Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material Extraction
2.3. HPLC Analysis and Antioxidant Activity
2.4. Ophthalmic In Situ Gels Formulation
2.5. Sterilization of Extracts and Ophthalmic Gels
2.6. Physicochemical Properties of Ophthalmic Gels
2.7. Sol-To-Gel Transition Temperature
2.8. In Vitro Release Test
2.9. Antioxidant Activity
2.10. Statistical Analysis
3. Results
3.1. HPLC Analysis of the Extracts
3.2. Composition of Formulations Ant Their Antioxidant Activity
3.3. Physicochemical Properties
3.4. Phenolic Acids Release
3.5. Salicin Release
3.6. Oftalmic Gels Stability
4. Discussion
4.1. Extraction of Phenolic Compounds from Balsam Poplar Buds
4.2. Antioxidant Activity of Ophthalmic Formulations
4.3. Sterilization of Formulations
4.4. Physicochemical Properties of the Formulations
4.5. Release of the Active Compounds
4.6. Stability of Formulations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yapar, E.A.; Durgun, M.; Esentürk, I.; Güngör, S.; Özsoy, Y. Herbal bioactives for ocular drug delivery systems. In Herbal Bioactive-Based Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2022; pp. 25–61. [Google Scholar] [CrossRef]
- Krstić, L.; González-García, M.J.; Diebold, Y. Ocular Delivery of Polyphenols: Meeting the Unmet Needs. Molecules 2021, 26, 370. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lee, J.B.; Cui, L.; Li, Y.; Li, Z.; Choi, J.S.; Lee, H.S.; Yoon, K.C. Therapeutic Efficacy of Topically Applied Antioxidant Medicinal Plant Extracts in a Mouse Model of Experimental Dry Eye. Oxidative Med. Cell. Longev. 2016, 2016, 4727415. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.-Q. Oxidative Stress Markers Induced by Hyperosmolarity in Primary Human Corneal Epithelial Cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef]
- Pokkalath, A.S.; Sawant, A.; Sawarkar, S.P. Herbal medicine for ocular diseases: An age old therapy and its future perspective. J. Drug Deliv. Sci. Technol. 2021, 68, 102979. [Google Scholar] [CrossRef]
- Yang, C.-C.; Su, S.-H.; Ho, T.-J. Retrospective evaluation of the curative effect of traditional Chinese medicine on dry eye disease. Tzu Chi Med. J. 2021, 33, 365. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Pharmacological activities of an eye drop containing Matricaria chamomilla and Euphrasia officinalis extracts in UVB-induced oxidative stress and inflammation of human corneal cells. J. Photochem. Photobiol. B Biol. 2017, 173, 618–625. [Google Scholar] [CrossRef]
- Arana, L.; Salado, C.; Vega, S.; Aizpurua-Olaizola, O.; de la Arada, I.; Suarez, T.; Usobiaga, A.; Arrondo, J.L.R.; Alonso, A.; Goñi, F.M.; et al. Solid lipid nanoparticles for delivery of Calendula officinalis extract. Colloids Surf. B Biointerfaces 2015, 135, 18–26. [Google Scholar] [CrossRef]
- Fraunfelder, F.W. Ocular side effects from herbal medicines and nutritional supplements. Am. J. Ophthalmol. 2004, 138, 639–647. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Zheng, H.-Q.; Liu, G.; Hu, F.-L. Development and validation of HPLC method for determination of salicin in poplar buds: Application for screening of counterfeit propolis. Food Chem. 2011, 127, 345–350. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Soles, B.B.; Lopes, A.R.; Vaz, B.F.; Rodrigues, C.M.; Alves, T.F.R.; Klensporf-Pawlik, D.; Durazzo, A.; Lucarini, M.; Severino, P.; et al. Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology 2020, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, B.T.; Reimers, M.; Ernst, N.; Bova, G.; Nowakowski, E.; Bukowski, J.; Ellis, B.C.; Smith, C.; Sauer, L.; Dionne, K.; et al. Validation of Autoclave Protocols for Successful Decontamination of Category A Medical Waste Generated from Care of Patients with Serious Communicable Diseases. J. Clin. Microbiol. 2017, 55, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Galante, R.; Pinto, T.D.J.A.; Colaco, R.; Serro, A.P. Sterilization of hydrogels for biomedical applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Castillo, F.; Hernández, D.; Gallegos, G.; Mendez, M.; Rodríguez, R.; Reyes, A.; Aguilar, C.N. In vitro antifungal activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind. Crop. Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Gajger, I.T.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous Polyethylene Glycol as a Safer Alternative to Ethanolic Propolis Extracts with Comparable Antioxidant and Antimicrobial Activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.M. Polyethylene glycol as a green chemical solvent. Curr. Opin. Colloid Interface Sci. 2022, 57, 101537. [Google Scholar] [CrossRef]
- Llorens, E.; Ibañez, H.; del Valle, L.; Puiggalí, J. Biocompatibility and drug release behavior of scaffolds prepared by coaxial electrospinning of poly(butylene succinate) and polyethylene glycol. Mater. Sci. Eng. C 2015, 49, 472–484. [Google Scholar] [CrossRef]
- Vrandečić, N.S.; Erceg, M.; Jakić, M.; Klarić, I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Singh, T.R. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef]
- Tundisi, L.; Mostaço, G.; Carricondo, P.; Petri, D. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Kim, H.; Woo, S. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Majeed, A.; Khan, N.A. Ocular in situ gel: An overview. J. Drug Deliv. Ther. 2019, 9, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.-H.; Jung, K.; Kim, A. Poloxamer-Based Thermoreversible Gel for Topical Delivery of Emodin: Influence of P407 and P188 on Solubility of Emodin and Its Application in Cellular Activity Screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, P.; Li, J. Elucidation of Colloid Performances of Thermosensitive In Situ–Forming Ophthalmic Gel Formed by Poloxamer 407 for Loading Drugs. J. Pharm. Sci. 2020, 109, 1703–1713. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic acid in ocular drug delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskienė, K.; Savickas, A.; Inkėnienė, A.; Vitkevičius, K.; Kasparavičienė, G.; Briedis, V.; Amšiejus, A. Analysis of content of phenolic acids in Lithuanian propolis using high-performance liquid chromatography technique. Medicina 2009, 45, 712. [Google Scholar] [CrossRef] [PubMed]
- Marksa, M.; Radušienė, J.; Jakštas, V.; Ivanauskas, L.; Marksienė, R. Development of an HPLC post-column antioxidant assay for Solidago canadensis radical scavengers. Nat. Prod. Res. 2016, 30, 536–543. [Google Scholar] [CrossRef]
- Xuan, J.-J.; Balakrishnan, P.; Oh, D.H.; Yeo, W.H.; Park, S.M.; Yong, C.S.; Choi, H.-G. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010, 395, 317–323. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, S.; Li, Y.; Wu, F.; Shen, C. Gelation of Konjac glucomannan crosslinked by organotitanium chelated with different ligands. J. Sol-Gel Sci. Technol. 2021, 98, 401–410. [Google Scholar] [CrossRef]
- Yim, S.-H.; Nam, S.-H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar] [CrossRef]
- Simpson, M.G. 8—Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics, 2nd ed.; Simpson, M.G., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 275–448. [Google Scholar] [CrossRef]
- Song, Y.; Tian, X.; Wang, X.; Feng, H. Vascular protection of salicin on IL-1β-induced endothelial inflammatory response and damages in retinal endothelial cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1995–2002. [Google Scholar] [CrossRef]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the effectiveness of willow bark for musculoskeletal pain. Phytother. Res. 2009, 23, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Fiebich, B.; Black, A.; Pollak, S. Treating low back pain with a Salix extract that inhibits COX 2 and the release of cytokines. Eur. J. Anaesthesiol. 2002, 19, 209–210. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Magora, F.; Chrubasik, S. Willow Species and Aspirin: Different Mechanism of Actions. Phytother. Res. 2011, 25, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Wölfle, U.; Haarhaus, B.; Kersten, A.; Fiebich, B.; Hug, M.J.; Schempp, C.M. Salicin from Willow Bark can Modulate Neurite Outgrowth in Human Neuroblastoma SH-SY5Y Cells. Phytother. Res. 2015, 29, 1494–1500. [Google Scholar] [CrossRef]
- Wroblewska, K.; Kucinska, M.; Murias, M.; Lulek, J. Characterization of new eye drops with choline salicylate and assessment of their irritancy by in vitro short time exposure tests. Saudi Pharm. J. 2015, 23, 407–412. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; Plewa, S.; Długaszewska, J.; Froelich, A.; Muszalska-Kolos, I. Design and evaluation of pharmaceutical availability, stability and quality of modified viscosity eye drops with choline salicylate. Eur. J. Pharm. Sci. 2021, 159, 105725. [Google Scholar] [CrossRef]
- Yan, H.; Guo, Y.; Zhang, J.; Ding, Z.; Ha, W.; Harding, J.J. Effect of Carnosine, Aminoguanidine, and Aspirin Drops on the Prevention of Cataracts in Diabetic Rats. Mol. Vis. 2008, 14, 2282–2291. [Google Scholar]
- Librando, A.; Carlesimo, S.C.; Albanese, G.; Albanese, G.M.; Migliorini, R.; Pacella, E. Effectiveness of 0.1% topical salicylic acid on blepharoconjunctivitis affecting glaucoma patients treated with topical prostaglandin analogues: A prospective randomized trial. Int. J. Ophthalmol. 2018, 11, 1936–1940. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Babickaite, L.; Majiene, D.; Ramanauskiene, K. Comparison of Ethanolic and Aqueous Populus balsamifera L. Bud Extracts by Different Extraction Methods: Chemical Composition, Antioxidant and Antibacterial Activities. Pharmaceuticals 2021, 14, 1018. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.-I.; Ciucure, C.T.; Lavric, V. Polyphenolics profile effects upon the antioxidant and antimicrobial activity of propolis extracts. Sci. Rep. 2021, 11, 20113. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Lodovici, M.; Morbidelli, L.; Dolara, P. Hydrocaffeic and p-coumaric acids, natural phenolic compounds, inhibit UV-B damage in WKD human conjunctival cells in vitro and rabbit eye in vivo. Free Radic. Res. 2008, 42, 903–910. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Lodovici, M.; Caldini, S.; Morbidelli, L.; Akpan, V.; Ziche, M.; Dolara, P. Protective effect of 4-coumaric acid from UVB ray damage in the rabbit eye. Toxicology 2009, 255, 1–5. [Google Scholar] [CrossRef]
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Sudha, P.N.; Rose, M.H. Beneficial Effects of Hyaluronic Acid. In Marine Carbohydrates: Fundamentals and Applications, Part A; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 137–176. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, L.; Liu, M.; Huang, B.; Zhang, N.; Mehmood, R.; Nan, K.; Li, Q.; Chen, W.; Lin, S. In situ scavenging of mitochondrial ROS by anti-oxidative MitoQ/hyaluronic acid nanoparticles for environment-induced dry eye disease therapy. Chem. Eng. J. 2020, 398, 125621. [Google Scholar] [CrossRef]
- Juncan, A.; Moisă, D.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Beaussart, A.; Retourney, C.; Quilès, F.; Morais, R.D.S.; Gaiani, C.; Fiérobe, H.-P.; El-Kirat-Chatel, S. Supported lysozyme for improved antimicrobial surface protection. J. Colloid Interface Sci. 2020, 582, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Dekina, S.S.; Romanovskaya, I.I.; Ovsepyan, A.M.; Balashova, M.V. Sterilization of Ocular Medical Inserts with Immobilized Proteins. Pharm. Chem. J. 2015, 49, 275–279. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Beard, M.C.; Cobb, L.; Grant, C.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of Poloxamer 407 hydrogel and its use as a drug delivery vehicle. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 338–347. [Google Scholar] [CrossRef]
- Mandal, S.; Prabhushankar, G.; Thimmasetty, M.K.; Geetha, M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78–82. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Pandit, J.K. Therapeutic Effectiveness in the Treatment of Experimental Bacterial Keratitis with Ion-activated Mucoadhesive Hydrogel. Ocul. Immunol. Inflamm. 2016, 24, 489–492. [Google Scholar] [CrossRef]
- Rossatto, A.; dos Santos, J.T.; Arlindo, M.Z.F.; de Morais, M.S.; de Souza, T.D.; Ogrodowski, C.S. Hyaluronic acid production and purification techniques: A review. Prep. Biochem. Biotechnol. 2022, 1–11. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó, B.; Balogh, E.; Zelkó, R.; Antal, I. Structural elucidation of hyaluronic acid gels after heat sterilisation. Polym. Test. 2013, 32, 1322–1325. [Google Scholar] [CrossRef]
- Kuo, J.; Vladimir, P. Steam-Sterilizing Solid Hyaluronic Acid. U.S. Patent 5,621,093, 15 April 1997. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef] [PubMed]
- Barse, R.; Kokare, C.; Tagalpallewar, A. Influence of hydroxypropylmethylcellulose and poloxamer composite on developed ophthalmic in situ gel: Ex vivo and in vivo characterization. J. Drug Deliv. Sci. Technol. 2016, 33, 66–74. [Google Scholar] [CrossRef]
- Rahman, M.Q.; Chuah, K.-S.; A Macdonald, E.C.; Trusler, J.P.M.; Ramaesh, K. The effect of pH, dilution, and temperature on the viscosity of ocular lubricants—shift in rheological parameters and potential clinical significance. Eye 2012, 26, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Mahboobian, M.M.; Mohammadi, M.; Mansouri, Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020, 55, 101400. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Asasutjarit, R.; Thanasanchokpibull, S.; Fuongfuchat, A.; Veeranondha, S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharm. 2011, 411, 128–135. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.I.; Popa, L. Chitosan-Based In Situ Gels for Ocular Delivery of Therapeutics: A State-of-the-Art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef]
- Lim, L.T.; Ah-Kee, E.Y.; Collins, C.E. Common eye drops and their implications for pH measurements in the management of chemical eye injuries. Int. J. Ophthalmol. 2014, 7, 1067–1068. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Seah, I.; Loh, X.J.; Su, X. A topical gel for extended ocular drug release. Nat. Biomed. Eng. 2020, 4, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, C.; Zhu, Q.; Zhang, X.; Guan, J.; Mao, S. Comparison of thermosensitive in situ gels and drug-resin complex for ocular drug delivery: In vitro drug release and in vivo tissue distribution. Int. J. Pharm. 2020, 578, 119184. [Google Scholar] [CrossRef] [PubMed]
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Austermann, H.; Schaeffel, F.; Mathis, U.; Hund, V.; Mußhoff, F.; Ziemssen, F.; Schnichels, S. Corneal Penetration of Low-Dose Atropine Eye Drops. J. Clin. Med. 2021, 10, 588. [Google Scholar] [CrossRef]
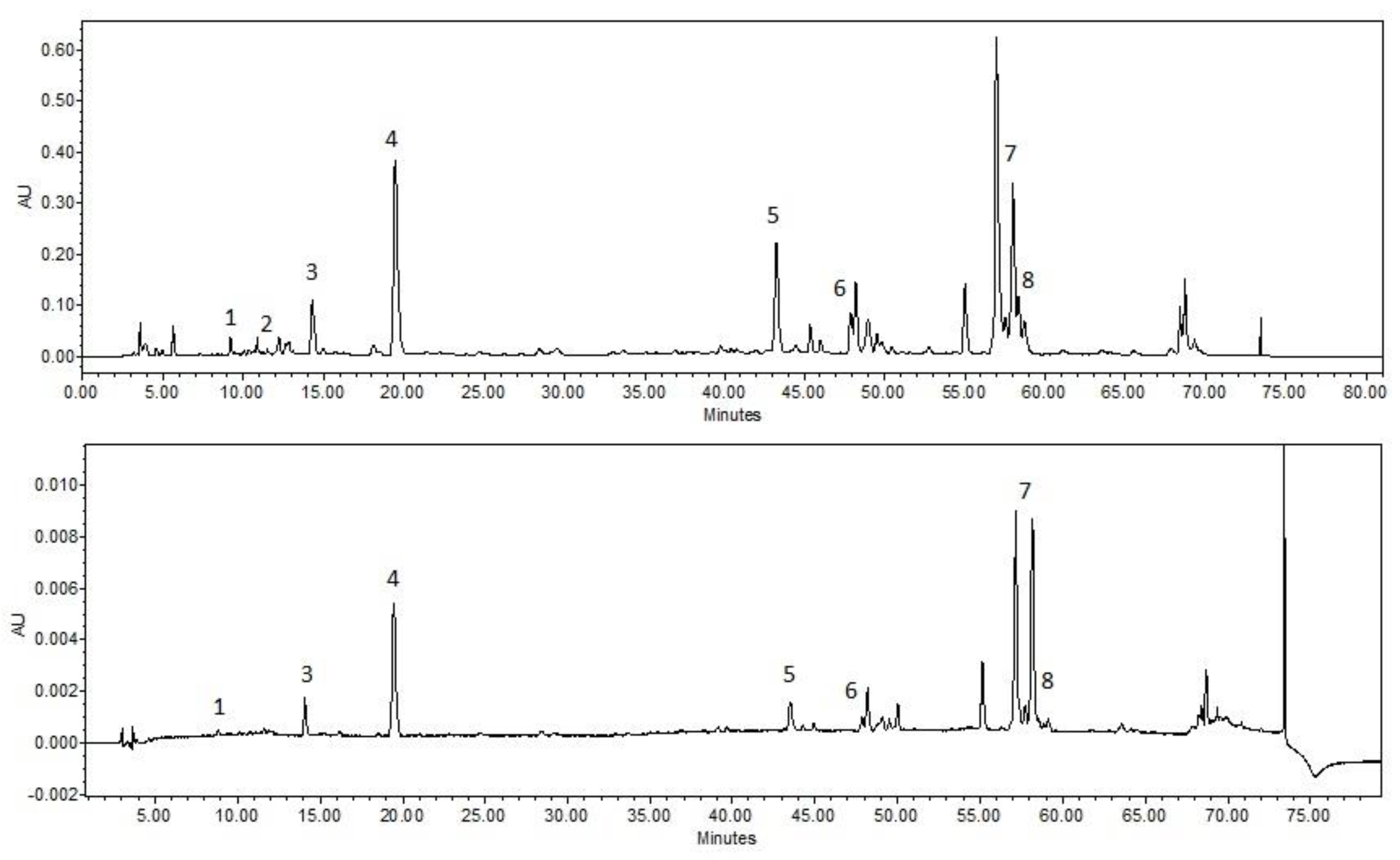
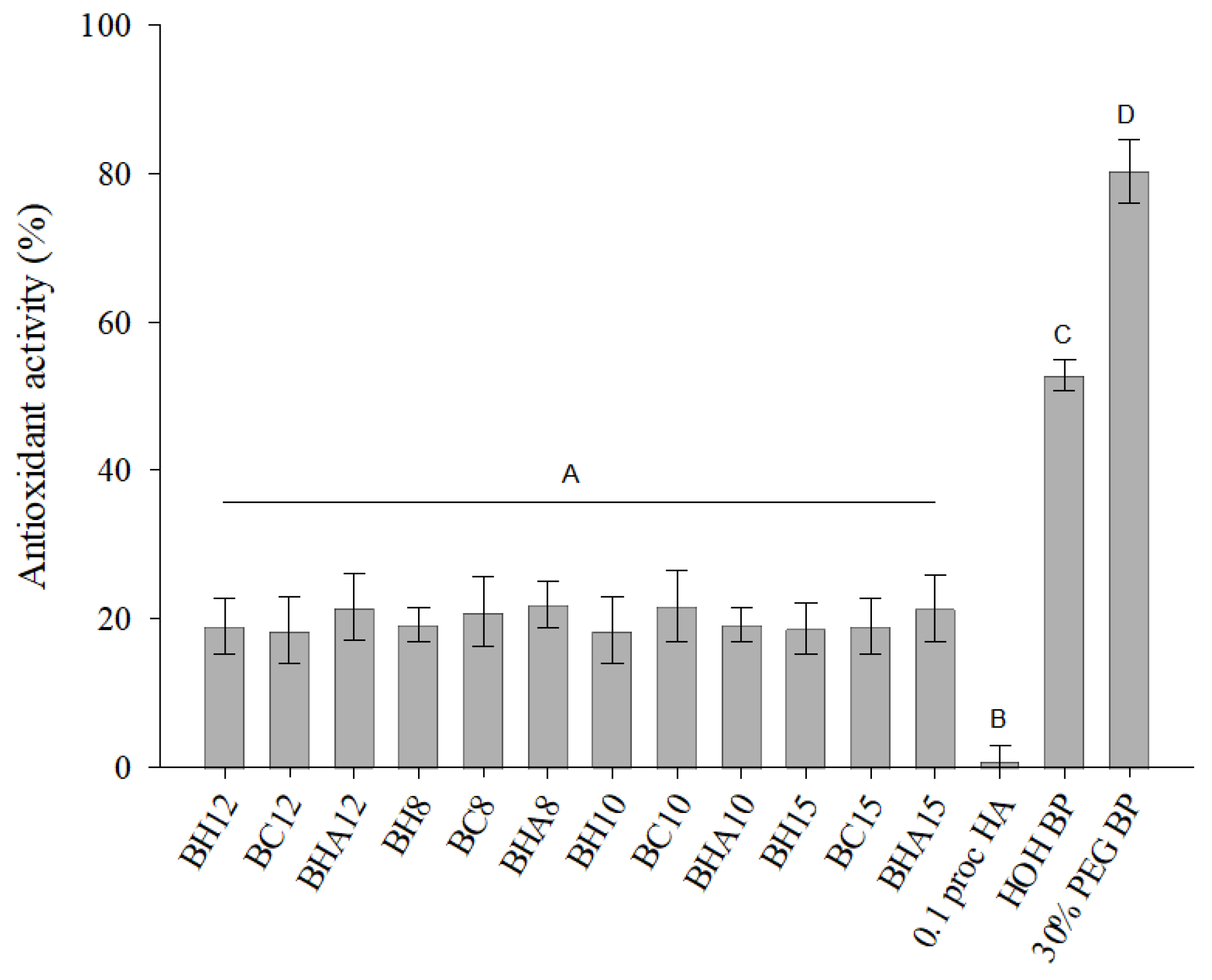
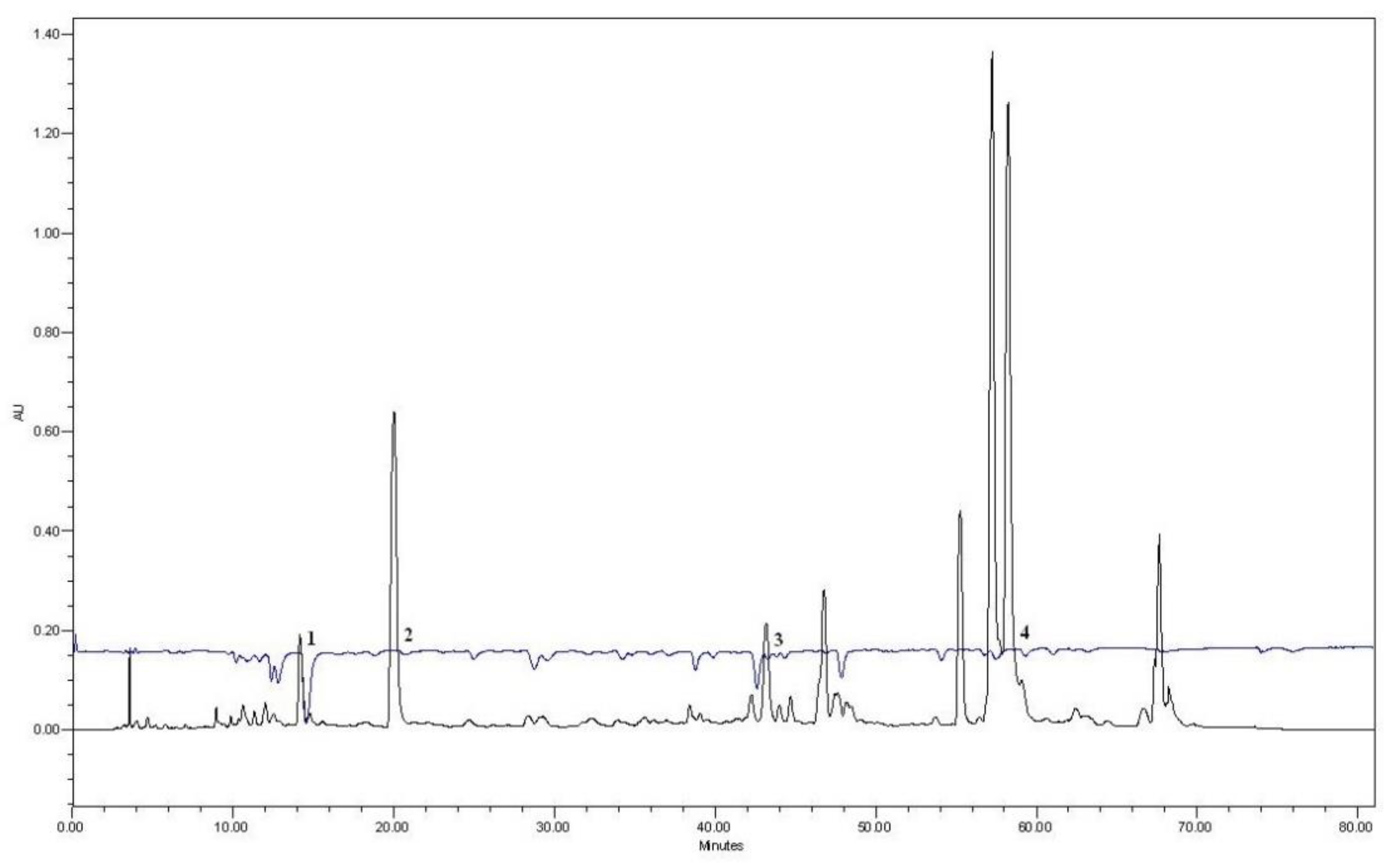

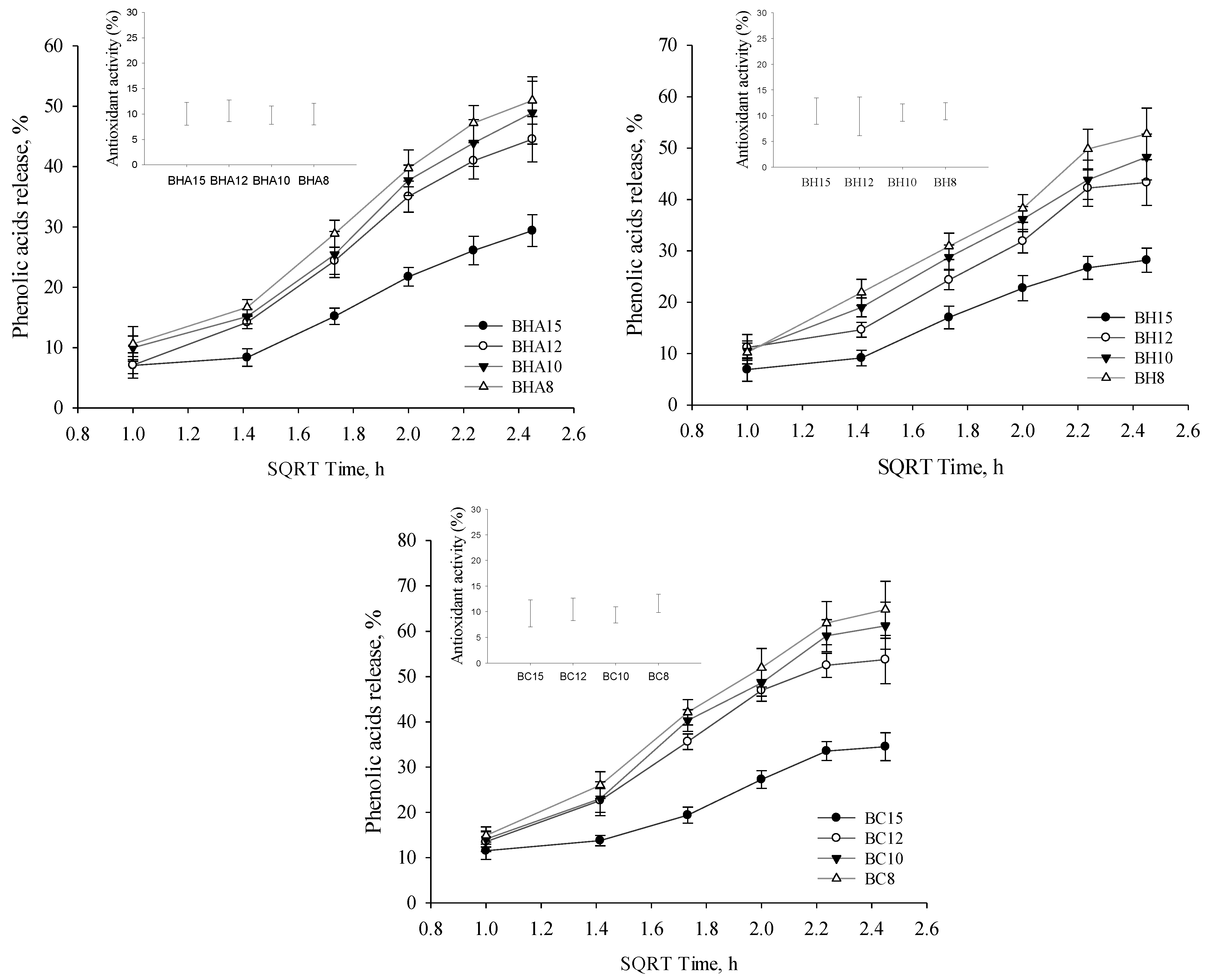

| A | HOH | PEG400 10% | PEG400 20% | PEG400 30% | ||||
| µg/mL | SD | µg/mL | SD | µg/mL | SD | µg/mL | SD | |
| Salicin | 135.80 | 12.35 | 108.91 | 9.91 | 117.95 | 10.73 | 138.00 | 9.61 |
| Chlorogenic acid | 1.41 | 0.10 | 1.39 | 0.10 | 1.84 | 0.13 | 2.95 | 0.22 |
| Caffeic acid | 38.68 | 2.75 | 91.90 | 6.54 | 90.17 | 6.42 | 121.55 | 9.01 |
| P-coumaric acid | 189.05 | 13.60 | 441.13 | 31.73 | 460.64 | 33.13 | 655.69 | 50.02 |
| Cinnamic acid | 19.22 | 1.42 | 30.74 | 2.27 | 44.10 | 3.26 | 85.11 | 5.49 |
| Pinobanksin | 2.92 | 0.29 | 61.67 | 6.23 | 91.64 | 9.25 | 174.43 | 10.72 |
| Pinocembrin | 0.00 | 0.00 | 2.15 | 0.20 | 6.05 | 0.55 | 12.97 | 1.15 |
| Galangin | 1.16 | 0.12 | 18.32 | 1.30 | 17.22 | 1.57 | 18.61 | 1.38 |
| Total amount of active compounds | 388.24 | 756.21 | 829.61 | 1209.31 | ||||
| B | HOH | PEG400 10% | PEG400 20% | PEG400 30% | ||||
| µg/mL | SD | µg/mL | SD | µg/mL | SD | µg/mL | SD | |
| Salicin | 124.86 | 11.36 | 101.52 | 8.26 | 129.05 | 11.74 | 144.83 | 13.18 |
| Chlorogenic acid | 1.50 | 0.10 | 1.29 | 0.10 | 2.03 | 0.14 | 3.02 | 0.34 |
| Caffeic acid | 44.13 | 3.14 | 85.01 | 5.92 | 101.02 | 7.19 | 133.39 | 9.50 |
| P-coumaric acid | 201.92 | 14.52 | 404.30 | 33.45 | 491.55 | 35.36 | 681.98 | 49.05 |
| Cinnamic acid | 15.92 | 1.18 | 27.27 | 1.57 | 40.46 | 2.99 | 81.41 | 6.02 |
| Pinobanksin | 3.09 | 0.31 | 55.17 | 5.46 | 83.07 | 8.39 | 157.21 | 15.88 |
| Pinocembrin | 0.00 | 0.00 | 2.11 | 0.17 | 4.16 | 0.38 | 10.23 | 0.93 |
| Galangin | 1.05 | 0.10 | 16.30 | 1.40 | 18.74 | 1.69 | 19.13 | 2.31 |
| Total amount of active compounds | 392.47 | 692.96 | 870.08 | 1231.20 | ||||
| Formulation (%) | P407 | CMC | HPMC | HA | Balsam Poplar Buds Extract | Benzalkonium Chloride | Purified Water |
|---|---|---|---|---|---|---|---|
| BH8 | 8 | - | 0.5 | - | 5 | 0.002 | Ad 100 |
| BH10 | 10 | - | 0.5 | - | 5 | 0.002 | Ad 100 |
| BH12 | 12 | - | 0.5 | - | 5 | 0.002 | Ad 100 |
| BH15 | 15 | - | 0.5 | - | 5 | 0.002 | Ad 100 |
| BC8 | 8 | 0.5 | - | - | 5 | 0.002 | Ad 100 |
| BC10 | 10 | 0.5 | - | - | 5 | 0.002 | Ad 100 |
| BC12 | 12 | 0.5 | - | - | 5 | 0.002 | Ad 100 |
| BC15 | 15 | 0.5 | - | - | 5 | 0.002 | Ad 100 |
| BHA8 | 8 | - | - | 0.1 | 5 | 0.002 | Ad 100 |
| BHA10 | 10 | - | - | 0.1 | 5 | 0.002 | Ad 100 |
| BHA12 | 12 | - | - | 0.1 | 5 | 0.002 | Ad 100 |
| BHA15 | 15 | - | - | 0.1 | 5 | 0.002 | Ad 100 |
| A | pH | SD | Vsc, mPa·s 22 ± 1 °C | SD | Appearance | ||
| BH12 | 6.60 | 0.06 | 33.90 | 4.36 | Clear/yellowish | ||
| BC12 | 6.64 | 0.07 | 32.57 | 4.23 | Clear/yellowish | ||
| BHA12 | 6.64 | 0.09 | 36.03 | 3.45 | Clear/yellowish | ||
| BH8 | 6.47 | 0.04 | 15.57 | 2.85 | Clear/yellowish | ||
| BC8 | 6.46 | 0.06 | 17.27 | 2.45 | Clear/yellowish | ||
| BHA8 | 6.55 | 0.04 | 18.47 | 2.61 | Clear/yellowish | ||
| BH10 | 6.50 | 0.04 | 18.03 | 2.18 | Clear/yellowish | ||
| BC10 | 6.46 | 0.07 | 24.27 | 2.70 | Clear/yellowish | ||
| BHA10 | 6.50 | 0.06 | 23.27 | 2.90 | Clear/yellowish | ||
| BH15 | 6.70 | 0.04 | 67.27 | 5.99 | Clear/yellowish | ||
| BC15 | 6.62 | 0.05 | 66.80 | 3.99 | Clear/yellowish | ||
| BHA15 | 6.67 | 0.06 | 62.27 | 6.45 | Clear/yellowish | ||
| B | pH | SD | Vsc, mPa·s 21 ± 1 °C | SD | Sol-to-gel | SD | Appearance |
| BH12 | 6.49 | 0.06 | 32.03 | 4.35 | 36.4 | 0.9 | Clear/yellowish |
| BC12 | 6.50 | 0.07 | 31.67 | 5.20 | 34.3 | 1.35 | Clear/yellowish |
| BHA12 | 6.56 | 0.04 | 31.20 | 3.38 | 37.9 | 1.11 | Clear/yellowish |
| BH8 | 6.40 | 0.06 | 16.23 | 2.29 | >50 °C | - | Clear/yellowish |
| BC8 | 6.40 | 0.07 | 15.60 | 2.26 | >50 °C | - | Clear/yellowish |
| BHA8 | 6.58 | 0.01 | 17.37 | 2.67 | >50 °C | - | Clear/yellowish |
| BH10 | 6.42 | 0.04 | 23.23 | 4.13 | >50 °C | - | Clear/yellowish |
| BC10 | 6.44 | 0.04 | 21.77 | 2.29 | >50 °C | - | Clear/yellowish |
| BHA10 | 6.49 | 0.06 | 22.20 | 5.13 | >50 °C | - | Clear/yellowish |
| BH15 | 6.62 | 0.05 | 63.80 | 6.66 | 27.4 | 1.1 | Clear/yellowish |
| BC15 | 6.56 | 0.04 | 61.67 | 6.27 | 28.7 | 1.28 | Clear/yellowish |
| BHA15 | 6.61 | 0.02 | 55.27 | 4.72 | 27.1 | 1.1 | Clear/yellowish |
| pH | SD | Vsc, mPa·s 21 ± 1 °C | SD | Active Compounds % | SD | Appearance | |
|---|---|---|---|---|---|---|---|
| BH12 | 6.54 | 0.08 | 30.93 | 3.15 | 98.95 | 4.07 | Clear/yellowish |
| BC12 | 6.57 | 0.06 | 29.42 | 2.47 | 98.75 | 2.94 | Clear/yellowish |
| BHA12 | 6.53 | 0.07 | 29.71 | 5.1 | 97.68 | 4.88 | Clear/yellowish |
| BH8 | 6.5 | 0.07 | 13.36 | 2.93 | 98.36 | 6.81 | Clear/yellowish |
| BC8 | 6.5 | 0.04 | 12.96 | 1.46 | 99.2 | 2.21 | Clear/yellowish |
| BHA8 | 6.49 | 0.08 | 14.4 | 3.96 | 98.81 | 3.46 | Clear/yellowish |
| BH10 | 6.48 | 0.04 | 18.17 | 3.68 | 97.19 | 4.17 | Clear/yellowish |
| BC10 | 6.49 | 0.08 | 17.5 | 2.48 | 96.94 | 4.87 | Clear/yellowish |
| BHA10 | 6.48 | 0.09 | 18.31 | 4.49 | 98.34 | 2.88 | Clear/yellowish |
| BH15 | 6.6 | 0.08 | 61.33 | 6.14 | 97.53 | 5.69 | Clear/yellowish |
| BC15 | 6.67 | 0.07 | 60.52 | 4.34 | 99.13 | 4.15 | Clear/yellowish |
| BHA15 | 6.68 | 0.07 | 54.38 | 2.76 | 98.56 | 3.75 | Clear/yellowish |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciauskaite, M.; Marksa, M.; Ivanauskas, L.; Ramanauskiene, K. Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations. Antioxidants 2022, 11, 1771. https://doi.org/10.3390/antiox11091771
Stanciauskaite M, Marksa M, Ivanauskas L, Ramanauskiene K. Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations. Antioxidants. 2022; 11(9):1771. https://doi.org/10.3390/antiox11091771
Chicago/Turabian StyleStanciauskaite, Monika, Mindaugas Marksa, Liudas Ivanauskas, and Kristina Ramanauskiene. 2022. "Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations" Antioxidants 11, no. 9: 1771. https://doi.org/10.3390/antiox11091771
APA StyleStanciauskaite, M., Marksa, M., Ivanauskas, L., & Ramanauskiene, K. (2022). Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations. Antioxidants, 11(9), 1771. https://doi.org/10.3390/antiox11091771






