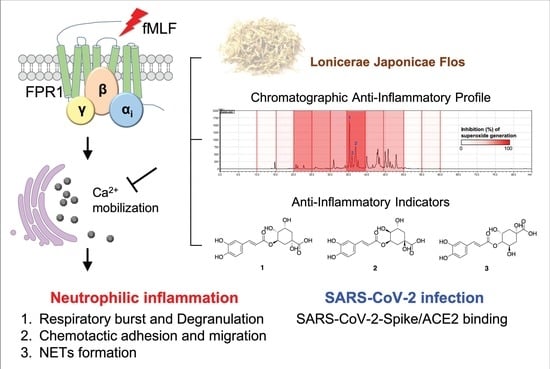
Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Human Neutrophils
2.3. Sample Extraction and Preparation
2.4. Fraction Preparation for Bioassay-Coupled High-Performance Liquid Chromatography (HPLC) Profile
2.5. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) Condition
2.6. Determination of Superoxide Anion (O2•−) Generation
2.7. Determination of Elastase Release
2.8. Determination of Lactate Dehydrogenase (LDH) Release
2.9. Determination of Superoxide Anion (O2•−) Scavenging Activity
2.10. Assessment of 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.11. CD11b Expression
2.12. Determination of Neutrophils Adhesion
2.13. Neutrophils Chemotactic Migration Assay
2.14. Analysis of Neutrophil Extracellular Trap (NET) Formation
2.15. Measurement of [Ca2+]i
2.16. Western Blotting
2.17. The Homogeneous Time-Resolved Fluorescence (HTRF) Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein (SARS-CoV-2 spike)/Angiotensin-Converting Enzyme 2 (ACE2) Binding Assay
2.18. Statistics
3. Results and Discussions
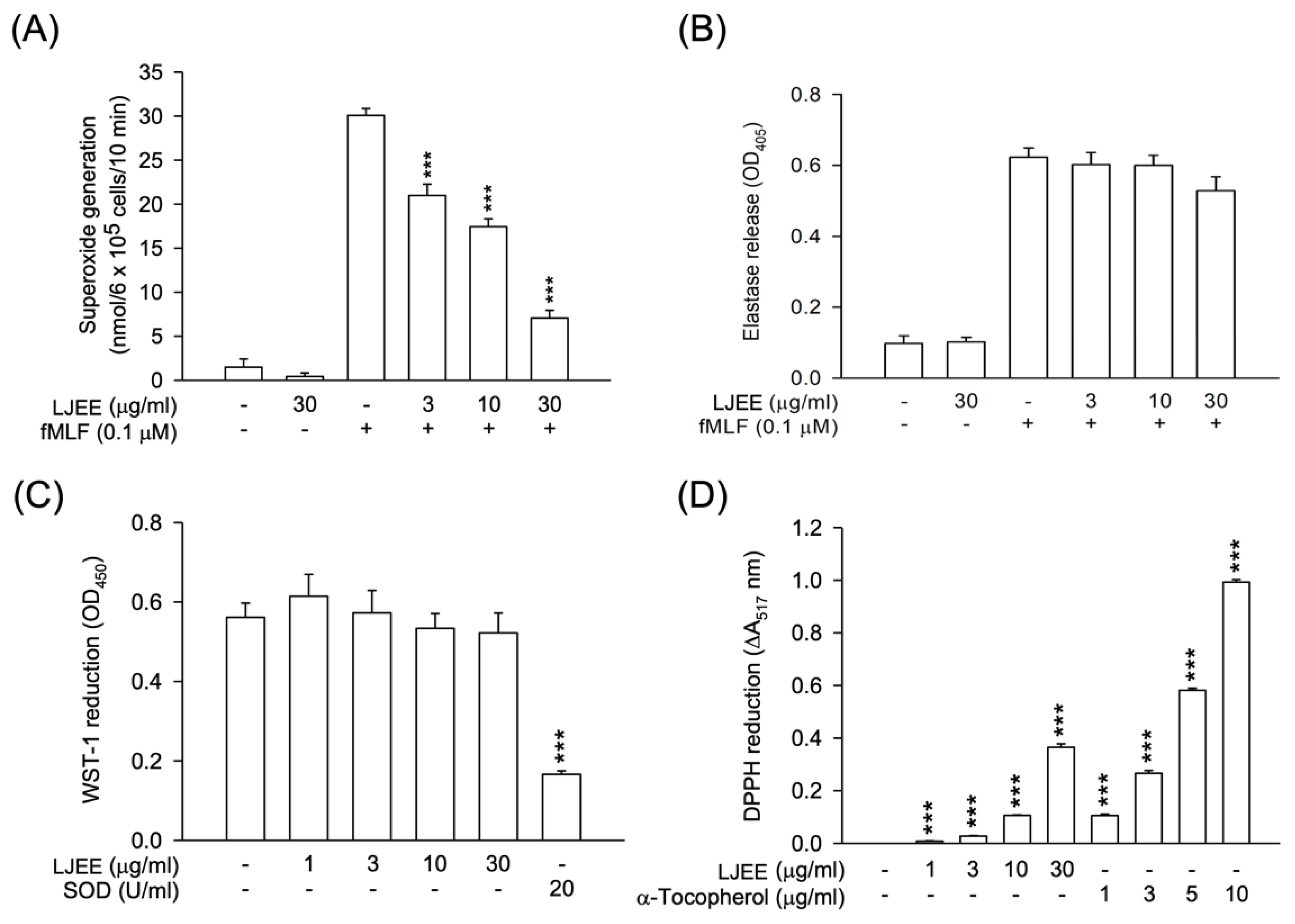
3.1. Lonicerae Japonicae Flos Ehanol Extract (LJEE) Inhibited the Superoxide Anion (O2•−) Generation but Not Elastase Release in N-Formyl-Methionyl-Leucyl-Phenylalanine (fMLF)-Activated Human Neutrophils
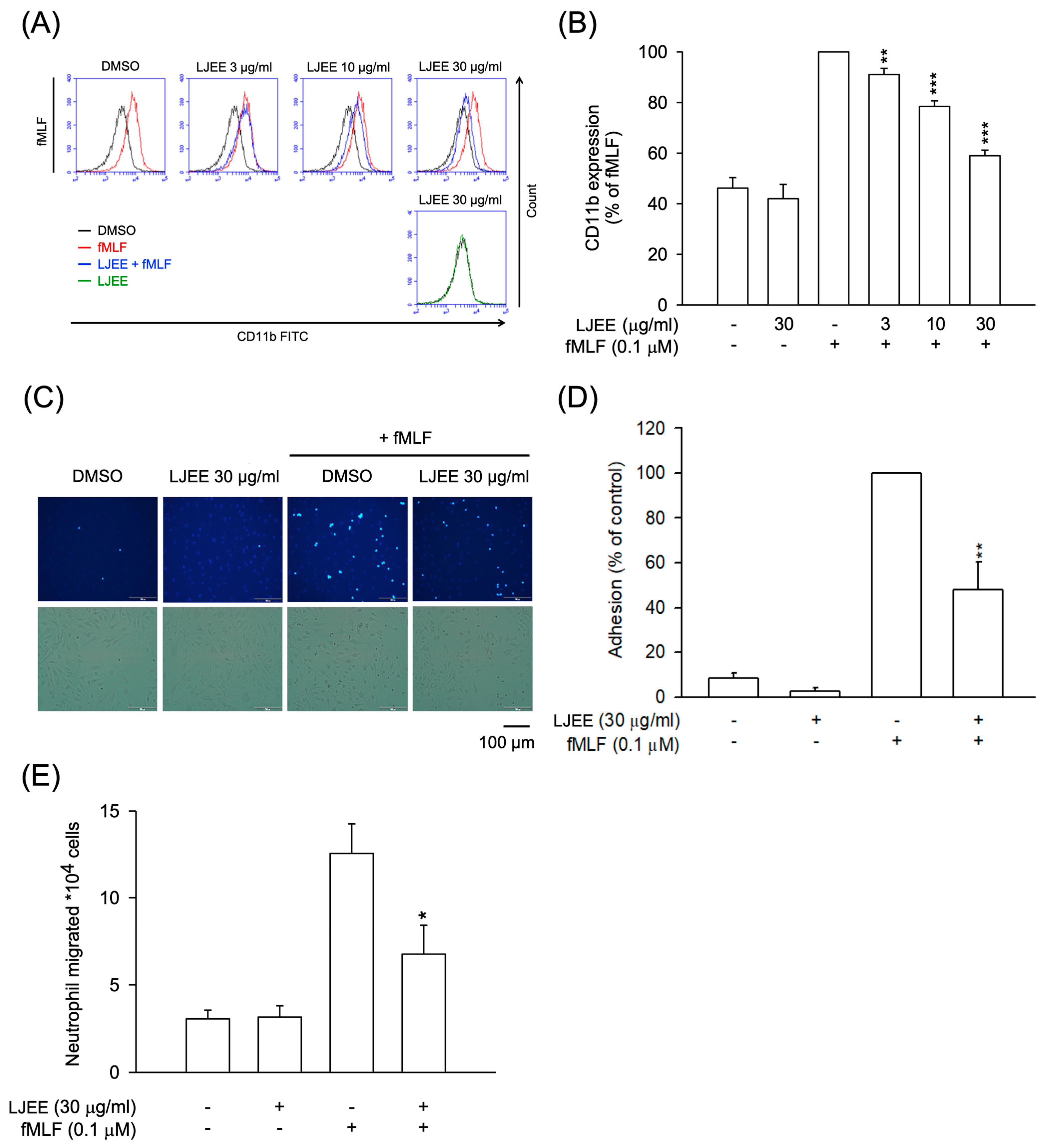
3.2. LJEE Ameliorated the fMLF-Induced Neutrophilic Adhesion, Migration, and CD11b Expression
3.3. LJEE Suppressed NET Formation

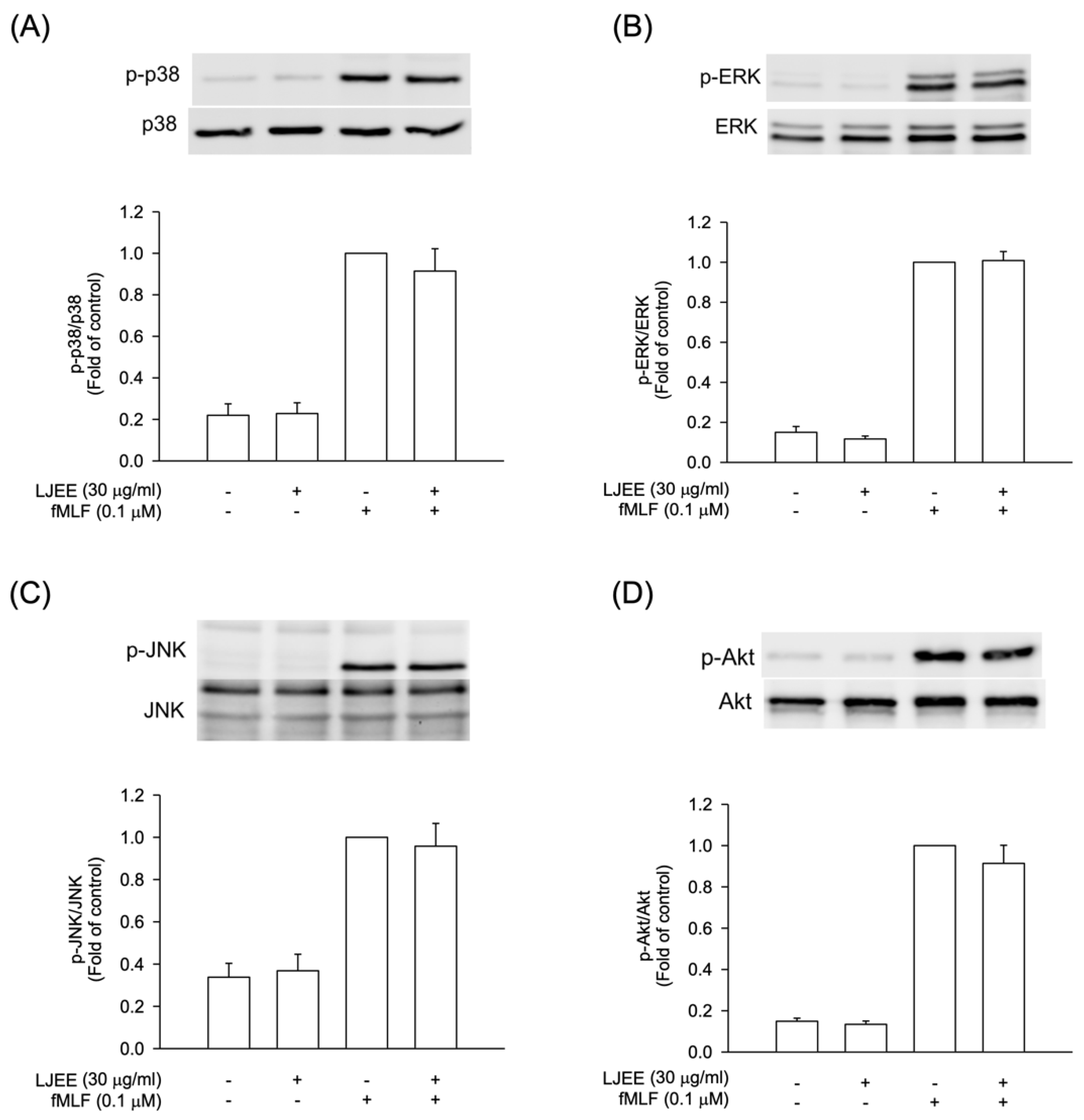
3.4. The Phosphorylation of Mitogen-Activated Protein Kinases (MAPKs) and Protein Kinase B (Akt) Signaling in Activated Human Neutrophils Were Not Affected by LJEE Treatment
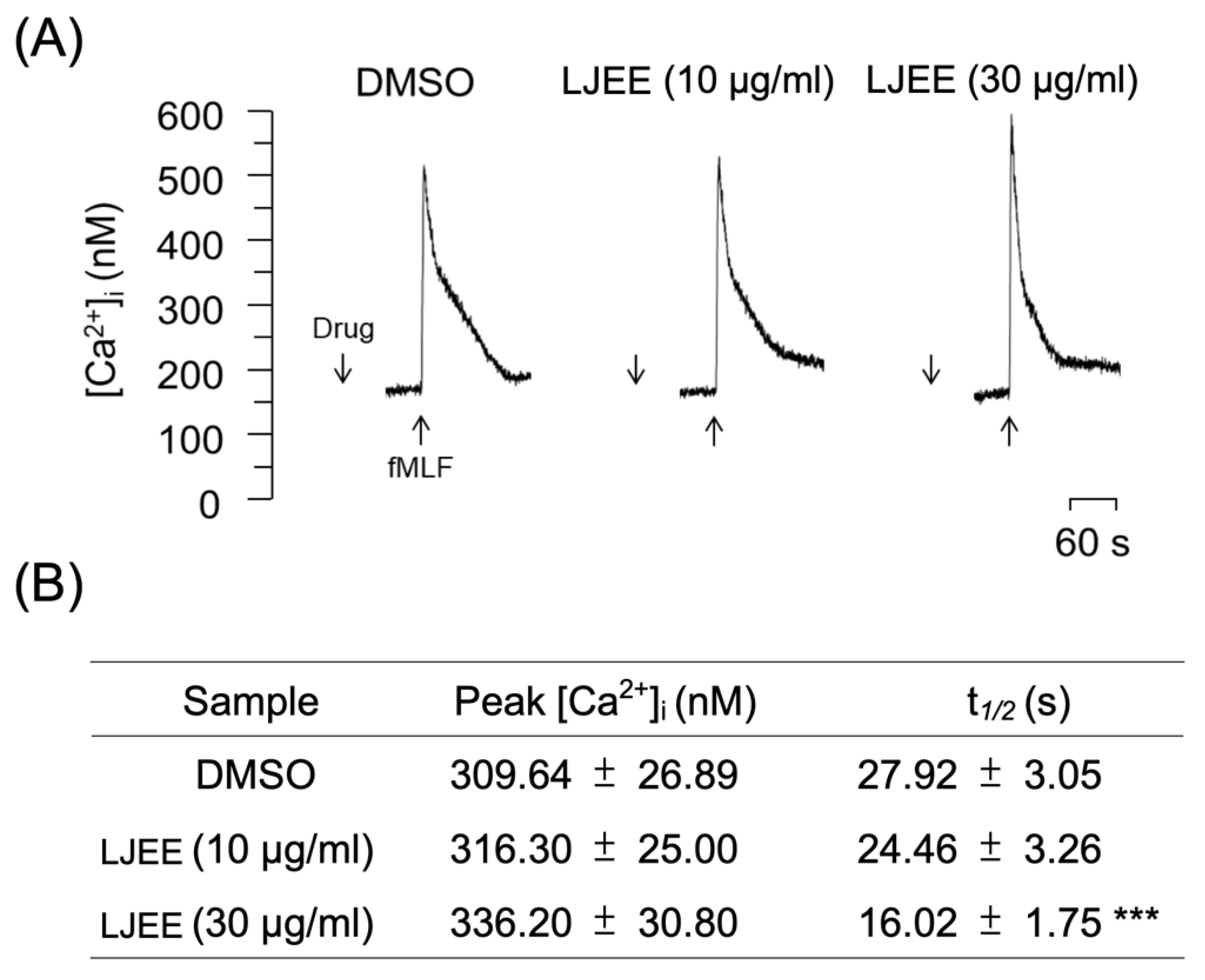
3.5. LJEE Decreased the fMLF-Induced Neutrophilic Intracellular Ca2+ Mobilization
3.6. Chlorogenic Acids Derivatives Dominated the Neutrophilic Inhibition of LJEE in fMLF-Induced Human Neutrophils

3.7. Lonicerae Japonicae Flos Water Extract (LJWE) Extracts Interrupted the Binding of SARS-CoV-2 Spike/ACE2

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, H.; Chen, J.; Yang, R.; Zou, L.; Wang, C.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Metabolomics characterizes the metabolic changes of Lonicerae Japonicae Flos under different salt stresses. PLoS ONE 2020, 15, e0243111. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.W.; Park, D.; Kwon, K.S.; Lee, D.H.; Jang, M.J.; Park, S.K.; Kim, J.Y. BST-104, a water extract of Lonicera japonica, has a gastroprotective effect via antioxidant and anti-inflammatory activities. J. Med. Food 2019, 22, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Xiao, L.; Wan, H.; Li, J.; Lv, K.; Peng, S.; Zhou, B.; Li, T.; Zeng, X. Chemical constituents from Lonicera japonica flower buds and their anti-hepatoma and anti-HBV activities. Bioorg. Chem. 2019, 92, 103198. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, W.S.; Han, M.H.; Song, K.S.; Hong, S.H.; Nagappan, A.; Kim, G.Y.; Kim, G.S.; Jung, J.M.; Ryu, C.H. Lonicera japonica Thunb. induces caspase-dependent apoptosis through death receptors and suppression of AKT in U937 human leukemic cells. Phytother. Res. 2018, 32, 504–513. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, e0204152. [Google Scholar] [CrossRef]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef]
- Kang, M.; Jung, I.; Hur, J.; Kim, S.H.; Lee, J.H.; Kang, J.-Y.; Jung, K.C.; Kim, K.S.; Yoo, M.C.; Park, D.-S. The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb. and Anemarrhena asphodeloides BUNGE in vivo. J. Ethnopharmacol. 2010, 131, 485–496. [Google Scholar] [CrossRef]
- Tian, J.; Che, H.; Ha, D.; Wei, Y.; Zheng, S. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr. Polym. 2012, 90, 1642–1647. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, Y.; Huang, Z.; Lu, B.; Ji, L. Lonicera japonica attenuates carbon tetrachloride-induced liver fibrosis in mice: Molecular mechanisms of action. Am. J. Chin. Med. 2019, 47, 351–367. [Google Scholar] [CrossRef]
- Ko, H.J.; Oh, S.K.; Jin, J.H.; Son, K.H.; Kim, H.P. Inhibition of experimental systemic inflammation (septic inflammation) and chronic bronchitis by new phytoformula BL containing Broussonetia papyrifera and Lonicera japonica. Biomol. Ther. 2013, 21, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.; Kim, Y.; Lee, G.; Kim, S.-J.; Jung, S.; Jung, H.; Bae, H. Lipopolysaccharide induced lung inflammation is inhibited by Lonicera japonica. Mol. Cell. Toxicol. 2011, 7, 87–93. [Google Scholar] [CrossRef]
- Park, Y.C.; Jin, M.; Kim, S.H.; Kim, M.H.; Namgung, U.; Yeo, Y. Effects of inhalable microparticle of flower of Lonicera japonica in a mouse model of COPD. J. Ethnopharmacol. 2014, 151, 123–130. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Q.; Hu, J.; Zhang, Y.; Li, J. Research progress on chemical constituents of Lonicerae japonicae flos. Biomed Res. Int. 2016, 2016, 1–18. [Google Scholar]
- Wu, X.Q.; Zhang, W.N.; Hao, M.Z.; Liu, X.P.; Xiao, J.; Wang, T.F.; Dong, Y.Z.; Zhao, J. How Chinese herbal medicine prevents epidemics: From ancient pestilences to COVID-19 pandemic. Am. J. Chin. Med. 2021, 49, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2021, 39, 3194–3203. [Google Scholar] [CrossRef]
- Yu, J.W.; Wang, L.; Bao, L.D. Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID-19) based on molecular docking method. J. Funct. Foods 2020, 71, 104016. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhang, Y.R.; Luo, S.Y.; Zhang, Y.S.; Zhang, Y.; Tang, C. Chlorogenic acid, a natural product as potential inhibitor of COVID-19: Virtual screening experiment based on network pharmacology and molecular docking. Nat. Prod. Res. 2021, 36, 2580–2584. [Google Scholar] [CrossRef]
- Jan, J.T.; Cheng, T.R.; Juang, Y.P.; Ma, H.H.; Wu, Y.T.; Yang, W.B.; Cheng, C.W.; Chen, X.; Chou, T.H.; Shie, J.J. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef]
- Collaborative, C.O. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. Lancet 2020, 396, 27–38. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E. Neutrophils and acute lung injury. Crit. Care Med. 2003, 31, S195–S199. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Mitsios, A.; Arampatzioglou, A.; Arelaki, S.; Mitroulis, I.; Ritis, K. NETopathies? unraveling the dark side of old diseases through neutrophils. Front. Immunol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Z.; Wang, G.; Guan, X.; Fang, K.; Wang, Z.; Wang, F. Advanced role of neutrophils in common respiratory diseases. J. Immunol. Res. 2017, 2017, 6710278. [Google Scholar] [CrossRef]
- Rebetz, J.; Semple, J.W.; Kapur, R. The pathogenic involvement of neutrophils in acute respiratory distress syndrome and transfusion-related acute lung injury. Transfus. Med. Hemother. 2018, 45, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Cheng, W.J.; Korinek, M.; Lin, C.Y.; Hwang, T.L. Neutrophils in psoriasis. Front. Immunol. 2019, 10, 2376. [Google Scholar] [CrossRef]
- Chen, P.J.; Ko, I.L.; Lee, C.L.; Hu, H.C.; Chang, F.R.; Wu, Y.C.; Leu, Y.L.; Wu, C.C.; Lin, C.Y.; Pan, C.Y.; et al. Targeting allosteric site of AKT by 5,7-dimethoxy-1,4-phenanthrenequinone suppresses neutrophilic inflammation. EBioMedicine 2019, 40, 528–540. [Google Scholar] [CrossRef]
- Chiang, C.C.; Cheng, W.J.; Lin, C.Y.; Lai, K.H.; Ju, S.C.; Lee, C.; Yang, S.H.; Hwang, T.L. Kan-Lu-Hsiao-Tu-Tan, a traditional Chinese medicine formula, inhibits human neutrophil activation and ameliorates imiquimod-induced psoriasis-like skin inflammation. J. Ethnopharmacol. 2020, 246, 112246. [Google Scholar] [CrossRef]
- Peng, B.R.; Lai, K.H.; Lee, G.H.; Yu, S.S.; Duh, C.Y.; Su, J.H.; Zheng, L.G.; Hwang, T.L.; Sung, P.J. Scalarane-type sesterterpenoids from the marine sponge Lendenfeldia sp. alleviate inflammation in human neutrophils. Mar. Drugs 2021, 19, 561. [Google Scholar] [CrossRef]
- Peng, B.R.; Lai, K.H.; Chang, Y.C.; Chen, Y.Y.; Su, J.H.; Huang, Y.M.; Chen, P.J.; Yu, S.S.; Duh, C.Y.; Sung, P.J. Sponge-derived 24-homoscalaranes as potent anti-inflammatory agents. Mar. Drugs 2020, 18, 434. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Miralda, I.; Armstrong, C.L.; Uriarte, S.M.; Bagaitkar, J. The roles of NADPH oxidase in modulating neutrophil effector responses. Mol. Oral Microbiol. 2019, 34, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, L.; Sun, L.; Zhou, L.; Xu, Y. In comparison with vitamin C and butylated hydroxytoluene, the antioxidant capacity of aqueous extracts from buds and flowers of Lonicera japonica Thunb. J. Tradit. Chin. Med. 2018, 38, 373–379. [Google Scholar] [PubMed]
- Tang, D.; Li, H.J.; Chen, J.; Guo, C.W.; Li, P. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae Japonicae by DPPH-HPLC-DAD-TOF/MS. J. Sep. Sci. 2008, 31, 3519–3526. [Google Scholar] [CrossRef]
- Maas, S.L.; Soehnlein, O.; Viola, J.R. Organ-specific mechanisms of transendothelial neutrophil migration in the lung, liver, kidney, and aorta. Front. Immunol. 2018, 9, 2739. [Google Scholar] [CrossRef]
- Lai, K.H.; Chen, P.J.; Chen, C.C.; Yang, S.H.; El-Shazly, M.; Chang, Y.C.; Wu, Y.H.; Wu, Y.H.; Wang, Y.H.; Hsieh, H.L.; et al. Lophatherum gracile Brongn. attenuates neutrophilic inflammation through inhibition of JNK and calcium. J. Ethnopharmacol. 2021, 264, 113224. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Immler, R.; Simon, S.I.; Sperandio, M. Calcium signaling and related ion channels in neutrophil recruitment and function. Eur. J. Clin. Invest. 2018, 48 (Suppl. 2), e12964. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, Y.H.; Chang, S.H.; Tsai, Y.F.; Fang, J.Y.; Hwang, T.L. Oleic acid-loaded nanostructured lipid carrier inhibit neutrophil activities in the presence of albumin and alleviates skin inflammation. Int. J. Nanomed. 2019, 14, 6539–6553. [Google Scholar] [CrossRef]
- Lin, C.Y.; Hsu, C.Y.; Elzoghby, A.O.; Alalaiwe, A.; Hwang, T.L.; Fang, J.Y. Oleic acid as the active agent and lipid matrix in cilomilast-loaded nanocarriers to assist PDE4 inhibition of activated neutrophils for mitigating psoriasis-like lesions. Acta Biomater. 2019, 90, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Leu, Y.L.; Fang, Y.; Lin, C.F.; Kuo, L.M.; Sung, W.C.; Tsai, Y.F.; Chung, P.J.; Lee, M.C.; Kuo, Y.T.; et al. Anti-inflammatory effects of Perilla frutescens in activated human neutrophils through two independent pathways: Src family kinases and Calcium. Sci. Rep. 2015, 5, 18204. [Google Scholar] [CrossRef]
- Tintinger, G.; Steel, H.C.; Anderson, R. Taming the neutrophil: Calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin. Exp. Immunol. 2005, 141, 191–200. [Google Scholar] [CrossRef]
- Clemens, R.A.; Lowell, C.A. Store-operated calcium signaling in neutrophils. J. Leukoc. Biol. 2015, 98, 497–502. [Google Scholar] [CrossRef]
- Korchak, H.M.; Dorsey, L.B.; Li, H.; Mackie, D.; Kilpatrick, L.E. Selective roles for alpha-PKC in positive signaling for O-(2) generation and calcium mobilization but not elastase release in differentiated HL60 cells. Biochim. Biophys. Acta 2007, 1773, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, L.; Shang, Y.; Yang, X.; Li, J.; He, J.; Gao, X.; Chang, Y.X. A strategy for intelligent chemical profiling-guided precise quantitation of multi-components in traditional Chinese medicine formulae-QiangHuoShengShi decoction. J. Chromatogr. A 2021, 1649, 462178. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. Am. J. Chin. Med. 2012, 33, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-W.; Jung, H.A.; Kang, S.S.; Choi, J.S. Antioxidant constituents and a new triterpenoid glycoside from Flos Lonicerae. Arch. Pharm. Res. 2007, 30, 1–7. [Google Scholar] [CrossRef]
- Son, M.J.; Moon, T.C.; Lee, E.K.; Son, K.H.; Kim, H.P.; Kang, S.S.; Son, J.K.; Lee, S.H.; Chang, H.W. Naturally occurring biflavonoid, ochanflavone, inhibits cyclo-oxygenases-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2006, 29, 282–286. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yoon, S.-J.; Kim, Y.-M.; Hong, S.-W.; Yeon, S.H.; Choe, K.-I.; Lee, S.-M. HS-23, Lonicera japonica extract, attenuates septic injury by suppressing toll-like receptor 4 signaling. J. Ethnopharmacol. 2014, 155, 256–266. [Google Scholar] [CrossRef]
- Ou, J.; Zhou, Z.; Dai, R.; Zhang, J.; Zhao, S.; Wu, X.; Lan, W.; Ren, Y.; Cui, L.; Lan, Q.; et al. V367F mutation in SARS-CoV-2 spike RBD emerging during the early transmission phase enhances viral infectivity through increased human ACE2 receptor binding affinity. J. Virol. 2021, 95, e0061721. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Superoxide Anion | Elastase Release | ||
|---|---|---|---|---|
| IC50 (μM) a | Inh (%) b | IC50 (μM) a | Inh (%) b | |
| Chlorogenic acid | 3.91 ± 0.35 | 74.63 ± 1.82 | 8.51 ± 0.47 | 75.41 ± 3.22 |
| Neochlorogenic acid | 3.97 ± 0.58 | 76.78 ± 3.73 | >20 | 23.82 ± 2.44 |
| Cryptochlorogenic acid | 3.76 ± 0.63 | 75.85 ± 1.85 | 12.14 ± 3.97 | 62.05 ± 6.07 |
| Cynaroside | ≥20 | 52.63 ± 5.00 | – c | |
| LY294002 d | 1.91 ± 0.56 | 88.99 ± 1.10 | 2.94 ± 0.12 | 83.49 ± 3.47 |
| Compounds | Ion Adduct | Precursor/ Product ion (m/z) a | CE | Calibration Curve | R2 | Amount (%) |
|---|---|---|---|---|---|---|
| Chlorogenic acid | [M-H]− | 353/191 | 31 | Y = 249,834X − 6253 | 0.9997 | 3.32 |
| Neochlorogenic acid | [M-H]− | 353/135 | 17 | Y = 1,113,295X + 3620 | 0.9997 | 1.23 |
| Cryptochlorogenic acid | [M-H]− | 353/173 | 16 | Y = 218,121X + 10,620 | 0.9993 | 0.56 |
| Cynaroside | [M-H]− | 447/285 | 28 | Y = 642,917 X + 167,946 | 0.9954 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, K.-H.; Chen, Y.-L.; Lin, M.-F.; El-Shazly, M.; Chang, Y.-C.; Chen, P.-J.; Su, C.-H.; Chiu, Y.-C.; Illias, A.M.; Chen, C.-C.; et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. https://doi.org/10.3390/antiox11091781
Lai K-H, Chen Y-L, Lin M-F, El-Shazly M, Chang Y-C, Chen P-J, Su C-H, Chiu Y-C, Illias AM, Chen C-C, et al. Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants. 2022; 11(9):1781. https://doi.org/10.3390/antiox11091781
Chicago/Turabian StyleLai, Kuei-Hung, Yu-Li Chen, Mei-Fang Lin, Mohamed El-Shazly, Yu-Chia Chang, Po-Jen Chen, Chun-Han Su, Yen-Chun Chiu, Amina M. Illias, Chih-Chuan Chen, and et al. 2022. "Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress" Antioxidants 11, no. 9: 1781. https://doi.org/10.3390/antiox11091781
APA StyleLai, K.-H., Chen, Y.-L., Lin, M.-F., El-Shazly, M., Chang, Y.-C., Chen, P.-J., Su, C.-H., Chiu, Y.-C., Illias, A. M., Chen, C.-C., Chen, L.-Y., & Hwang, T.-L. (2022). Lonicerae Japonicae Flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants, 11(9), 1781. https://doi.org/10.3390/antiox11091781