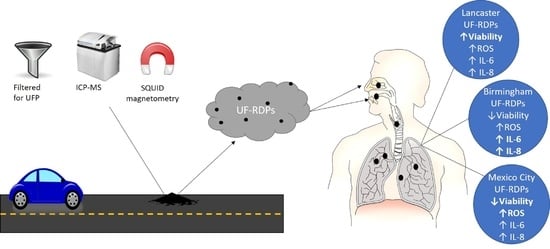
Oxidative Stress, Cytotoxic and Inflammatory Effects of Urban Ultrafine Road-Deposited Dust from the UK and Mexico in Human Epithelial Lung (Calu-3) Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Ultrafine Particle Extraction from Road-Deposited Dust
2.3. Inductively Coupled Plasma (ICP) Mass Spectrometry (MS) and Optical Emission Spectroscopy (OES)
2.4. Superconducting Quantum Interference Device (SQUID) Magnetometry
2.5. Endotoxin Quantification
2.6. Cell Culture
2.7. MTS Assay
2.8. Reactive Oxygen Species (ROS) Assay
2.9. Cytokine ELISAs
2.10. Statistical Analysis
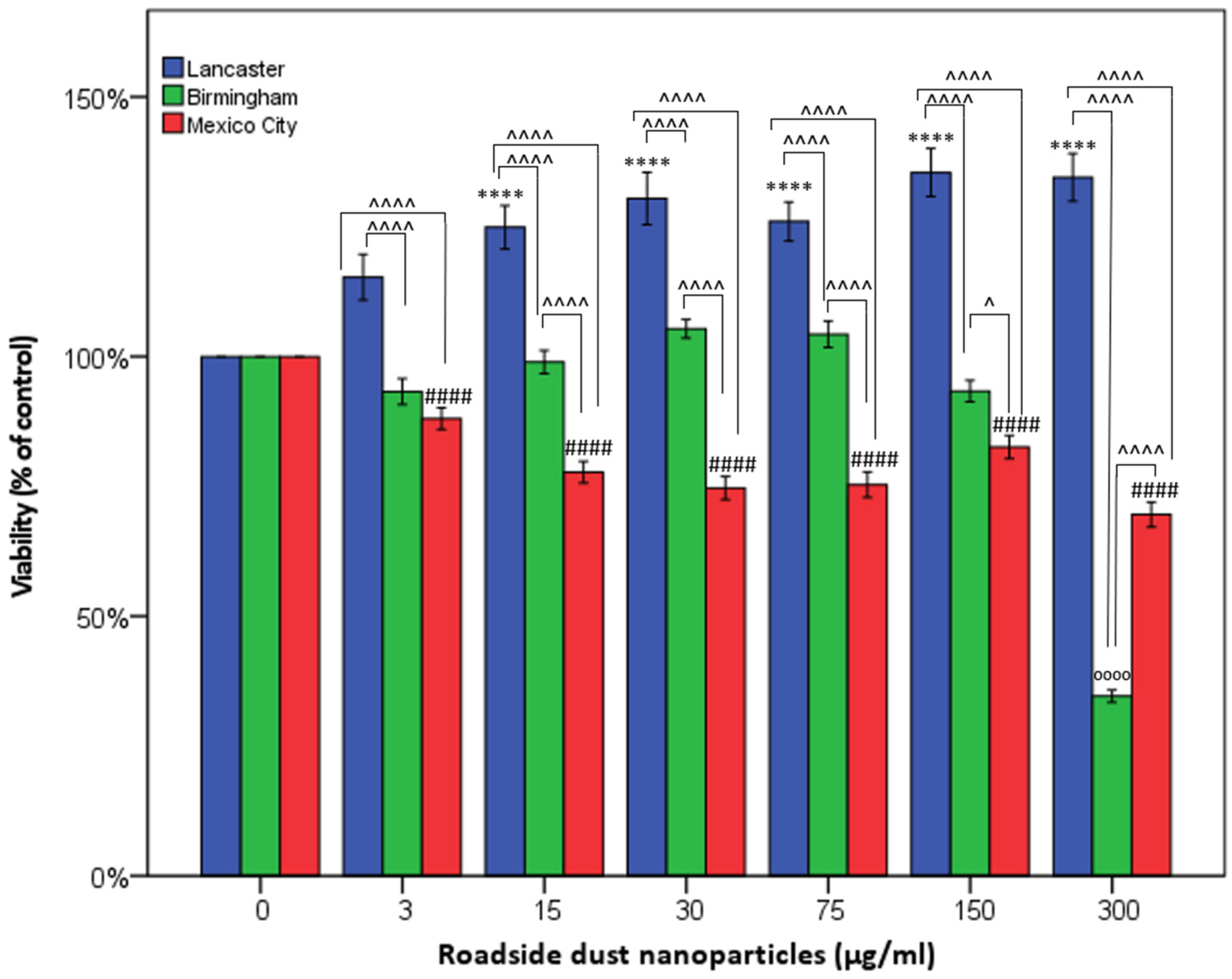
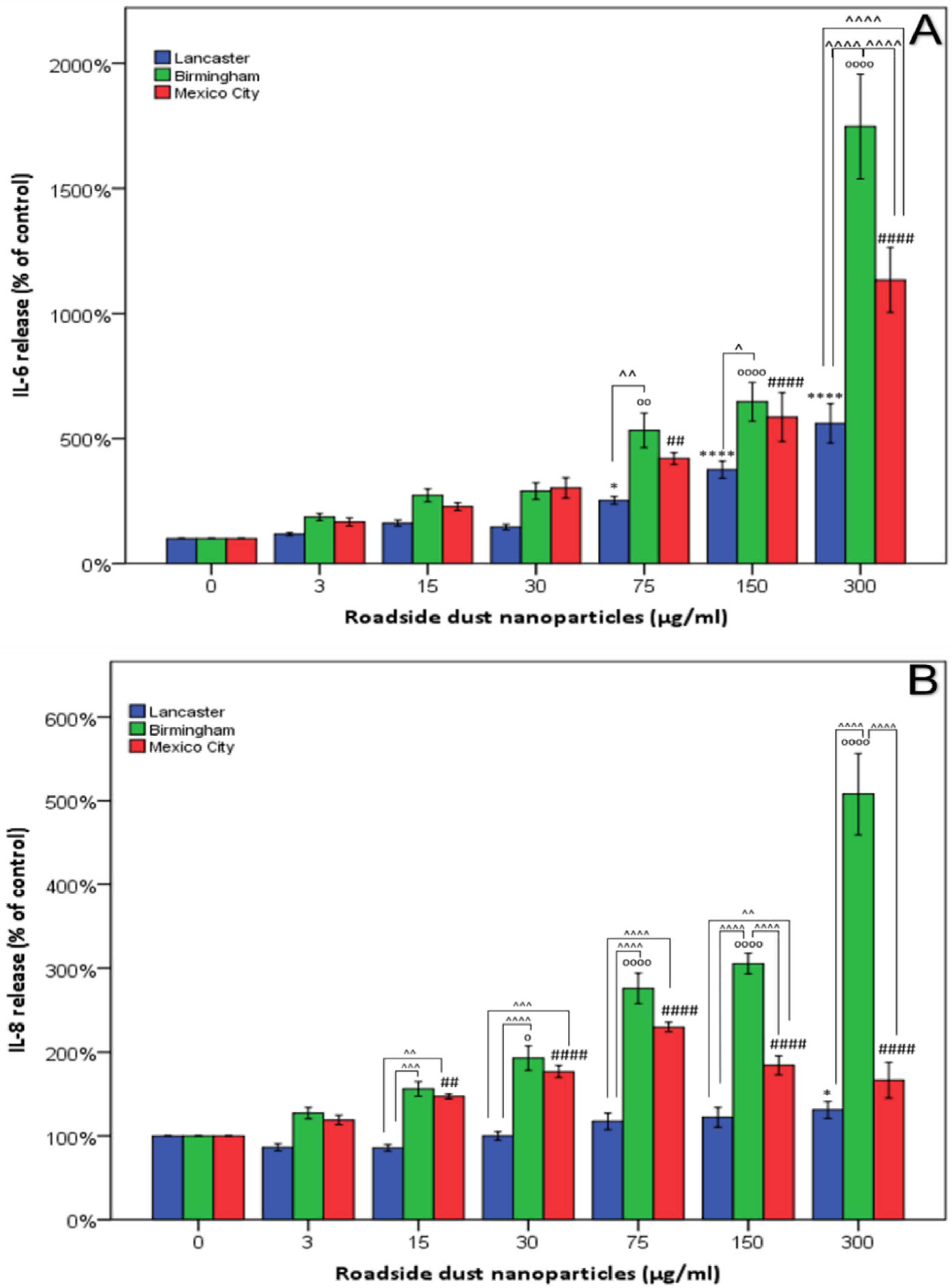
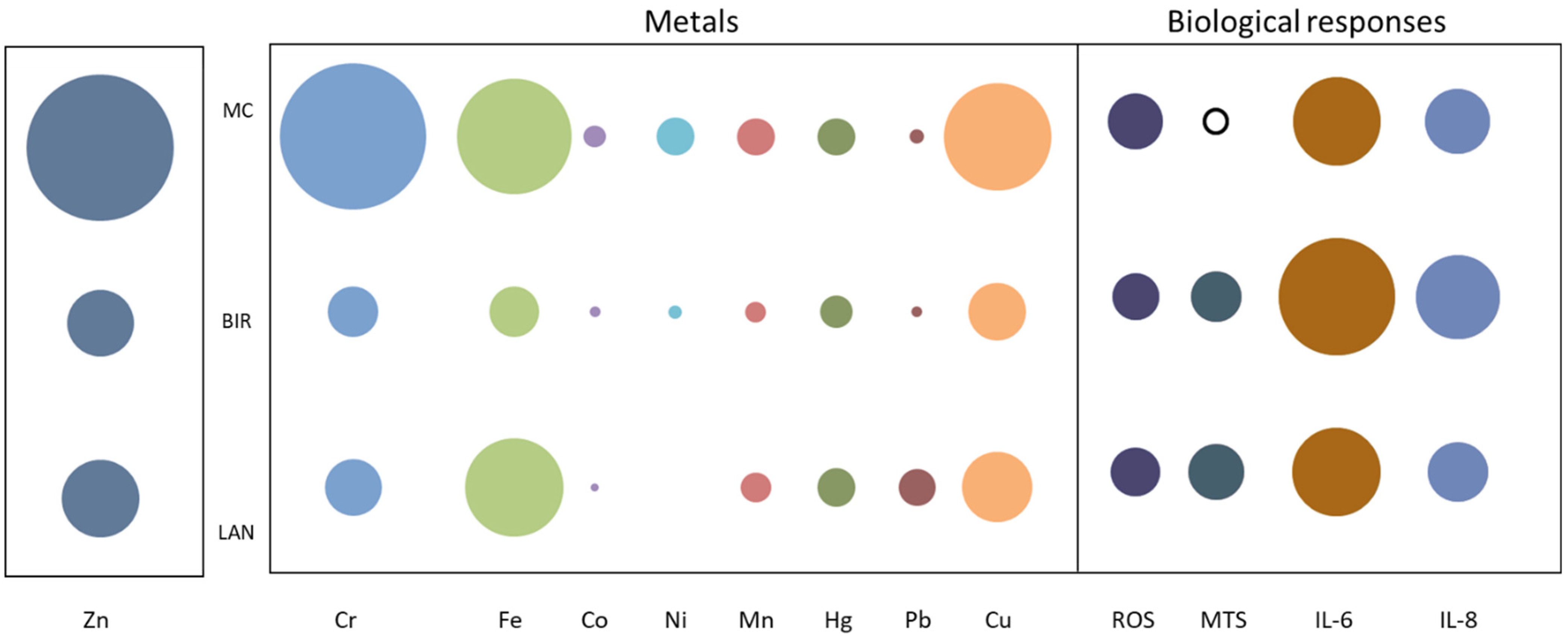
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Southerland, V.A.; Brauer, M.; Mohegh, A.; Hammer, M.S.; van Donkelaar, A.; Martin, R.V.; Apte, J.S.; Anenberg, S.C. Global urban temporal trends in fine particulate matter (PM2·5) and attributable health burdens: Estimates from global datasets. Lancet Planet. Health 2022, 6, e139–e146. [Google Scholar] [CrossRef]
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 28 September 2021).
- Zhang, Z.; Zhu, D.; Cui, B.; Ding, R.; Shi, X.; He, P. Association between particulate matter air pollution and lung cancer. Thorax 2020, 75, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-J.; Lee, S.H.; Kang, S.-H.; Kim, S.-Y. Incident cardiovascular disease and particulate matter air pollution in South Korea using a population-based and nationwide cohort of 0.2 million adults. Environ. Health 2020, 19, 113. [Google Scholar] [CrossRef]
- Weichenthal, S.; Olaniyan, T.; Christidis, T.; Lavigne, E.; Hatzopoulou, M.; Van Ryswyk, K.; Tjepkema, M.; Burnett, R. Within-city Spatial Variations in Ambient Ultrafine Particle Concentrations and Incident Brain Tumors in Adults. Epidemiology 2020, 31, 177–183. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Mortamais, M.; Gutierrez, L.-A.; de Hoogh, K.; Chen, J.; Vienneau, D.; Carrière, I.; Letellier, N.; Helmer, C.; Gabelle, A.; Mura, T.; et al. Long-term exposure to ambient air pollution and risk of dementia: Results of the prospective Three-City Study. Environ. Int. 2021, 148, 106376. [Google Scholar] [CrossRef]
- Heal, M.R.; Hammonds, M.D. Insights into the composition and sources of rural, urban and roadside carbonaceous PM10. Environ. Sci. Technol. 2014, 48, 8995–9003. [Google Scholar] [CrossRef]
- Hong, N.; Guan, Y.; Yang, B.; Zhong, J.; Zhu, P.; Ok, Y.S.; Hou, D.; Tsang, D.C.W.; Guan, Y.; Liu, A. Quantitative source tracking of heavy metals contained in urban road deposited sediments. J. Hazard. Mater. 2020, 393, 122362. [Google Scholar] [CrossRef]
- Mon, E.E.; Phay, N.; Agusa, T.; Bach, L.T.; Yeh, H.-M.; Huang, C.-H.; Nakata, H. Polycyclic Aromatic Hydrocarbons (PAHs) in Road Dust Collected from Myanmar, Japan, Taiwan, and Vietnam. Arch. Environ. Contam. Toxicol. 2020, 78, 34–45. [Google Scholar] [CrossRef]
- Gonet, T.; Maher, B.A.; Kukutschová, J. Source apportionment of magnetite particles in roadside airborne particulate matter. Sci. Total Environ. 2021, 752, 141828. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Vance, M.; Tou, F.; Tiwari, A.; Liu, M.; Hochella, M.F. Nanoparticles in road dust from impervious urban surfaces: Distribution, identification, and environmental implications. Environ. Sci. Nano 2016, 3, 534–544. [Google Scholar] [CrossRef]
- Pant, P.; Baker, S.J.; Shukla, A.; Maikawa, C.; Godri Pollitt, K.J.; Harrison, R.M. The PM10 fraction of road dust in the UK and India: Characterization, source profiles and oxidative potential. Sci. Total Environ. 2015, 530–531, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Mantecca, P.; Corvaja, V.; Longhin, E.; Perrone, M.G.; Bolzacchini, E.; Camatini, M. Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol. Lett. 2009, 188, 52–62. [Google Scholar] [CrossRef]
- Harrison, R.M.; Jones, A.M.; Gietl, J.; Yin, J.; Green, D.C. Estimation of the Contributions of Brake Dust, Tire Wear, and Resuspension to Nonexhaust Traffic Particles Derived from Atmospheric Measurements. Environ. Sci. Technol. 2012, 46, 6523–6529. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Wichser, A.; Vonbank, R.; Brunner, S.; Ulrich, A.; Zuin, S.; Nowack, B. Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environ. Sci. Process. Impacts 2013, 15, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Prichard, H.M.; Fisher, P.C. Identification of Platinum and Palladium Particles Emitted from Vehicles and Dispersed into the Surface Environment. Environ. Sci. Technol. 2012, 46, 3149–3154. [Google Scholar] [CrossRef]
- Kim, E.-A.; Koh, B. Utilization of road dust chemical profiles for source identification and human health impact assessment. Sci. Rep. 2020, 10, 14259. [Google Scholar] [CrossRef]
- Liao, C.-M.; Chiang, K.-C. Probabilistic risk assessment for personal exposure to carcinogenic polycyclic aromatic hydrocarbons in Taiwanese temples. Chemosphere 2006, 63, 1610–1619. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Levesque, C.; Rasmussen, P.E. Characterizing the sources, concentrations and resuspension potential of metals and metalloids in the thoracic fraction of urban road dust. Sci. Total Environ. 2021, 786, 147467. [Google Scholar] [CrossRef]
- Halsall, C.J.; Maher, B.A.; Karloukovski, V.V.; Shah, P.; Watkins, S.J. A novel approach to investigating indoor/outdoor pollution links: Combined magnetic and PAH measurements. Atmos. Environ. 2008, 42, 8902–8909. [Google Scholar] [CrossRef] [Green Version]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderón-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [PubMed]
- Dadras, A.; Riazi, G.H.; Afrasiabi, A.; Naghshineh, A.; Ghalandari, B.; Mokhtari, F. In vitro study on the alterations of brain tubulin structure and assembly affected by magnetite nanoparticles. JBIC J. Biol. Inorg. Chem. 2013, 18, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Plascencia-Villa, G.; Ponce, A.; Collingwood, J.F.; Arellano-Jiménez, M.J.; Zhu, X.; Rogers, J.T.; Betancourt, I.; José-Yacamán, M.; Perry, G. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci. Rep. 2016, 6, 24873. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A. Airborne Magnetite- and Iron-Rich Pollution Nanoparticles: Potential Neurotoxicants and Environmental Risk Factors for Neurodegenerative Disease, Including Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 361–375. [Google Scholar] [CrossRef]
- Ramesh, V.; Ravichandran, P.; Copeland, C.L.; Gopikrishnan, R.; Biradar, S.; Goornavar, V.; Ramesh, G.T.; Hall, J.C. Magnetite induces oxidative stress and apoptosis in lung epithelial cells. Mol. Cell. Biochem. 2012, 363, 225–234. [Google Scholar] [CrossRef]
- Pereira, G.M.; Teinilä, K.; Custódio, D.; Gomes Santos, A.; Xian, H.; Hillamo, R.; Alves, C.A.; Bittencourt de Andrade, J.; Olímpio da Rocha, G.; Kumar, P.; et al. Particulate pollutants in the Brazilian city of São Paulo: 1-year investigation for the chemical composition and source apportionment. Atmos. Chem. Phys. 2017, 17, 11943–11969. [Google Scholar] [CrossRef]
- Sahu, S.K.; Beig, G.; Parkhi, N.S. Emissions inventory of anthropogenic PM2.5 and PM10 in Delhi during Commonwealth Games 2010. Atmos. Environ. 2011, 45, 6180–6190. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Lin, J.; Huang, J.; Zhao, D.; Yuan, T.; Huang, K.; Luo, Y.; Jia, Z.; Zang, Z.; et al. Fugitive Road Dust PM2.5 Emissions and Their Potential Health Impacts. Environ. Sci. Technol. 2019, 53, 8455–8465. [Google Scholar] [CrossRef]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. Acta 2016, 1860, 2844–2855. [Google Scholar] [CrossRef]
- DEFRA. Non-Exhaust Emissions from Road Traffic; Department for Environment, Food and Rural Affairs: London, UK, 2019; p. 89.
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994, 102 (Suppl. S5), 173–179. [Google Scholar]
- Wang, B.; Yin, J.-J.; Zhou, X.; Kurash, I.; Chai, Z.; Zhao, Y.; Feng, W. Physicochemical Origin for Free Radical Generation of Iron Oxide Nanoparticles in Biomicroenvironment: Catalytic Activities Mediated by Surface Chemical States. J. Phys. Chem. C 2013, 117, 383–392. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109 (Suppl. S4), 523–527. [Google Scholar] [PubMed]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles. The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract likely a key brainstem portal. Environ. Res. 2020, 110139. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Global Perspective on the Oxidative Potential of Airborne Particulate Matter: A Synthesis of Research Findings. Environ. Sci. Technol. 2014, 48, 7576–7583. [Google Scholar] [CrossRef]
- Yu, S.-H.; Tang, D.-W.; Hsieh, H.-Y.; Wu, W.-S.; Lin, B.-X.; Chuang, E.-Y.; Sung, H.-W.; Mi, F.-L. Nanoparticle-induced tight-junction opening for the transport of an anti-angiogenic sulfated polysaccharide across Caco-2 cell monolayers. Acta Biomater. 2013, 9, 7449–7459. [Google Scholar] [CrossRef]
- Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Snow, S.J.; Henriquez, A.R.; Costa, D.L.; Kodavanti, U.P. Neuroendocrine Regulation of Air Pollution Health Effects: Emerging Insights. Toxicol. Sci. 2018, 164, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Mukherjee, P.S.; Reynoso-Robles, R.; Pérez-Guillé, B.; Gayosso-Chávez, C.; Torres-Jardón, R.; Cross, J.V.; Ahmed, I.A.M.; Karloukovski, V.V.; et al. Combustion- and friction-derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019, 176, 108567. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Calderón-Garcidueñas, L. Iron-rich air pollution nanoparticles: An unrecognised environmental risk factor for myocardial mitochondrial dysfunction and cardiac oxidative stress. Environ. Res. 2020, 188, 109816. [Google Scholar] [CrossRef]
- Chen, R.; Li, H.; Cai, J.; Wang, C.; Lin, Z.; Liu, C.; Niu, Y.; Zhao, Z.; Li, W.; Kan, H. Fine Particulate Air Pollution and the Expression of microRNAs and Circulating Cytokines Relevant to Inflammation, Coagulation, and Vasoconstriction. Environ. Health Perspect. 2018, 126, 017007. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Chen, W.L.; Wang, C.C.; Lai, C.H. Associations between Personal Exposure to Metals in Fine Particulate Matter and Autonomic Nervous System Dysfunction among Healthy Adults. Aerosol Air Qual. Res. 2020, 20, 1842–1849. [Google Scholar] [CrossRef]
- Hammond, J.; Maher, B.A.; Ahmed, I.A.M.; Allsop, D. Variation in the concentration and regional distribution of magnetic nanoparticles in human brains, with and without Alzheimer’s disease, from the UK. Sci. Rep. 2021, 11, 9363. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.-H.; Amyai, N.; Marques-Vidal, P.; Wang, J.-L.; Riediker, M.; Mooser, V.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of particulate matter on inflammatory markers in the general adult population. Part. Fibre Toxicol. 2012, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Andreau, K.; Leroux, M.; Bouharrour, A. Health and cellular impacts of air pollutants: From cytoprotection to cytotoxicity. Biochem. Res. Int. 2012, 2012, 493894. [Google Scholar] [CrossRef]
- Puisney, C.; Oikonomou, E.K.; Nowak, S.; Chevillot, A.; Casale, S.; Baeza-Squiban, A.; Berret, J.-F. Brake wear (nano)particle characterization and toxicity on airway epithelial cells in vitro. Environ. Sci. Nano 2018, 5, 1036–1044. [Google Scholar] [CrossRef]
- Abbas, I.; Garçon, G.; Saint-Georges, F.; Billet, S.; Verdin, A.; Gosset, P.; Mulliez, P.; Shirali, P. Occurrence of molecular abnormalities of cell cycle in L132 cells after in vitro short-term exposure to air pollution PM2.5. Chem.-Biol. Interact. 2010, 188, 558–565. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Torres, V.; Miranda, J.; Martínez, L.; García-Cuellar, C.; Nawrot, T.S.; Vanaudenaerde, B.; Hoet, P.; Ramírez-López, P.; Rosas, I.; et al. Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environ. Res. 2009, 109, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Kang, Y.; Wang, W.; Chan, C.Y.; Wang, X.; Wong, M.H. Potential cytotoxicity of water-soluble fraction of dust and particulate matters and relation to metal(loid)s based on three human cell lines. Chemosphere 2015, 135, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, J.; Shen, Z.; Niu, X.; Wang, D.; Wang, X.; Xu, H.; Chuang, H.-C.; Cao, J.; Ho, K.-F. Oxidative stress–inducing effects of various urban PM2.5 road dust on human lung epithelial cells among 10 Chinese megacities. Ecotoxicol. Environ. Saf. 2021, 224, 112680. [Google Scholar] [CrossRef]
- Koh, B.; Kim, E.-A. Comparative analysis of urban road dust compositions in relation to their potential human health impacts. Environ. Pollut. 2019, 255, 113156. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 17007. [Google Scholar] [CrossRef]
- Tung, N.T.; Ho, K.-F.; Niu, X.; Sun, J.; Shen, Z.; Wu, F.; Cao, J.; Dung, H.B.; Thuy, T.P.C.; Hsiao, T.-C.; et al. Loss of E-cadherin due to road dust PM2.5 activates the EGFR in human pharyngeal epithelial cells. Environ. Sci. Pollut. Res. 2021, 28, 53872–53887. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Han, S.; Jeon, K.J.; Kwon, S. Effects of collected road dusts on cell viability, inflammatory response, and oxidative stress in cultured human corneal epithelial cells. Toxicol. Lett. 2018, 284, 152–160. [Google Scholar] [CrossRef]
- Morgan, T.E.; Davis, D.A.; Iwata, N.; Tanner, J.A.; Snyder, D.; Ning, Z.; Kam, W.; Hsu, Y.-T.; Winkler, J.W.; Chen, J.-C.; et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ. Health Perspect. 2011, 119, 1003–1009. [Google Scholar] [CrossRef]
- Woodward, N.C.; Levine, M.C.; Haghani, A.; Shirmohammadi, F.; Saffari, A.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflamm. 2017, 14, 84. [Google Scholar] [CrossRef]
- Birmingham City Council. 2019 Air Quality Annual Status Report (ASR). 2019. Available online: http://62.65.40.208/birmingham//Reports/2019_Birmingham_City_Council_ASR.pdf (accessed on 26 August 2021).
- Lancaster City Council. 2019 Air Quality Annual Status Report (ASR) for Lancaster City Council. 2020. Available online: https://www.lancaster.gov.uk/assets/attach/6104/Air%20Quality%20Annual%20Status%20Report%20-%20Lancaster%202020.pdf (accessed on 26 August 2021).
- SEDEMA. Mexico City Air Quality Monitoring System Public Database. Available online: http://www.aire.cdmx.gob.mx/default.php (accessed on 26 August 2021).
- ONS. UK Population Pyramid Interactive. 2019. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/ukpopulationpyramidinteractive/2020-01-08 (accessed on 26 August 2021).
- Birmingham City Council. Birmingham Population. 2018. Available online: https://www.birmingham.gov.uk/info/20057/about_birmingham/1294/population_and_census/2 (accessed on 26 August 2021).
- Romero, T. Total Population in Mexico City between 2008 and 2018. 2021. Available online: https://www.statista.com/statistics/1038080/mexico-city-total-population/ (accessed on 26 August 2021).
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surf. B 2010, 75, 275–281. [Google Scholar] [CrossRef]
- Gorr, M.W.; Youtz, D.J.; Eichenseer, C.M.; Smith, K.E.; Nelin, T.D.; Cormet-Boyaka, E.; Wold, L.E. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H53–H62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.-W.; Gerlofs-Nijland, M.E.; Boere, J.; Fokkens, P.; Leseman, D.; Janssen, N.A.H.; Cassee, F.R. Comparative toxicity of ultrafine particles around a major airport in human bronchial epithelial (Calu-3) cell model at the air–liquid interface. Toxicol. In Vitro 2020, 68, 104950. [Google Scholar] [CrossRef] [PubMed]
- Cooney, D.J.; Hickey, A.J. Cellular response to the deposition of diesel exhaust particle aerosols onto human lung cells grown at the air–liquid interface by inertial impaction. Toxicol. In Vitro 2011, 25, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Vietti, G.; Ibouraadaten, S.; Palmai-Pallag, M.; Yakoub, Y.; Bailly, C.; Fenoglio, I.; Marbaix, E.; Lison, D.; van den Brule, S. Towards predicting the lung fibrogenic activity of nanomaterials: Experimental validation of an in vitro fibroblast proliferation assay. Part. Fibre Toxicol. 2013, 10, 52. [Google Scholar] [CrossRef]
- Kocbach, A.; Totlandsdal, A.I.; Låg, M.; Refsnes, M.; Schwarze, P.E. Differential binding of cytokines to environmentally relevant particles: A possible source for misinterpretation of in vitro results? Toxicol. Lett. 2008, 176, 131–137. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J. Hallmarks of maghemitization in low-temperature remanence cycling of partially oxidized magnetite nanoparticles. J. Geophys. Res. Solid Earth 2010, 115. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Rousseau, M.-C.; Straif, K.; Siemiatycki, J. IARC carcinogen update. Environ. Health Perspect. 2005, 113, A580–A581. [Google Scholar] [CrossRef]
- Botsou, F.; Moutafis, I.; Dalaina, S.; Kelepertzis, E. Settled bus dust as a proxy of traffic-related emissions and health implications of exposures to potentially harmful elements. Atmos. Pollut. Res. 2020, 11, 1776–1784. [Google Scholar] [CrossRef]
- Churg, A.; Brauer, M.; del Carmen Avila-Casado, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2003, 111, 714–718. [Google Scholar] [CrossRef]
- Dumková, J.; Smutná, T.; Vrlíková, L.; Le Coustumer, P.; Večeřa, Z.; Dočekal, B.; Mikuška, P.; Čapka, L.; Fictum, P.; Hampl, A.; et al. Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs. Part. Fibre Toxicol. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Horie, M.; Tabei, Y.; Kashiwada, S. Proinflammatory response caused by lead nanoparticles triggered by engulfed nanoparticles. Environ. Toxicol. 2021, 36, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Stochel, G.; Brindell, M. Variations in Reactive Oxygen Species Generation by Urban Airborne Particulate Matter in Lung Epithelial Cells—Impact of Inorganic Fraction. Front. Chem. 2020, 8, 581752. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; Kalai, M.; Denecker, G.; Meeus, A.; Saelens, X.; Vandenabeele, P. Necrosis is associated with IL-6 production but apoptosis is not. Cell. Signal. 2006, 18, 328–335. [Google Scholar] [CrossRef]
- de Caritat, P.; Reimann, C. Comparing results from two continental geochemical surveys to world soil composition and deriving Predicted Empirical Global Soil (PEGS2) reference values. Earth Planet. Sci. Lett. 2012, 319–320, 269–276. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, G.; Gao, X.; Zhang, Y.; Fan, W.; Jiang, J.; An, Z.; Li, J.; Song, J.; Wu, W. Oxidative stress-mediated epidermal growth factor receptor activation regulates PM(2.5)-induced over-secretion of pro-inflammatory mediators from human bronchial epithelial cells. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129672. [Google Scholar] [CrossRef]
- Shukla, A.; Timblin, C.; BeruBe, K.; Gordon, T.; McKinney, W.; Driscoll, K.; Vacek, P.; Mossman, B.T. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am. J. Respir. Cell Mol. Biol. 2000, 23, 182–187. [Google Scholar] [CrossRef]
- Veranth, J.M.; Moss, T.A.; Chow, J.C.; Labban, R.; Nichols, W.K.; Walton, J.C.; Watson, J.G.; Yost, G.S. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ. Health Perspect. 2006, 114, 341–349. [Google Scholar] [CrossRef]
- Thorn, J. The inflammatory response in humans after inhalation of bacterial endotoxin: A review. Inflamm. Res. 2001, 50, 254–261. [Google Scholar] [CrossRef]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, H.; Setyan, A.; Elser, M.; Dietrich, M.; Li, J.; Zhang, T.; Zhang, X.; Zheng, Y.; Wang, J.; et al. Size-Resolved Endotoxin and Oxidative Potential of Ambient Particles in Beijing and Zürich. Environ. Sci. Technol. 2018, 52, 6816–6824. [Google Scholar] [CrossRef] [PubMed]
- Gonet, T.; Maher, B.A.; Nyirő-Kósa, I.; Pósfai, M.; Vaculík, M.; Kukutschová, J. Size-resolved, quantitative evaluation of the magnetic mineralogy of airborne brake-wear particulate emissions. Environ. Pollut. 2021, 288, 117808. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.; Blomqvist, G.; Järlskog, I.; Lundberg, J.; Janhäll, S.; Elmgren, M.; Johansson, C.; Norman, M.; Silvergren, S. Road dust load dynamics and influencing factors for six winter seasons in Stockholm, Sweden. Atmos. Environ. X 2019, 2, 100014. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.P.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2021, 751, 142235. [Google Scholar] [CrossRef] [PubMed]
- Salo, L.; Hyvärinen, A.; Jalava, P.; Teinilä, K.; Hooda, R.K.; Datta, A.; Saarikoski, S.; Lintusaari, H.; Lepistö, T.; Martikainen, S.; et al. The characteristics and size of lung-depositing particles vary significantly between high and low pollution traffic environments. Atmos. Environ. 2021, 255, 118421. [Google Scholar] [CrossRef]



| City | Population Size (2018) | Site | Traffic (Vehicles/Day) | Date Collected | Avg. Annual PM10 (µg/m3) 2018 | Avg. Annual PM2.5 (µg/m3) 2018 |
|---|---|---|---|---|---|---|
| Lancaster | 144,426 | (A6) Cable Street | ~12,000 | 18/10/18 | 22 | No data * |
| Birmingham | 1,141,400 | (A38) Bristol Road | ~32,000 | 20/09/19 | 18 | 12 |
| Observation Site | ||||||
| Mexico City | 8,781,300 | Constitución de la | ~19,200 | 06/03/17 | 47 | 22 |
| República Avenue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, J.; Maher, B.A.; Gonet, T.; Bautista, F.; Allsop, D. Oxidative Stress, Cytotoxic and Inflammatory Effects of Urban Ultrafine Road-Deposited Dust from the UK and Mexico in Human Epithelial Lung (Calu-3) Cells. Antioxidants 2022, 11, 1814. https://doi.org/10.3390/antiox11091814
Hammond J, Maher BA, Gonet T, Bautista F, Allsop D. Oxidative Stress, Cytotoxic and Inflammatory Effects of Urban Ultrafine Road-Deposited Dust from the UK and Mexico in Human Epithelial Lung (Calu-3) Cells. Antioxidants. 2022; 11(9):1814. https://doi.org/10.3390/antiox11091814
Chicago/Turabian StyleHammond, Jessica, Barbara A. Maher, Tomasz Gonet, Francisco Bautista, and David Allsop. 2022. "Oxidative Stress, Cytotoxic and Inflammatory Effects of Urban Ultrafine Road-Deposited Dust from the UK and Mexico in Human Epithelial Lung (Calu-3) Cells" Antioxidants 11, no. 9: 1814. https://doi.org/10.3390/antiox11091814
APA StyleHammond, J., Maher, B. A., Gonet, T., Bautista, F., & Allsop, D. (2022). Oxidative Stress, Cytotoxic and Inflammatory Effects of Urban Ultrafine Road-Deposited Dust from the UK and Mexico in Human Epithelial Lung (Calu-3) Cells. Antioxidants, 11(9), 1814. https://doi.org/10.3390/antiox11091814