Antifungal Peptide P852 Controls Fusarium Wilt in Faba Bean (Viciafaba L.) by Promoting Antioxidant Defense and Isoquinoline Alkaloid, Betaine, and Arginine Biosyntheses
Abstract
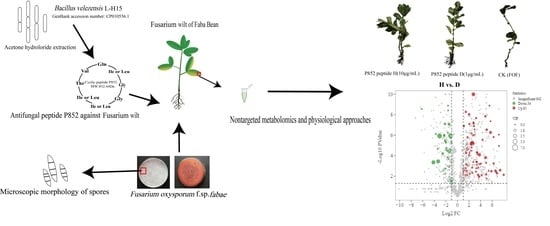
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Experimental Design
2.2. Field Trials
2.3. Evaluation of the Incidence of Fusarium Wilt
2.4. Measurement of Chlorophyll Content
2.5. Measurement of Plant Physiological Parameters
2.6. Measurement of Antioxidant Enzyme Activities
2.7. Malondialdehyde (MDA) Content and Electrolyte Leakage
2.8. Metabolite Extraction and Metabolite Profiling
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the Incidence of Fusarium Wilt
3.2. Effect of Different Treatments on Faba Bean Plant Growth and Chlorophyll Content
3.3. Effect of Antifungal Peptide P852 Treatments on Antioxidant Enzyme Activities, MDA Content, and Electrolyte Leakage
3.4. Effect of Antifungal Peptide P852 Treatments on β-1,3-GA and Chitinase Activities
3.5. Effect of P852 Treatments on the Overall Metabolic Profile of Faba Bean Leaf
3.6. Metabolic Pathways in Leaves of Faba Bean in Response to P852 Treratment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Lv, J.; Zhao, Q.; Dong, Y.; Dong, K. Cinnamic Acid Increased the Incidence of Fusarium Wilt by Increasing the Pathogenicity of Fusarium oxysporum and Reducing the Physiological and Biochemical Resistance of Faba bean, Which Was Alleviated by Intercropping with Wheat. Front. Plant Sci. 2020, 11, 608389. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Migdadi, H.M.; Ammar, M.H.; Paull, J.G.; Siddique, K.H.M. Faba bean Genomics: Current Status and Future Prospects. Euphytica 2012, 186, 609–624. [Google Scholar] [CrossRef]
- Lv, J.; Xiao, J.; Guo, Z.; Dong, K.; Dong, Y. Nitrogen Supply and Intercropping Control of Fusarium Wilt in Faba bean Depend on Organic Acids Exuded from the Roots. Sci. Rep. 2021, 11, 9589. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.W.; Wilson, M.H.; Derks, M.F.L.; Smit, S.; Kunert, K.J.; Cullis, C.; Foyer, C.H. Enhancing Faba bean (Vicia faba L.) Genome Resources. J. Exp. Bot. 2017, 68, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Liu, Z.; Luo, X. A Metabolomic, Transcriptomic Profiling, and Mineral Nutrient Metabolism Study of the Phytotoxicity Mechanism of Uranium. J. Hazard. Mater. 2020, 386, 121437. [Google Scholar] [CrossRef]
- Asai, S.; Ayukawa, Y.; Gan, P.; Shirasu, K. Draft Genome Resources for Brassicaceae Pathogens Fusarium oxysporum f. Sp. Raphani and Fusarium oxysporum f. Sp. rapae. Mol. Plant-Microbe Interact. 2021, 34, 1316–1319. [Google Scholar] [CrossRef]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J. Biological Control Agents Against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Ye, X.; Li, Z.; Luo, X.; Wang, W.; Li, Y.; Li, R.; Zhang, B.; Qiao, Y.; Zhou, J.; Fan, J.; et al. A Predatory Myxobacterium Controls Cucumber Fusarium Wilt by Regulating the Soil Microbial Community. Microbiome 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Mbasa, W.V.; Nene, W.A.; Kapinga, F.A.; Lilai, S.A.; Tibuhwa, D.D. Characterization and Chemical Management of Cashew Fusarium Wilt Disease Caused by Fusarium oxysporum in Tanzania. Crop Prot. 2021, 139, 105379. [Google Scholar] [CrossRef]
- Cai, X.; Zhao, H.; Liang, C.; Li, M.; Liu, R. Effects and Mechanisms of Symbiotic Microbial Combination Agents to Control Tomato Fusarium Crown and Root Rot Disease. Front. Microbiol. 2021, 12, 629793. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lv, J.; Dong, Y.; Dong, K. Exploration of the Potential Mechanism of Faba bean–Wheat Intercropping to Control Faba bean Fusarium Wilt Due to Allelopathic Plant Extracts. ACS Omega 2021, 6, 15590–15600. [Google Scholar] [CrossRef]
- Zhang, L.; Gallo, R.L. Antimicrobial Peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-Assisted Green Synthesis and Characterization of Silver Nanoparticles Using Melia Azedarach for the Management of Fusarium Wilt in Tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Cang, T.; Liu, Z.; Xie, Y.; Zhao, H.; Qi, P.; Wang, Z.; Xu, H.; Wang, X. Comprehensive Evaluation of Chiral Pydiflumetofen from the Perspective of Reducing Environmental Risks. Sci. Total Environ. 2022, 826, 154033. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Na, H.; Seo, C.H.; Park, Y. Antifungal Activity of (KW)n or (RW)n Peptide against Fusarium solani and Fusarium oxysporum. Int. J. Mol. Sci. 2012, 13, 15042–15053. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kang, H.K.; Kwon, K.-D.; Seo, C.H.; Lee, H.B.; Park, Y. Antagonistic Activities of Novel Peptides from Bacillus amyloliquefaciens PT14 against Fusarium solani and Fusarium oxysporum. J. Agric. Food Chem. 2015, 63, 10380–10387. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Han, Y.; Shang, Q.; Li, P. Complete Genome Sequence of Bacillus amyloliquefaciens L-H15, a Plant Growth Promoting Rhizobacteria Isolated from Cucumber Seedling Substrate. J. Biotechnol. 2015, 200, 59–60. [Google Scholar] [CrossRef]
- Han, Y.; Deng, L. Optimization of Fermentation Conditions for Production of Antifungal Peptides by Bacillus amyloliquefaciens H15 and Comparison of Extraction Methods for Antifungal Peptide in China. Food Sci. 2015, 36, 135–141. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SPKX201515030&DbName=CJFQ2015 (accessed on 5 May 2022).
- Han, Y.; Zhao, J.; Zhang, B.; Shen, Q.; Shang, Q.; Li, P. Effect of a Novel Antifungal Peptide P852 on Cell Morphology and Membrane Permeability of Fusarium oxysporum. Biochim. Biophys. Acta (BBA)—Biomembr. 2019, 1861, 532–539. [Google Scholar] [CrossRef]
- Fang, Z. Research Methods of Plant Pathology; China Agriculture Press: Beijing, China, 2007; ISBN 978-7-10905-255-0. [Google Scholar]
- Zhang, C.; Cai, K.; Li, M.; Zheng, J.; Han, Y. Plant-Growth-Promoting Potential of PGPE Isolated from Dactylis glomerata L. Microorganisms 2022, 10, 731. [Google Scholar] [CrossRef]
- Xuekui, W.; Jianliang, H. Principle and Technology of Plant Physiology and Biochemistry Experiment, 3rd ed.; China High Education Press: Berlin, Germany, 2018; ISBN 978-7-04039-646-1. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hassan, S.E.-D. Plant Growth-Promoting Activities for Bacterial and Fungal Endophytes Isolated from Medicinal Plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, Y.; Nie, Z.; Liu, H.; Gao, W.; Li, C.; Zhao, P. Metabolomic and Antioxidant Enzyme Activity Changes in Response to Cadmium Stress under Boron Application of Wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 34701–34713. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, Y.; Wang, Q.; Gu, Z.; Wang, P.; Han, Y.; Yang, R. Mechanism of Nitric Oxide Enhancing NaCl Tolerance of Barley Seedlings Based on Physiol-Biochemical Analysis and LC-MS Metabolomics. Environ. Exp. Bot. 2021, 189, 104533. [Google Scholar] [CrossRef]
- Chai, N.; Xu, J.; Zuo, R.; Sun, Z.; Cheng, Y.; Sui, S.; Li, M.; Liu, D. Metabolic and Transcriptomic Profiling of Lilium Leaves Infected with Botrytis elliptica Reveals Different Stages of Plant Defense Mechanisms. Front. Plant Sci. 2021, 12, 2048. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhou, C.; Jing, J.; Zhuang, M.; Zhang, J.; Wang, K.; Zhang, H. Metabolomics Analysis of Cucumber Fruit in Response to Foliar Fertilizer and Pesticides Using UHPLC-Q-Orbitrap-HRMS. Food Chem. 2022, 369, 130960. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Tao, M.; Li, M.; Wu, Y.; Qi, Q.; Yang, H.; Wan, X. Comparative Metabolic Responses and Adaptive Strategies of Tea Leaves (Camellia sinensis) to N2 and CO2 Anaerobic Treatment by a Nontargeted Metabolomics Approach. J. Agric. Food Chem. 2018, 66, 9565–9572. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Zhang, C.; Li, C.; Fang, Z.; Zeng, Z.; Hu, B.; Chen, H.; Wu, W.; Wang, T.; et al. Targeted and Untargeted Metabolomic Analyses and Biological Activity of Tibetan Tea. Food Chem. 2022, 384, 132517. [Google Scholar] [CrossRef]
- Shen, S.; Huang, J.; Li, T.; Wei, Y.; Xu, S.; Wang, Y.; Ning, J. Untargeted and Targeted Metabolomics Reveals Potential Marker Compounds of an Tea during Storage. LWT 2022, 154, 112791. [Google Scholar] [CrossRef]
- Khademi, M.; Varasteh-Shams, M.; Nazarian-Firouzabadi, F.; Ismaili, A. New Recombinant Antimicrobial Peptides Confer Resistance to Fungal Pathogens in Tobacco Plants. Front. Plant Sci. 2020, 11, 1236. [Google Scholar] [CrossRef]
- Han, J.; Wang, F.; Gao, P.; Ma, Z.; Zhao, S.; Lu, Z.; Lv, F.; Bie, X. Mechanism of Action of AMP-Jsa9, a LI-F-Type Antimicrobial Peptide Produced by Paenibacillus polymyxa JSa-9, against Fusarium moniliforme. Fungal Genet. Biol. 2017, 104, 45–55. [Google Scholar] [CrossRef]
- Zhang, Q.-X.; Zhang, Y.; Shan, H.-H.; Tong, Y.-H.; Chen, X.-J.; Liu, F.-Q. Isolation and Identification of Antifungal Peptides from Bacillus amyloliquefaciens W10. Environ. Sci. Pollut. Res. 2017, 24, 25000–25009. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sun, S.; Zhao, N.; Yang, W.; Shi, Q.; Gong, B. COMT1 Overexpression Resulting in Increased Melatonin Biosynthesis Contributes to the Alleviation of Carbendazim Phytotoxicity and Residues in Tomato Plants. Environ. Pollut. 2019, 252, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, Y.; Qu, Q.; Yu, Y.; Jin, M.; Lu, T.; Qian, H. Enantioselective Metabolomic Modulations in Arabidopsis thaliana Leaf Induced by the Herbicide Dichlorprop. Sci. Total Environ. 2021, 797, 149015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Ma, X.; Ouyang, Z.; Deng, L.; Shen, S.; Dong, X.; Du, N.; Dong, H.; Guo, Z.; et al. Melatonin Alleviates Copper Toxicity via Improving ROS Metabolism and Antioxidant Defense Response in Tomato Seedlings. Antioxidants 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Q.; Yang, F.; Xie, X.; Goodwin, P.H.; Deng, X.; Tian, B.; Yang, L. Evaluation and Genome Analysis of Bacillus subtilis YB-04 as a Potential Biocontrol Agent Against Fusarium Wilt and Growth Promotion Agent of Cucumber. Front. Microbiol. 2022, 13, 616. [Google Scholar] [CrossRef]
- Ali, M.; Ahmad, H.; Hayat, S.; Ghani, M.I.; Amin, B.; Atif, M.J.; Wali, K.; Cheng, Z. Application of Garlic Allelochemicals Improves Growth and Induces Defense Responses in Eggplant (Solanum melongena) against Verticillium dahliae. Ecotoxicol. Environ. Saf. 2021, 215, 112132. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS Generation in Plants: Boon or Bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Q.; Lu, T.; Ke, M.; Zhu, Y.; Zhang, M.; Zhang, Z.; Du, B.; Pan, X.; Sun, L.; et al. The Combined Toxicity Effect of Nanoplastics and Glyphosate on Microcystis aeruginosa Growth. Environ. Pollut. 2018, 243, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA Enhances Physio-Biochemical Metabolism and Antioxidant Capacity of Germinated Hulless Barley under NaCl Stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Schlumbaum, A.; Mauch, F.; Vögeli, U.; Boller, T. Plant Chitinases Are Potent Inhibitors of Fungal Growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Fan, Q.; Tian, S.; Liu, H.; Xu, Y. Production of β-1,3-Glucanase and Chitinase of Two Biocontrol Agents and Their Possible Modes of Action. Chin. Sci. Bull. 2002, 47, 292–296. [Google Scholar] [CrossRef]
- Fu, J.; Sun, J.; Zhou, R.; Yan, X. Effect of Methyl Jasmonate on the Activities of β-1, 3-Glucanase and Chitinase in Ginseng. Chin. Agric. Sci. Bull. 2012, 28, 214–217. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZNTB201209044&DbName=CJFQ2012 (accessed on 5 May 2022).
- Wang, Y.; Ren, W.; Li, Y.; Xu, Y.; Teng, Y.; Christie, P.; Luo, Y. Nontargeted Metabolomic Analysis to Unravel the Impact of Di (2-Ethylhexyl) Phthalate Stress on Root Exudates of Alfalfa (Medicago sativa). Sci. Total Environ. 2019, 646, 212–219. [Google Scholar] [CrossRef]
- Zhou, C.; Tan, Y.; Goßner, S.; Li, Y.; Shu, Q.; Engel, K.-H. Impact of Crossing Parent and Environment on the Metabolite Profiles of Progenies Generated from a Low Phytic Acid Rice (Oryza sativa L.) Mutant. J. Agric. Food Chem. 2019, 67, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Zhang, W.; Li, S.; Gao, Y.; Ai, X.; Zhang, D.; Liu, B.; Li, Q. Metabolomics Analysis Reveals That Elevated Atmospheric CO2 Alleviates Drought Stress in Cucumber Seedling Leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef]
- Niu, L.; Zhong, X.; Zhang, Y.; Yang, J.; Xing, G.; Li, H.; Liu, D.; Ma, R.; Dong, Y.; Yang, X. Enhanced Tolerance to Phytophthora Root and Stem Rot by Over-Expression of the Plant Antimicrobial Peptide CaAMP1 Gene in Soybean. BMC Genet. 2020, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Ward, J.L.; Galster, A.M.; Beale, M.H.; Powell, G. The Effects of a Plant Defence Priming Compound, β-Aminobutyric Acid, on Multitrophic Interactions with an Insect Herbivore and a Hymenopterous Parasitoid. BioControl 2011, 56, 699–711. [Google Scholar] [CrossRef]
- Hibberd, K.A.; Green, C.E. Inheritance and Expression of Lysine plus Threonine Resistance Selected in Maize Tissue Culture. Proc. Natl. Acad. Sci. USA 1982, 79, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K.M.; Phillips, R. Polyamines in Relation to Growth in Carrot Cell Cultures. Plant Physiol. 1988, 88, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, S.; Dou, Q.; Wang, Y.; Cui, X. The effect of exogenous NO on L-arginine Metabolism in Tomato Seed lings under Copper Stress. Asian J. Ecotoxicol. 2015, 10, 112–122. (In Chinese) [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, Ornithine, Arginine, Proline, and Polyamine Metabolic Interactions: The Pathway Is Regulated at the Post-Transcriptional Level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef]
- Sharma, S.; Dietz, K.-J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P. Auxin Driven Indoleamine Biosynthesis and the Role of Tryptophan as an Inductive Signal in Hypericum perforatum (L.). PLoS ONE 2019, 14, e0223878. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.M.; Errafi, S.; Bucher, R.; Dobrev, P.; Geisler, M.; Bigler, L.; Zažímalová, E.; Ringli, C. 7-Rhamnosylated Flavonols Modulate Homeostasis of the Plant Hormone Auxin and Affect Plant Development*. J. Biol. Chem. 2016, 291, 5385–5395. [Google Scholar] [CrossRef] [PubMed]
- Deleu, M.; Deboever, E.; Nasir, M.N.; Crowet, J.-M.; Dauchez, M.; Ongena, M.; Jijakli, H.; Fauconnier, M.-L.; Lins, L. Linoleic and Linolenic Acid Hydroperoxides Interact Differentially with Biomimetic Plant Membranes in a Lipid Specific Manner. Colloids Surf. B Biointerfaces 2019, 175, 384–391. [Google Scholar] [CrossRef]
- Llorens, E.; Camañes, G.; Lapeña, L.; García-Agustín, P. Priming by Hexanoic Acid Induce Activation of Mevalonic and Linolenic Pathways and Promotes the Emission of Plant Volatiles. Front. Plant Sci. 2016, 7, 495. [Google Scholar] [CrossRef]
- Kim, P.T.A.; An, L.T.B.; Chung, N.T.; Truong, N.T.; Thu, L.T.A.; Huan, L.V.T.; Loc, N.H. Growth and Oleanolic Acid Accumulation of Polyscias fruticosa Cell Suspension Cultures. Curr. Pharm. Biotechnol. 2020, 22, 1266–1272. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, S.; Guardiola, L.; Hernández-Sánchez, P.; Núñez-Delicado, E. Complexation between Oleanolic and Maslinic Acids with Native and Modified Cyclodextrins. Food Chem. 2018, 240, 139–146. [Google Scholar] [CrossRef]
- Chong, J.; Pierrel, M.-A.; Atanassova, R.; Werck-Reichhart, D.; Fritig, B.; Saindrenan, P. Free and Conjugated Benzoic Acid in Tobacco Plants and Cell Cultures. Induced Accumulation upon Elicitation of Defense Responses and Role as Salicylic Acid Precursors. Plant Physiol. 2001, 125, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Fernie, A.R.; Martinoia, E. Malate. Jack of All Trades or Master of a Few? Phytochemistry 2009, 70, 828–832. [Google Scholar] [CrossRef]
- Tramontano, W.A.; Jouve, D. Trigonelline Accumulation in Salt-Stressed Legumes and the Role of Other Osmoregulators as Cell Cycle Control Agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar] [CrossRef]
- Khamtache-Abderrahim, S.; Lequart-Pillon, M.; Gontier, E.; Gaillard, I.; Pilard, S.; Mathiron, D.; Djoudad-Kadji, H.; Maiza-Benabdesselam, F. Isoquinoline Alkaloid Fractions of Fumaria Officinalis: Characterization and Evaluation of Their Antioxidant and Antibacterial Activities. Ind. Crops Prod. 2016, 94, 1001–1008. [Google Scholar] [CrossRef]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant Isoquinoline Alkaloids: Advances in the Chemistry and Biology of Berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef]
- Mello, A.L.d.N.; Zancan, P. Isoquinolines Alkaloids and Cancer Metabolism: Pathways and Targets to Novel Chemotherapy. Chem. Biol. Drug Des. 2022, 99, 944–956. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Liu, Y.-J.; He, G.-R.; Cao, Y.-W.; Bi, M.-M.; Song, M.; Yang, P.-P.; Xu, L.-F.; Ming, J. Comprehensive Analysis of Secondary Metabolites in the Extracts from Different Lily Bulbs and Their Antioxidant Ability. Antioxidants 2021, 10, 1634. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.; Wu, S.; Zheng, L.; Wang, Q.; Wang, G.; Yan, S. Defense Responses of Arbuscular Mycorrhizal Fungus-Colonized Poplar Seedlings against Gypsy Moth Larvae: A Multiomics Study. Hortic. Res. 2021, 8, 245. [Google Scholar] [CrossRef]
- Quan, J.; Zheng, W.; Wu, M.; Shen, Z.; Tan, J.; Li, Z.; Zhu, B.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; et al. Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L.) Seedlings with Improved Photosynthetic Performance and Antioxidant System. Plants 2022, 11, 1213. [Google Scholar] [CrossRef]
- Warhade, M.I.; Badere, R.S. Fusarium oxysporum Cell Elicitor Enhances Betalain Content in the Cell Suspension Culture of Celosia Cristata. Physiol. Mol. Biol. Plants 2018, 24, 285–293. [Google Scholar] [CrossRef]
- Glenz, R.; Kaiping, A.; Göpfert, D.; Weber, H.; Lambour, B.; Sylvester, M.; Fröschel, C.; Mueller, M.J.; Osman, M.; Waller, F. The Major Plant Sphingolipid Long Chain Base Phytosphingosine Inhibits Growth of Bacterial and Fungal Plant Pathogens. Sci. Rep. 2022, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, H. Role of Carnitine in Non-Alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef]
- Eijkelkamp, B.A.; Begg, S.L.; Pederick, V.G.; Trapetti, C.; Gregory, M.K.; Whittall, J.J.; Paton, J.C.; McDevitt, C.A. Arachidonic Acid Stress Impacts Pneumococcal Fatty Acid Homeostasis. Front. Microbiol. 2018, 9, 813. [Google Scholar] [CrossRef]
- Rosler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize Phenylalanine Ammonia-Lyase Has Tyrosine Ammonia-Lyase Activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Khakdan, F.; Alizadeh, H.; Ranjbar, M. Molecular Cloning, Functional Characterization and Expression of a Drought Inducible Phenylalanine Ammonia-Lyase Gene (ObPAL) from Ocimum basilicum L. Plant Physiol. Biochem. 2018, 130, 464–472. [Google Scholar] [CrossRef]
- Suzen, S.; Bozkaya, P.; Coban, T.; Nebioğlu, D. Investigation of the in Vitro Antioxidant Behaviour of Some 2-Phenylindole Derivatives: Discussion on Possible Antioxidant Mechanisms and Comparison with Melatonin. J. Enzym. Inhib. Med. Chem. 2006, 21, 405–411. [Google Scholar] [CrossRef]
- Long, M.; Shou, J.; Wang, J.; Hu, W.; Hannan, F.; Mwamba, T.M.; Farooq, M.A.; Zhou, W.; Islam, F. Ursolic Acid Limits Salt-Induced Oxidative Damage by Interfering with Nitric Oxide Production and Oxidative Defense Machinery in Rice. Front. Plant Sci. 2020, 11, 697. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef] [Green Version]









| Different Treatments | Artificial Climate Chamber | Field Test | ||
|---|---|---|---|---|
| Incidence% | Disease Index | Incidence% | Disease Index | |
| CK | 91.67 ± 0.17 a | 18.33 ± 2.72 a | 83.33 ± 0.08 a | 5.9 ± 0.61 a |
| D | 58.33 ± 0.17 b | 9.58 ± 3.15 b | 52.78 ± 0.10 d | 2.9 ± 0.32 c |
| H | 58.33 ± 0.17 b | 6.67 ± 4.08 b | 55.56 ± 0.05 c | 2.5 ± 0.82 c |
| C | 66.67 ± 0.27 b | 10.42 ± 7.86 b | 58.40 ± 0.67 b | 3.5 ± 0.32 b |
| Different Treatments | Chlorophyll Content (mg/g) | Plant Height (cm) | Dry Mass of Stem with Leaves (g) | Dry Mass of Roots (g) | Fresh Mass of Stem with Leaves (g) | Fresh Mass of Roots (g) | Stem Diameter (cm) |
|---|---|---|---|---|---|---|---|
| CK | 0.89 ± 0.45 c | 34.86 ± 1.60 c | 1.05 ± 0.13 b | 0.18 ± 0.01 c | 11.06 ± 0.84 b | 1.42 ± 0.89 b | 0.47 ± 0.06 b |
| D | 1.82 ± 0.08 ab | 46.17 ± 5.58 a | 2.70 ± 0.17 a | 0.69 ± 0.09 a | 18.30 ± 7.42 ab | 2.99 ± 1.24 ab | 0.70 ± 0.14 a |
| H | 2.43 ± 0.48 a | 45.43 ± 1.00 ab | 2.86 ± 0.46 a | 0.61 ± 0.61 a | 20.86 ± 7.81 a | 3.31 ± 0.1 a | 0.79 ± 0.05 a |
| C | 1.55 ± 0.11 bc | 36.87 ± 1.60 bc | 1.65 ± 0.49 b | 0.43 ± 0.06 b | 16.54 ± 0.83 ab | 2.68 ± 0.37 ab | 0.67 ± 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Ou, X.; Wang, J.; Wang, Z.; Du, W.; Zhao, J.; Han, Y. Antifungal Peptide P852 Controls Fusarium Wilt in Faba Bean (Viciafaba L.) by Promoting Antioxidant Defense and Isoquinoline Alkaloid, Betaine, and Arginine Biosyntheses. Antioxidants 2022, 11, 1767. https://doi.org/10.3390/antiox11091767
Zhang C, Ou X, Wang J, Wang Z, Du W, Zhao J, Han Y. Antifungal Peptide P852 Controls Fusarium Wilt in Faba Bean (Viciafaba L.) by Promoting Antioxidant Defense and Isoquinoline Alkaloid, Betaine, and Arginine Biosyntheses. Antioxidants. 2022; 11(9):1767. https://doi.org/10.3390/antiox11091767
Chicago/Turabian StyleZhang, Chaowen, Xuan Ou, Jingyi Wang, Zhaoling Wang, Wenting Du, Jianjun Zhao, and Yuzhu Han. 2022. "Antifungal Peptide P852 Controls Fusarium Wilt in Faba Bean (Viciafaba L.) by Promoting Antioxidant Defense and Isoquinoline Alkaloid, Betaine, and Arginine Biosyntheses" Antioxidants 11, no. 9: 1767. https://doi.org/10.3390/antiox11091767
APA StyleZhang, C., Ou, X., Wang, J., Wang, Z., Du, W., Zhao, J., & Han, Y. (2022). Antifungal Peptide P852 Controls Fusarium Wilt in Faba Bean (Viciafaba L.) by Promoting Antioxidant Defense and Isoquinoline Alkaloid, Betaine, and Arginine Biosyntheses. Antioxidants, 11(9), 1767. https://doi.org/10.3390/antiox11091767