Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Semen
2.2. Motility Analysis
2.3. Mitochondrial Activity
2.4. Detection of Essential and Heavy Metals in Seminal Plasma
2.5. Ferric Reducing Ability of Plasma (FRAP)
2.6. Total Oxidant Status (TOS)
2.7. Superoxide Dismutase (SOD)
2.8. Glutathione Peroxidase (GPx)
2.9. Lipid Peroxidation (LPO)
2.10. Biochemical Analysis of Seminal Plasma
2.11. Statistical Analysis
3. Results
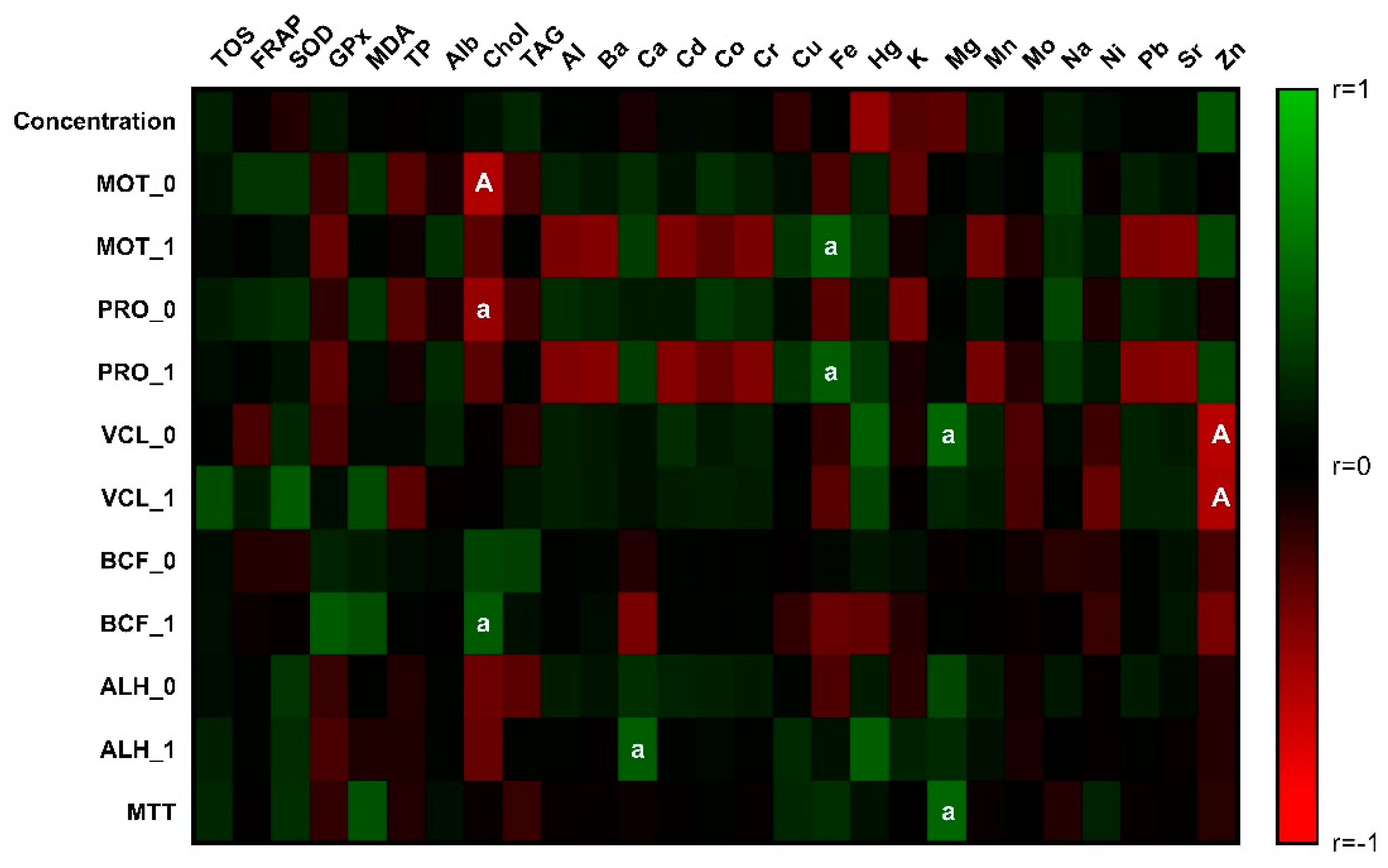
3.1. Sperm Quality
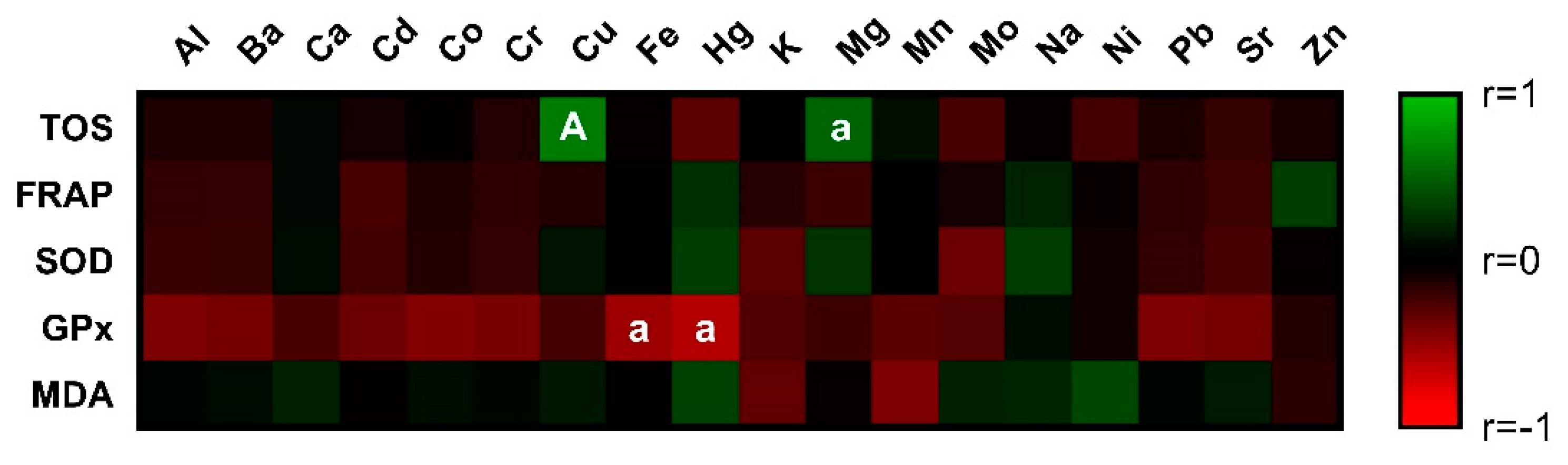
3.2. Markers of Oxidative Stress in Seminal Plasma
3.3. Biochemical Composition of Bovine Seminal Plasma
3.4. Chemical Composition of Bovine Seminal Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baruselli, P.S.; Ferreira, R.M.; Filho, M.F.S.; Bó, G.A. Review: Using Artificial Insemination v. Natural Service in Beef Herds. Animal 2018, 12, s45–s52. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.S.E.; Hafez, B. Reproduction in Farm Animals; Wiley: Hoboken, New Jersey, USA, 2013; ISBN 9781118710708. [Google Scholar]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal Plasma Proteins: What Role Do They Play? Am. J. Reprod. Immunol. 2011, 66 (Suppl. 1), 11–22. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N.; Caberlotto, S. Composition and metabolism of carbohydrates and lipids in Sparus aurata semen and its relation to viability expressed as sperm motility when activated. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Beer-Ljubić, B.; Aladrović, J.; Marenjak, T.S.; Laškaj, R.; Majić-Balić, I.; Milinković-Tur, S. Cholesterol Concentration in Seminal Plasma as a Predictive Tool for Quality Semen Evaluation. Theriogenology 2009, 72, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Tirpák, F.; Greifová, H.; Lukáč, N.; Stawarz, R.; Massányi, P. Exogenous Factors Affecting the Functional Integrity of Male Reproduction. Life 2021, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Tirpák, F.; Halo, M.; Tokárová, K.; Binkowski, L.J.; Vašíček, J.; Svoradová, A.; Błaszczyk-Altman, M.; Kováčik, A.; Tvrdá, E.; Chrenek, P.; et al. Composition of Stallion Seminal Plasma and Its Impact on Oxidative Stress Markers and Spermatozoa Quality. Life 2021, 11, 1238. [Google Scholar] [CrossRef]
- Wong, W.Y.; Flik, G.; Groenen, P.M.; Swinkels, D.W.; Thomas, C.M.; Copius-Peereboom, J.H.; Merkus, H.M.; Steegers-Theunissen, R.P. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001, 15, 131–136. [Google Scholar] [CrossRef]
- Marzec-Wróblewska, U.; Kamiński, P.; Lakota, P. Influence of Chemical Elements on Mammalian Spermatozoa. Folia Biol. 2012, 58, 7–15. [Google Scholar]
- Aloosh, M.; Hassani, M.; Nikoobakht, M. Seminal plasma magnesium and premature ejaculation: A case-control study. Br. J. Urol. 2006, 98, 402–404. [Google Scholar] [CrossRef]
- Ghasemi, H.; Karimi, J.; Goodarzi, M.T.; Khodadadi, I.; Tavilani, H.; Moridi, H.; Kheiripour, N. Seminal Plasma Zinc and Magnesium Levels and Their Relation to Spermatozoa Parameters in Semen of Diabetic Men. Int. J. Diabetes Dev. Ctries. 2016, 36, 34–39. [Google Scholar] [CrossRef]
- Liang, H.; Miao, M.; Chen, J.; Chen, K.; Wu, B.; Dai, Q.; Wang, J.; Sun, F.; Shi, H.; Yuan, W. The Association between Calcium, Magnesium, and Ratio of Calcium/Magnesium in Seminal Plasma and Sperm Quality. Biol. Trace Elem. Res. 2016, 174, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Kilic, M.; Soylak, M. The Determination of Toxic Metals in Some Traditional Cosmetic Products and Health Risk Assessment. Biol. Trace Elem. Res. 2021, 199, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef]
- Mintziori, G.; Mousiolis, A.; Duntas, L.H.; Goulis, D.G. Evidence for a Manifold Role of Selenium in Infertility. Hormones 2020, 19, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Lukáč, N.; Schneidgenová, M.; Lukáčová, J.; Szabó, C.; Goc, Z.; Greń, A.; Massányi, P. Impact of Seminal Chemical Elements on the Oxidative Balance in Bovine Seminal Plasma and Spermatozoa. J. Vet. Med. 2013, 2013, 125096. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology 2021, 10, 882. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary Evidence on the Physiological Role of Reactive Oxygen Species in Human Sperm Function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Baszyński, J.; Kamiński, P.; Bogdzińska, M.; Mroczkowski, S.; Szymański, M.; Wasilow, K.; Stanek, E.; Hołderna-Bona, K.; Brodzka, S.; Bilski, R.; et al. Enzymatic Antioxidant Defense and Polymorphic Changes in Male Infertility. Antioxidants 2022, 11, 817. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Pesch, S.; Bergmann, M.; Bostedt, H. Determination of Some Enzymes and Macro- and Microelements in Stallion Seminal Plasma and Their Correlations to Semen Quality. Theriogenology 2006, 66, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Kareskoski, M.; Katila, T. Components of Stallion Seminal Plasma and the Effects of Seminal Plasma on Sperm Longevity. Anim. Reprod. Sci. 2008, 107, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Pipan, M.Z.; Mrkun, J.; Strajn, B.J.; Vrtač, K.P.; Kos, J.; Pišlar, A.; Zrimšek, P. The Influence of Macro- and Microelements in Seminal Plasma on Diluted Boar Sperm Quality. Acta Vet. Scand. 2017, 59, 11. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, L.; Liu, Z.; Wei, H.; Zhou, Y.; Tan, J.; Sun, H.; Li, S.; Jiang, S.; Peng, J. Microelements in Seminal and Serum Plasma Are Associated with Fresh Semen Quality in Yorkshire Boars. Theriogenology 2019, 132, 88–94. [Google Scholar] [CrossRef]
- Wu, Y.; Lai, W.; Liu, Z.; Wei, H.; Zhou, Y.; Tan, J.; Sun, H.; Li, S.; Peng, J. Serum and Seminal Plasma Element Concentrations in Relation to Semen Quality in Duroc Boars. Biol. Trace Elem. Res. 2019, 189, 85–94. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal Plasma Proteins as Markers of Sperm Fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Pipan, M.Z.; Zrimšek, P.; Strajn, B.J.; Vrtač, K.P.; Knific, T.; Mrkun, J. Macro- and microelements in serum and seminal plasma as biomarkers for bull sperm cryotolerance. Acta Veter-Scand. 2021, 63, 25. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Sánchez, J.M.; Recuero, S.; Bagés-Arnal, S.; McDonald, M.; Kenny, D.A.; Yeste, M.; Lonergan, P.; Fernandez-Fuertes, B. Effect of Exposure to Seminal Plasma through Natural Mating in Cattle on Conceptus Length and Gene Expression. Front. Cell Dev. Biol. 2020, 8, 341. [Google Scholar] [CrossRef]
- Ahmadi, H.; Csabai, T.; Gorgey, E.; Rashidiani, S.; Parhizkar, F.; Aghebati-Maleki, L. Composition and Effects of Seminal Plasma in the Female Reproductive Tracts on Implantation of Human Embryos. Biomed. Pharmacother. 2022, 151, 113065. [Google Scholar] [CrossRef]
- Slanina, T.; Miškeje, M.; Tirpák, F.; Baszczyk, M.; Stawarz, R.; Massányi, P. Effect of Taurine on Turkey (Meleagris gallopavo) Spermatozoa Viability and Motility. Czech J. Anim. Sci. 2018, 63, 127–135. [Google Scholar] [CrossRef]
- Kovacik, A.; Tirpak, F.; Tomka, M.; Miskeje, M.; Tvrda, E.; Arvay, J.; Andreji, J.; Slanina, T.; Gabor, M.; Hleba, L.; et al. Trace Elements Content in Semen and Their Interactions with Sperm Quality and RedOx Status in Freshwater Fish Cyprinus Carpio: A Correlation Study. J. Trace Elem. Med. Biol. 2018, 50, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Binkowski, Ł.J.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, Lead and Mercury Concentrations and Their Influence on Morphological Parameters in Blood Donors from Different Age Groups from Southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Greifová, H.; Abdramanov, A.; Lukáč, N. Curcumin Has Protective and Antioxidant Properties on Bull Spermatozoa Subjected to Induced Oxidative Stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kňažická, Z.; Lukáčová, J.; Schneidgenová, M.; Goc, Z.; Greń, A.; Szabó, C.; Massányi, P.; Lukáč, N. The Impact of Lead and Cadmium on Selected Motility, Prooxidant and Antioxidant Parameters of Bovine Seminal Plasma and Spermatozoa. J. Environ. Sci. Health Part A 2013, 48, 1292–1300. [Google Scholar] [CrossRef]
- Kňažická, Z.; Lukáčová, J.; Greń, A.; Formicki, G.; Massányi, P.; Lukáč, N. Relationship between Level of Copper in Bovine Seminal Plasma and Spermatozoa Motility. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1351–1362. [Google Scholar]
- Aguiar, G.; Batista, B.; Rodrigues, J.; Silva, L.; Campiglia, A.; Barbosa, R.; Barbosa, F. Determination of trace elements in bovine semen samples by inductively coupled plasma mass spectrometry and data mining techniques for identification of bovine class. J. Dairy Sci. 2012, 95, 7066–7073. [Google Scholar] [CrossRef]
- Skiba, T.V.; Gou, H. Anodic Stripping Voltammetry for Direct Determination of Heavy Metals in Bovine Seminal Plasma Using Thick Film Modified Graphite Electrodes. Microchem. J. 2019, 147, 818–823. [Google Scholar] [CrossRef]
- Tvrda, E.; Knazicka, Z.; Lukac, N. Selected Heavy Metals versus Antioxidant Parameters in Bull Seminal Plasma—A Comparative Study. J. Environ. Sci. Health Part A 2012, 47, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Hardneck, F.; de Villiers, C.; Maree, L. Effect of Copper Sulphate and Cadmium Chloride on Non-Human Primate Sperm Function in Vitro. Int. J. Environ. Res. Public Health 2021, 18, 6200. [Google Scholar] [CrossRef] [PubMed]
- Hardneck, F.; Israel, G.; Pool, E.; Maree, L. Quantitative Assessment of Heavy Metal Effects on Sperm Function Using Computer-Aided Sperm Analysis and Cytotoxicity Assays. Andrologia 2018, 50, e13141. [Google Scholar] [CrossRef] [PubMed]
- Knazicka, Z.; Tvrda, E.; Bardos, L.; Lukac, N. Dose- and Time-Dependent Effect of Copper Ions on the Viability of Bull Spermatozoa in Different Media. J. Environ. Sci. Health Part A 2012, 47, 1294–1300. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Muiño-Blanco, T.; Pérez-Pé, R.; Cebrián-Pérez, J.A. Seminal Plasma Proteins and Sperm Resistance to Stress. Reprod. Domest. Anim. 2008, 43, 18–31. [Google Scholar]
- Arabi, M. The Role of Mercury in the Etiology of Sperm Dysfunction in Holstein Bulls. Asian-Australas. J. Anim. Sci. 2006, 19, 335–340. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; Gopalakrishnan, A.V. Heavy metal and metalloid—Induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef]
- Zemanová, J.; Lukáč, N.; Massányi, P.; Trandžik, J.; Burócziová, M.; Naď, P.; Capcarová, M.; Stawarz, R.; Skalická, M.; Toman, R.; et al. Nickel Seminal Concentrations in Various Animals and Correlation to Spermatozoa Quality. J. Vet. Med. 2007, 54, 281–286. [Google Scholar] [CrossRef]
- Chen, C.; Li, B.; Huang, R.; Dong, S.; Zhou, Y.; Song, J.; Zeng, X.; Zhang, X. Involvement of Ca2+ and ROS Signals in Nickel-Impaired Human Sperm Function. Ecotoxicol. Environ. Saf. 2022, 231, 113181. [Google Scholar] [CrossRef] [PubMed]
- Janicki, B.; Cygan-Szczegielniak, D. Zn and Pb Concentration in Seminal Plasma in Reference to Selected Parameters of Semiological Assessment of Bull Semen. Folia Biol. 2008, 56, 97–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arabi, M.; Mohammadpour, A.A. Adverse Effects of Cadmium on Bull Spermatozoa. Vet. Res. Commun. 2006, 30, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Kňažická, Z.; Lukáčová, J.; Schneidgenová, M.; Massányi, P.; Goc, Z.; Stawarz, R.; Lukáč, N. Relationships between Iron and Copper Content, Motility Characteristics and Antioxidant Status in Bovine Seminal. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 536–547. [Google Scholar]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and Copper in Male Reproduction: A Double-Edged Sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxid. Med. Cell Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef]
- Tribulo, P.; Bogle, O.; Mapletoft, R.; Adams, G. Bioactivity of Ovulation Inducing Factor (or Nerve Growth Factor) in Bovine Seminal Plasma and Its Effects on Ovarian Function in Cattle. Theriogenology 2015, 83, 1394–1401. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V.; Arangasamy, A.; Kastelic, J. Sperm and Seminal Plasma Proteomics of High- versus Low-Fertility Holstein Bulls. Theriogenology 2019, 126, 41–48. [Google Scholar] [CrossRef]
- Grant, K.E.; de Oliveira, R.V.; Hennington, B.S.; Govindaraju, A.; Perkins, A.; Stokes, J.; Rowe, D.; Topper, E.; Kaya, A.; Moura, A.; et al. Sperm Superoxide Dismutase Is Associated with Bull Fertility. Reprod. Fertil. Dev. 2016, 28, 1405–1413. [Google Scholar] [CrossRef]
- Elzanaty, S.; Erenpreiss, J.; Becker, C. Seminal Plasma Albumin: Origin and Relation to the Male Reproductive Parameters. Andrologia 2007, 39, 60–65. [Google Scholar] [CrossRef]
- Žaja, I.Ž.; Samardžija, M.; Vince, S.; Vilić, M.; Majić-Balić, I.; Đuričić, D.; Milinković-Tur, S. Differences in Seminal Plasma and Spermatozoa Antioxidative Systems and Seminal Plasma Lipid and Protein Levels among Boar Breeds and Hybrid Genetic Traits. Anim. Reprod. Sci. 2016, 170, 75–82. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Love, C.; Bauer, J.; Macpherson, M.; Varner, D. Cholesterol-to-Phospholipid Ratio in Whole Sperm and Seminal Plasma from Fertile Stallions and Stallions with Unexplained Subfertility. Anim. Reprod. Sci. 2007, 99, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Neergaard, R.D.; Nielsen, J.E.; Jørgensen, A.; Toft, B.G.; Goetze, J.P.; Jørgensen, N. Positive Association between Cholesterol in Human Seminal Plasma and Sperm Counts: Results from a Cross-Sectional Cohort Study and Immunohistochemical Investigations. Andrology 2018, 6, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Juyena, N.S.; Stelletta, C. Seminal Plasma: An Essential Attribute to Spermatozoa. J. Androl. 2012, 33, 536–551. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Koziorowski, M.; Strzezek, J.; Fraser, L. Seasonal Changes in Antioxidant Defence of the Boar Reproductive Tract. Reprod. Biol. 2011, 11, 37–47. [Google Scholar] [CrossRef]
| LOQ | Unit | |
|---|---|---|
| Al | 0.0254 | mg/kg |
| Ba | 0.0110 | mg/kg |
| Ca | 0.0009 | mg/kg |
| Cd | 0.0012 | mg/kg |
| Co | 0.0060 | mg/kg |
| Cr | 0.0397 | mg/kg |
| Cu | 0.0110 | mg/kg |
| Fe | 0.0038 | mg/kg |
| Hg | 0.0200 | μg/kg |
| K | 2.0508 | mg/kg |
| Mg | 0.0013 | mg/kg |
| Mn | 0.0011 | mg/kg |
| Mo | 0.0115 | mg/kg |
| Na | 0.6116 | mg/kg |
| Ni | 0.0057 | mg/kg |
| Pb | 0.0332 | mg/kg |
| Sr | 0.0060 | mg/kg |
| Zn | 0.0331 | mg/kg |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Concentration (109/mL) | 2.26 | 0.80 | 0.63 | 3.96 |
| MOT_0 (%) | 90.64 | 6.04 | 77.98 | 96.66 |
| MOT_1 (%) | 69.45 | 18.87 | 37.99 | 94.31 |
| PRO_0 (%) | 86.24 | 6.87 | 69.26 | 93.58 |
| PRO_1 (%) | 62.81 | 18.99 | 32.75 | 88.17 |
| VCL_0 (µm/s) | 133.05 | 16.40 | 104.00 | 171.63 |
| VCL_1 (µm/s) | 124.27 | 23.40 | 97.21 | 206.96 |
| BCF_0 (Hz) | 31.10 | 3.34 | 27.22 | 38.78 |
| BCF_1 (Hz) | 29.06 | 3.15 | 25.24 | 34.99 |
| ALH_0 (µm) | 5.37 | 0.71 | 3.83 | 6.41 |
| ALH_1 (µm) | 5.30 | 0.69 | 3.96 | 6.70 |
| MTT (Abs) | 0.20 | 0.13 | 0.03 | 0.46 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| TOS (μmol H2O2/g TP) | 0.23 | 0.15 | 0.06 | 0.59 |
| FRAP (μmol Fe2+/g TP) | 32.68 | 7.63 | 24.65 | 53.02 |
| GPx (U/g TP) | 1.66 | 1.02 | 0.62 | 4.73 |
| SOD (U/mg TP) | 0.15 | 0.04 | 0.09 | 0.27 |
| MDA (µmol/g TP) | 0.92 | 0.80 | 0.01 | 2.80 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| TP (g/L) | 49.03 | 10.07 | 26.15 | 63.59 |
| Alb (g/L) | 16.69 | 4.16 | 10.84 | 26.43 |
| Chol (mmol/L) | 1.81 | 0.80 | 0.84 | 3.96 |
| TAG (mmol/L) | 1.10 | 1.08 | 0.19 | 4.50 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Al (mg/kg) | 0.80 | 0.01 | 0.79 | 0.81 |
| Ba (mg/kg) | 0.35 | 0.00 | 0.34 | 0.35 |
| Ca (mg/kg) | 330.32 | 72.85 | 159.43 | 470.98 |
| Cd (mg/kg) | 1.08 | 0.01 | 1.06 | 1.09 |
| Co (µg/kg) | 190.50 | 1.58 | 188.00 | 192.00 |
| Cr (mg/kg) | 1.25 | 0.01 | 1.24 | 1.27 |
| Cu (mg/kg) | 0.43 | 0.12 | 0.35 | 0.81 |
| Fe (mg/kg) | 2.46 | 0.17 | 2.11 | 2.82 |
| Hg (µg/kg) | 0.46 | 0.06 | 0.33 | 0.55 |
| K (g/kg) | 3.27 | 0.65 | 2.10 | 4.35 |
| Mg (mg/kg) | 80.43 | 35.96 | 40.39 | 175.49 |
| Mn (µg/kg) | 35.44 | 0.51 | 35.00 | 36.00 |
| Mo (mg/kg) | 1.07 | 0.03 | 1.02 | 1.13 |
| Na (g/kg) | 1.31 | 0.40 | 0.75 | 2.17 |
| Ni (mg/kg) | 2.50 | 0.58 | 1.49 | 3.87 |
| Pb (mg/kg) | 1.05 | 0.01 | 1.03 | 1.06 |
| Sr (µg/kg) | 188.28 | 1.64 | 185.00 | 190.00 |
| Zn (mg/kg) | 3.60 | 0.74 | 2.13 | 5.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirpák, F.; Halo, M.; Tomka, M.; Slanina, T.; Tokárová, K.; Błaszczyk-Altman, M.; Dianová, L.; Ivanič, P.; Kirchner, R.; Greń, A.; et al. Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma. Antioxidants 2022, 11, 1796. https://doi.org/10.3390/antiox11091796
Tirpák F, Halo M, Tomka M, Slanina T, Tokárová K, Błaszczyk-Altman M, Dianová L, Ivanič P, Kirchner R, Greń A, et al. Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma. Antioxidants. 2022; 11(9):1796. https://doi.org/10.3390/antiox11091796
Chicago/Turabian StyleTirpák, Filip, Marko Halo, Marián Tomka, Tomáš Slanina, Katarína Tokárová, Martyna Błaszczyk-Altman, Lucia Dianová, Peter Ivanič, Róbert Kirchner, Agnieszka Greń, and et al. 2022. "Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma" Antioxidants 11, no. 9: 1796. https://doi.org/10.3390/antiox11091796
APA StyleTirpák, F., Halo, M., Tomka, M., Slanina, T., Tokárová, K., Błaszczyk-Altman, M., Dianová, L., Ivanič, P., Kirchner, R., Greń, A., Lukáč, N., & Massányi, P. (2022). Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma. Antioxidants, 11(9), 1796. https://doi.org/10.3390/antiox11091796