the main phenolic antioxidants of essential oils.
Terpenes and their derivatives (Figure 3) interact with electrogenerated bromine via its electrophilic addition to double bonds.
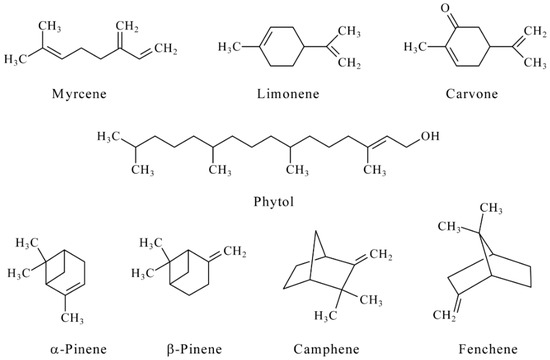
Figure 3.
The structure of the terpenes under consideration.
Thus, reactions of all studied compounds except myrcene and limonene proceed with the participation of two electrons that agree well with the number of double bonds in their structure. Similar behavior has been found for limonene, for which four electrons are involved in the reaction with titrant that means bromination on both double bonds. Myrcene also reacts with electrogenerated bromine with four electrons transfer i.e., one of the double bonds is inactive, which is probably caused by sterical effects. Therefore, conjugated double bonds undergo a reaction (Scheme 1).
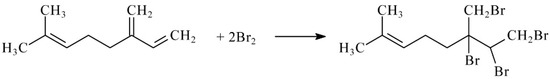
Scheme 1.
Reaction of myrcene with electrogenerated bromine.
3.2.2. Reactivity of Essential Oils Antioxidants towards Electrogenerated Ferricyianide Ions
Ferricyanide ions react with phenolic antioxidants, only allowing to conclude that phenolic hydroxyl groups are oxidized that agree with data for hydroxybenzoic acids [42] and flavonoids [43]. Eugenol, carvacrol and thymol undergo one electron oxidation that coincides with the number of hydroxyl groups in their structure. As long as titration with ferricyanide ions is performed in basic medium, the relatively easy electron detachment from phenolate ions takes place with the formation of the phenoxyl radical according to Scheme 2. Similar data have been reported for the reactions of these phenolic antioxidants in micellar medium of Triton X-100 [44] and Brij® 35 [45].
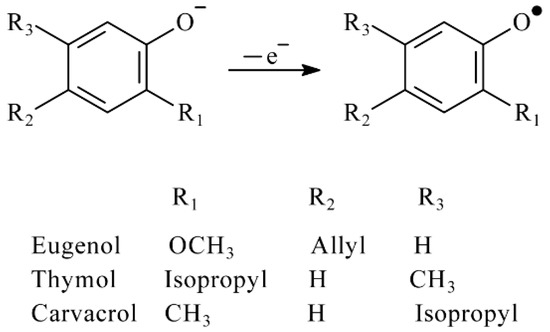
Scheme 2.
Reaction of phenolic antioxidants with electrogenerated ferricyanide ions.
The number of electrons participating in the reactions of all antioxidants under consideration with electrogenerated titrants are reproduced in repeated measurements and are consistent with that reported for some of them [25], which confirms the accuracy of the data obtained. Thus, coulometric titration with electrogenerated bromine and ferricyanide ions can be applied to evaluate the antioxidant properties of essential oils.
3.3. Evaluation of Total Antioxidant Parameters of Essential Oils
Total antioxidant parameters of the essential oils in particular TAC and FRP based on the sample’s titration with electrogenerated bromine and ferricyanide ions, respectively, have been estimated. A screening of 15 types of essential oils has been carried out (Table 3).

Table 3.
Total antioxidant parameters of the essential oils based on the coulometric titration data (n = 5; p = 0.95).
The highest values of both parameters have been obtained for clove essential oils, which is caused by the high content of eugenol being the major constituent. Its concentration in clove essential oil is usually at least 50% [46]. The lower eugenol content in the essential oils of cinnamon, nutmeg and basil (2.46–0.023% according to the chromatographic determination) is reflected in their antioxidant parameters. In the case of the essential oil of thyme, a high FRP is caused by the presence of carvacrol (54.6 ± 0.1%), thymol (0.31 ± 0.05%) and trace eugenol. Isopropylmethylphenols (thymol and carvacrol) in marjoram, thymol in clary sage and trace amounts of eugenol in jasmine are the major contributors to the FRP of these essential oils. Other essential oils are almost free of phenolic antioxidants, and their components do not react with the electrogenerated ferricyanide ions. Thus, the FRP parameter reflects the contents of phenolic antioxidants in the essential oils that agree with reported for spices micellar extracts [44,45] and other plant-derived products [42,43].
High TAC (1220 ± 28 C mL−1) of the neroli essential oil is caused by the presence of terpenoids, in particular β-pinene, limonene, linalool and linalyl acetate reacting with electrogenerated bromine. Other essential oils that are free of volatile phenolics show significantly less TAC (83–459 C mL−1), which is reflected in their terpene constituents.
As can be seen in Table 3, the antioxidant parameters for the different samples of the same type of essential oils agree well with each other in the case of clove, cinnamon, lavender, and rosemary. Significantly different TAC values have been obtained for anise, bergamot, jasmine, and marjoram samples of various trademarks. This phenomenon can be caused by plant material characteristics that are strongly dependent on the origin, growing and harvesting conditions, vegetation stage, storage, and so on, as well as conditions of essential oil preparation [47]. Probably, these factors affect more strongly the terpenoid constituents and their contents as can be seen from the TAC and FRP values.
The difference in the TAC and FRP allows for concluding that TAC is a preferable parameter for screening purposes as long as it is applicable to all types of essential oils and allows for evaluating a wider range of antioxidants presented in the sample due to the different mechanisms of coulometric titrant action.
3.4. Correlation of Essential Oils TAC and FRP with Standard Antioxidant Parameters
Standard antioxidant parameters (antioxidant activity by reaction with DPPH• and total phenolic contents) based on spectrophotometric data have been evaluated (Table 4). It should be noted that spectrophotometric determination of total phenolic content by the Folin–Ciocalteu method could not be applied to all samples under investigation due to the formation of turbid solutions after the addition of photometric reagents. Therefore, only essential oils of clove, cinnamon, nutmeg and thyme can be studied. Furthermore, the method is time-consuming (more than 1 h), requiring application of several additional reagents.

Table 4.
Standard antioxidant parameters of essential oils based on spectrophotometry data (n = 3; p = 0.95).
The major disadvantages of the evaluation of antioxidant activity by reaction with DPPH• are the following:
- Instability of the reagent solution under light exposure;
- Necessity to use toxic methanol;
- Limited range of antioxidants reacting with the DPPH•;
- Certain antioxidants interact with DPPH• slowly and/or reversibly that leads to distortion of results and their incorrect interpretation;
- Necessity to prepare a blank solution for each sample makes the method more tedious.
Coulometric titration does not have the limitations mentioned above, which is a favorable advantage over standard procedures. Moreover, the RSD values for coulometric determination are less than those for spectrophotometry, especially in the case of DPPH•-test, confirming better reproducibility of the determination.
The validation of TAC and FRP of essential oils has been carried out by comparison with antioxidant activity by reaction with DPPH• and total phenolic contents. Positive significant correlations have been obtained for both parameters (Figure S1). The r-values (Table 5) indicate a strong correlation between the parameters according to the Chaddock scale [48].

Table 5.
Correlation coefficients of the antioxidant parameters of essential oils.
In general, correlations obtained allow for concluding that coulometric titration with electrogenerated bromine and ferricyianide ions is a promising tool in the essential oils analysis, in particular, for the screening of their antioxidant properties.
4. Conclusions
Coulometric titration with electrogenerated bromine and ferricyanide ions has been suggested for the evaluation of the essential oils’ total antioxidant parameters. Data for the reaction of individual antioxidants (volatile phenolics and terpenes) with coulometric titrants confirm the applicability of the method for the characterization of essential oils TAC and FRP. Screening of 27 samples from 15 types of essential oils has been performed and statistically significant difference between the samples has been observed. The data obtained agree well with the standard spectrophotometric approaches confirming the accuracy of the coulometric methods. Disadvantages of spectrophotometric methods like instability of DPPH• solution, application of several reagents, time-consuming procedures and limited applicability to essential oils samples are successfully overcome in coulometry. Moreover, the method can be easily automated, which provides high throughput (the average time of one titration is approximately 1 min) and applicability in routine analysis.
Thus, coulometric titration has been shown to be an effective and promising tool for the characterization of the essential oils via total antioxidant parameters that enlarge the practical application area of the method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11091749/s1, Figure S1: Correlation plots of the essential oils antioxidant parameters. (a) TAC vs. DPPH•; (b) FRP vs. DPPH•; (c) TAC vs. total phenolics; (d) FRP vs. total phenolics.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z. and O.K.; validation, G.Z., A.K. and O.K.; formal analysis, G.Z., A.K. and O.K.; investigation, G.Z., A.K. and O.K.; writing—original draft preparation, G.Z. and A.K.; writing—review and editing, G.Z.; visualization, G.Z., A.K. and O.K.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
This paper has been supported by the Kazan Federal University Strategic Academic Leadership Program (‘PRIORITY-2030’).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer: Cham, Switzerland, 2015; pp. 19–61. [Google Scholar] [CrossRef]
- Esposito, E.R.; Bystrek, M.V.; Klein, J.S. An elective course in aromatherapy science. Am. J. Pharm. Educ. 2014, 78, 79. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M. Introduction to the special issue: Application of essential oils in food systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef]
- Schiller, C.; Schiller, D. 500 Formulas for Aromatherapy: Mixing Essential Oils for Every Use; Sterling Publications: New York, NY, USA, 1994; 158p. [Google Scholar]
- Wildwood, C. The Encyclopedia of Aromatherapy; Healing Arts Press: Rochester, NY, USA, 1996; 320p. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Anthony, K.P.; Deolu-Sobogun, S.A.; Saleh, M.A. Comprehensive assessment of antioxidant activity of essential oils. Food Sci. 2012, 77, C839–C843. [Google Scholar] [CrossRef]
- Olmedo, R.; Ribotta, P.; Grosso, N.R. Antioxidant activity of essential oils extracted from Aloysia triphylla and Minthostachys mollis that improve the oxidative stability of sunflower oil under accelerated storage conditions. Eur. J. Lipid Sci. Technol. 2018, 120, 1700374. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Yucel, E.; Ozturk, B.; Kilic, G. Chemical compositions, total phenolic contents, antimicrobial and antioxidant activities of the extract and essential oil of Thymbra spicata L. growing wild in Turkey. Food Meas. 2021, 15, 386–393. [Google Scholar] [CrossRef]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2017, 13, S875–S880. [Google Scholar]
- Hashemi, S.M.B.; Khorram, S.B.; Sohrabi, M. Antioxidant activity of essential oils in foods in essential oils in food processing. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Hashemi, S.M.B., Mousavi Khaneghah, A., de Souza Sant’Ana, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 247–265. [Google Scholar] [CrossRef]
- Crespo, Y.A.; Sánchez, L.R.B.; Quintana, Y.G.; Cabrera, A.S.T.; del Sol, A.B.; Mayancha, D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Helyon 2019, 5, e01942. [Google Scholar] [CrossRef]
- Boveiri Dehsheikh, A.; Mahmoodi Sourestani, M.; Boveiri Dehsheikh, P.; Vitalini, S.; Iriti, M.; Mottaghipisheh, J. A Comparative Study of Essential Oil Constituents and Phenolic Compounds of Arabian Lilac (Vitex trifolia var. Purpurea): An Evidence of Season Effects. Foods 2019, 8, 52. [Google Scholar] [CrossRef]
- Semiz, G.; Semiz, A.; Mercan-Doğan, N. Essential oil composition, total phenolic content, antioxidant and antibiofilm activities of four Origanum species from southeastern Turkey. Int. J. Food Prop. 2018, 21, 194–204. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kozlova, E.; Morozova, E.; Budnikov, H. Chronocoulometric method for the evaluation of antioxidant capacity of medicinal plant tinctures. Anal. Methods 2018, 10, 4995–5003. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Snegureva, Y.; Budnikov, H. Novel approach for the voltammetric evaluation of antioxidant activity using DPPH•-modified electrode. Electrochim. Acta 2017, 247, 97–106. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Zelenova, Y.; Budnikov, H. Novel modified electrode with immobilized galvinoxyl radical for the voltammetric determination of antioxidant activity. J. Electroanal. Chem. 2020, 586, 113677. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Gaisina, G.K.; Budnikov, G.K. Use of electrogenerated bromine for estimating the total antioxidant capacity of plant raw materials and plant-based medicinal preparations. J. Anal. Chem. 2002, 57, 557–560. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Turova, E.N.; Budnikov, G.K.; Ziyatdinova, G.K.; Gajsina, G.K. Electrogenerated bromine-reagent for determination of antioxidant capacity of juices and extracts. Zavod. Lab. Diagn. Mater. 2002, 68, 12–15. [Google Scholar]
- Ziyatdinova, G.K.; Zakharova, S.P.; Budnikov, H.C. Reactions of phenolic antioxidants with electrogenerated superoxide anion radical and their analytical application. Uch. Zap. Kazan. Univ. Ser. Estestv. Nauki 2015, 157, 129–142. [Google Scholar]
- Ghiaba, Z.; Yousfi, M.; Hadjadj, M.; Saidi, M.; Dakmouche, M. Study of antioxidant properties of five algerian date (Phoenix dactylifera L.) cultivars by cyclic voltammetric technique. Int. J. Electrochem. Sci. 2014, 9, 909–920. [Google Scholar]
- Gonçalves, R.S.; Battistin, A.; Pauletti, G.; Rota, L.; Serafini, L.A. Antioxidant properties of essential oils from Mentha species evidenced by electrochemical methods. Rev. Bras. Plantas Med. 2009, 11, 372–382. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. Analytical capabilities of coulometric sensor systems in the antioxidants analysis. Chemosensors 2021, 9, 91. [Google Scholar] [CrossRef]
- Abdullin, I.F.; Budnikov, G.K. Coulometric analysis of organic compounds (review). Ind. Lab. 1998, 64, 1–11. [Google Scholar]
- Abdullin, I.F.; Budnikov, G.K.; Gorbunova, T.S. Electrochemical generation of hypohalogenite ions and their application to determining pharmaceuticals. J. Anal. Chem. 1997, 52, 264–268. [Google Scholar]
- Howes, M.-J.R.; Simmonds, M.S.J.; Kite, G.C. Evaluation of the quality of sandalwood essential oils by gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1028, 307–312. [Google Scholar] [CrossRef]
- Kupriyanova, O.V.; Shevyrin, V.A.; Shafran, Y.M.; Lebedev, A.T.; Milyukov, V.A.; Rusinov, V.L. Synthesis and determination of analytical characteristics and differentiation of positional isomers in the series of N-(2-methoxybenzyl)-2-(dimethoxyphenyl)ethanamine using chromatography–mass spectrometry. Drug Test. Anal. 2020, 12, 1154–1170. [Google Scholar] [CrossRef]
- GOST 31665-2012; Vegetable Oils and Animal Fats. Preparation of Methyl Esters of Fatty Acids. Standartinform: Moscow, Russia, 2013; 6p.
- Toivo, J.; Piironen, V.; Kalo, P.; Varo, P. Gas chromatographic determination of major sterols in edible oils and fats using solid-phase extraction in sample preparation. Chromatographia 1998, 48, 745–750. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Tkachev, A.V. Issledovanie Letuchikh Veshchestv Rastenii; Study of Volatile Compounds of Plants; Ofset: Novosibirsk, Russia, 2008; pp. 230–781. [Google Scholar]
- NIST: National Institute of Standards and Technologies, Mass Spectra Libraries. Available online: http://www.sisweb.com/software/nist-gc-library.htm (accessed on 26 August 2017).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. (Eds.) Chemistry of Spices; CABI International: London, UK, 2008; 445p. [Google Scholar]
- Hüsnü, K.; Başer, K.H.C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 43–86. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007; 804p. [Google Scholar]
- Sabuzi, F.; Churakova, E.; Galloni, P.; Wever, R.; Hollmann, F.; Floris, B.; Conte, V. Thymol bromination—A comparison between enzymatic and chemical catalysis. Eur. J. Inorg. Chem. 2015, 2015, 3519–3525. [Google Scholar] [CrossRef]
- Günay, T.; Çimen, Y.; Karabacak, R.B.; Türk, H. Oxidation of thymol and carvacrol to thymoquinone with KHSO5 catalyzed by iron phthalocyanine tetrasulfonate in a methanol–water mixture. Catal. Lett. 2016, 146, 2306–2312. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Salikhova, I.; Budnikov, H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014, 150, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Nizamova, A.; Budnikov, H. Novel coulometric approach to evaluation of total free polyphenols in tea and coffee beverages in presence of milk proteins. Food Anal. Methods 2011, 4, 334–340. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Cong, F.N.; Budnikov, H.C. Assessment of the antioxidant properties of micellar spice extracts by galvanostatic coulometry with electrogenerated hexacyanoferrate(III) ions. J. Anal. Chem. 2015, 70, 974–982. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Ultrasound-assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Anal. Meth. 2016, 8, 7150–7157. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Archdeacon, T.J. Correlation and Regression Analysis: A Historian’s Guide, 1st ed.; University of Wisconsin Press: Madison, WI, USA, 1994; 352p. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).