Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Determination of 96h-LH50
2.3. Sample Collection
2.4. Determination of Serum Biochemical Indices
2.5. Histopathological Analysis of Intestine Samples
2.6. Detection of Intestinal Cell Apoptosis in Largemouth Bass
2.7. Sequencing and Analysis of Intestinal Transcriptome
2.7.1. Construction and Sequencing of mRNA Libraries
2.7.2. Data Filtering, Read Mapping and Detection of Differentially Expressed Genes
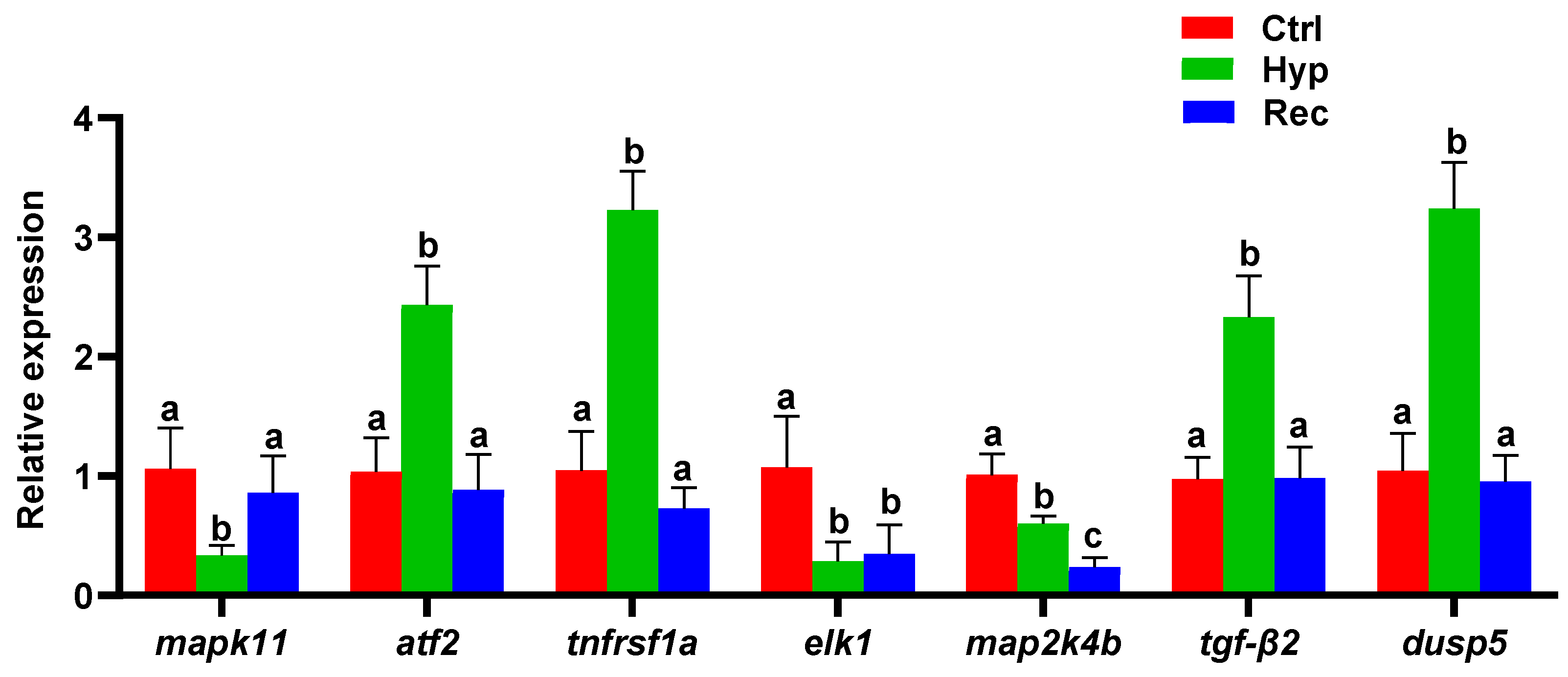
2.7.3. Identification of DEGs in Intestines by qRT-PCR
2.8. Sequencing and Analysis of Intestinal Microorganisms in 16S rRNA
2.8.1. DNA Extraction and Sequencing
2.8.2. Statistical and Bioinformatic Analysis
2.9. Transcriptome and Intestinal Microorganism Correlation Analyses
2.10. Statistical Analysis
3. Results
3.1. Determination of 96h-LH50 in Largemouth Bass
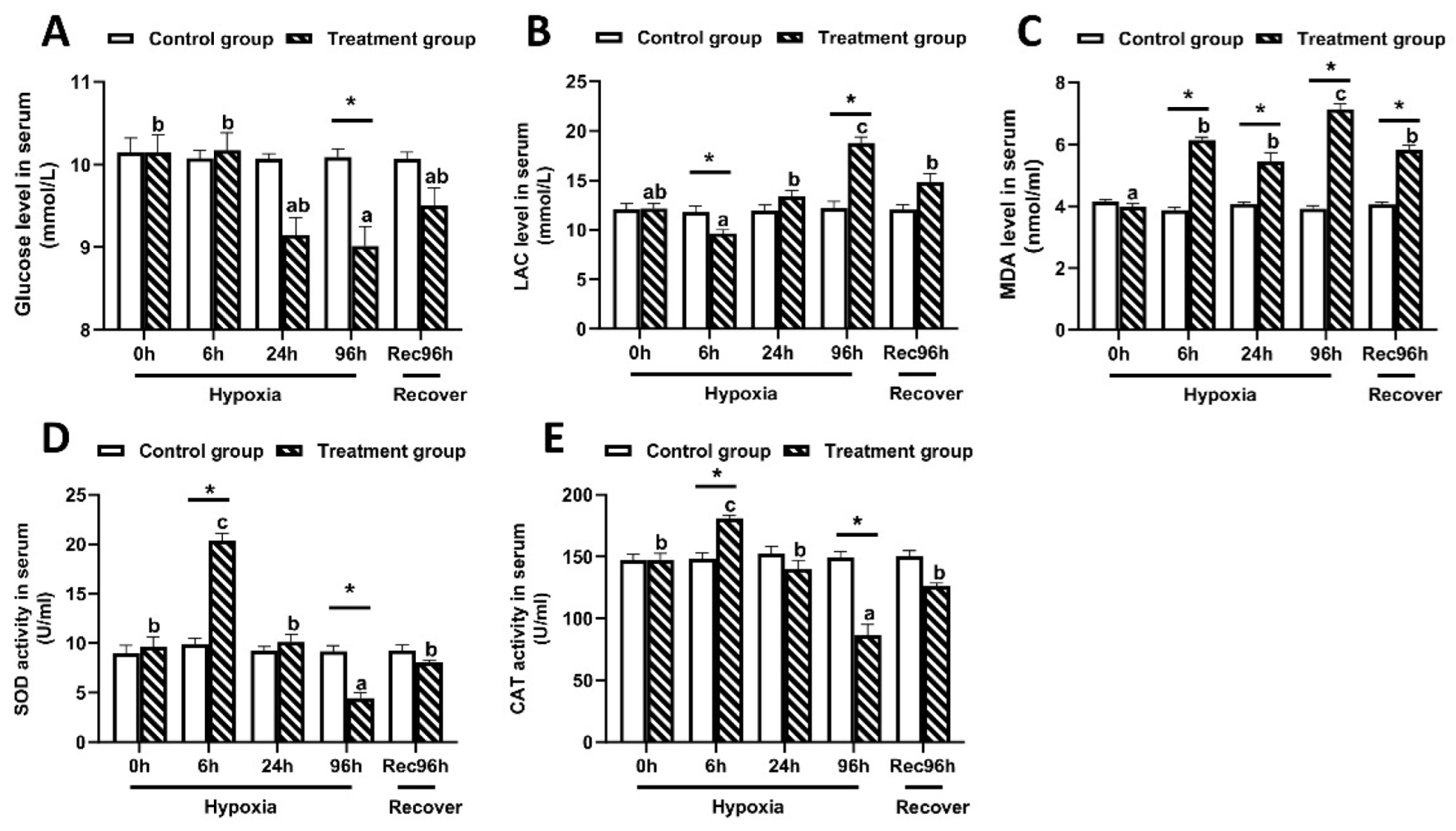
3.2. Determination of Antioxidant Capacity of Largemouth Bass under Hypoxic Stress and Reoxygenation
3.3. Intestinal Histological Structure of Largemouth Bass under Hypoxic Stress and Reoxygenation
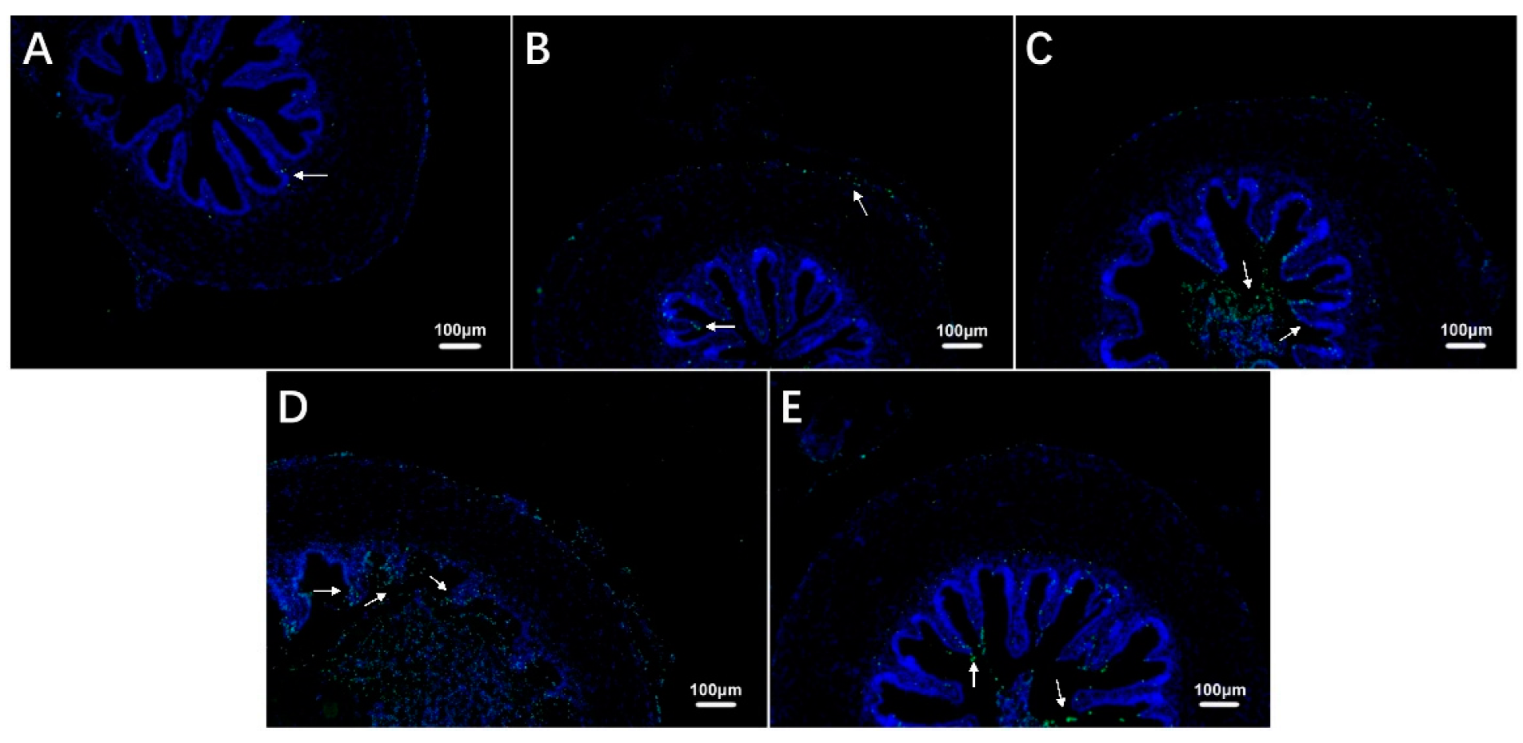
3.4. Intestinal Cell Apoptosis in Largemouth Bass under Hypoxic Stress and Recovery
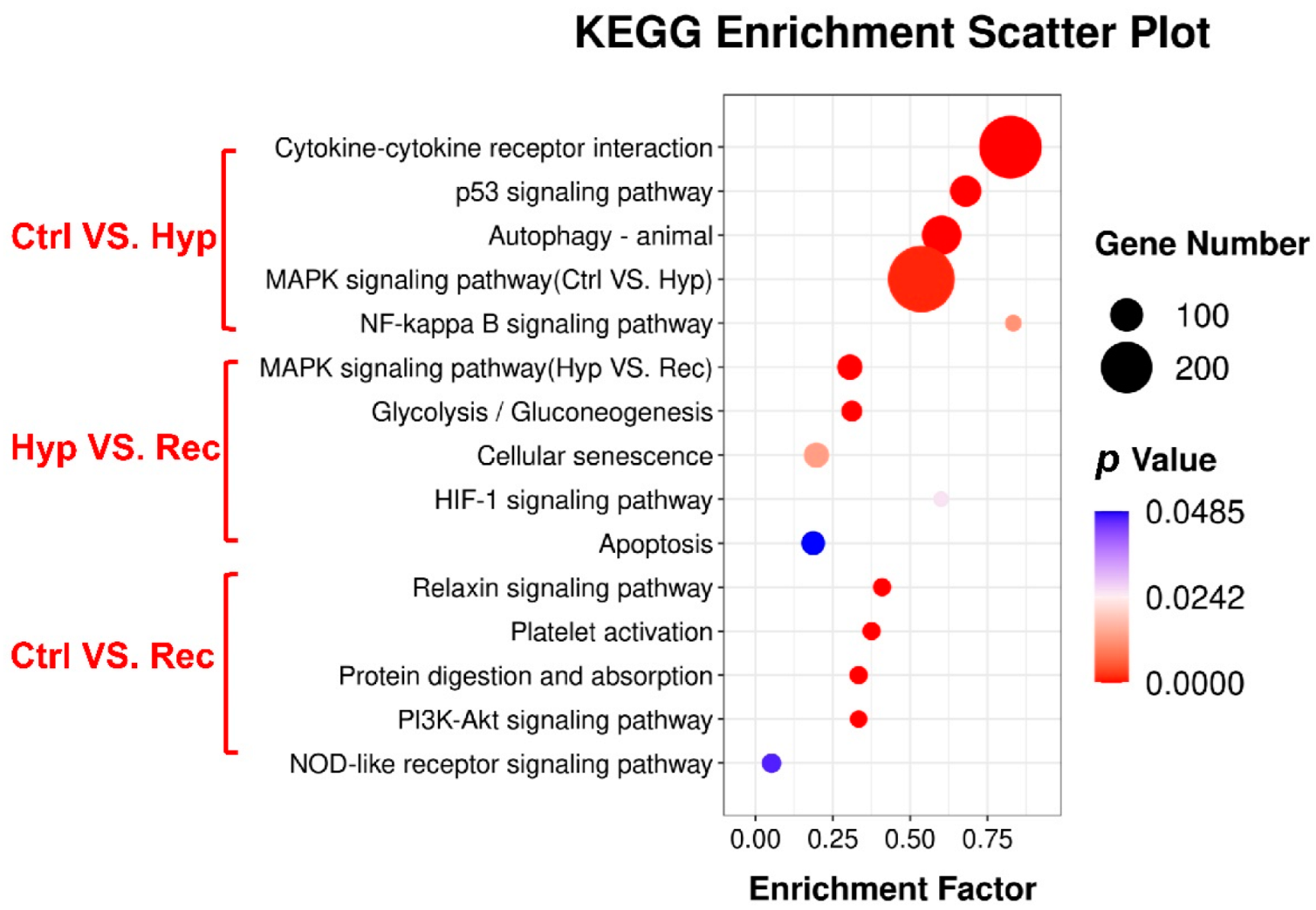
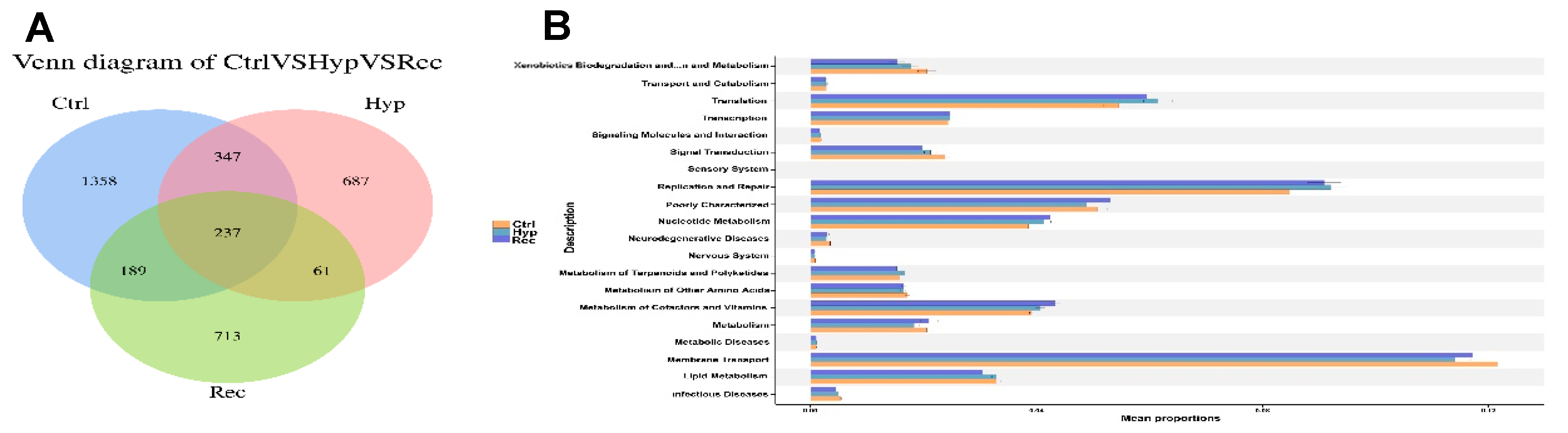
3.5. Transcriptome Analysis of Largemouth Bass under Hypoxic Stress and Reoxygenation
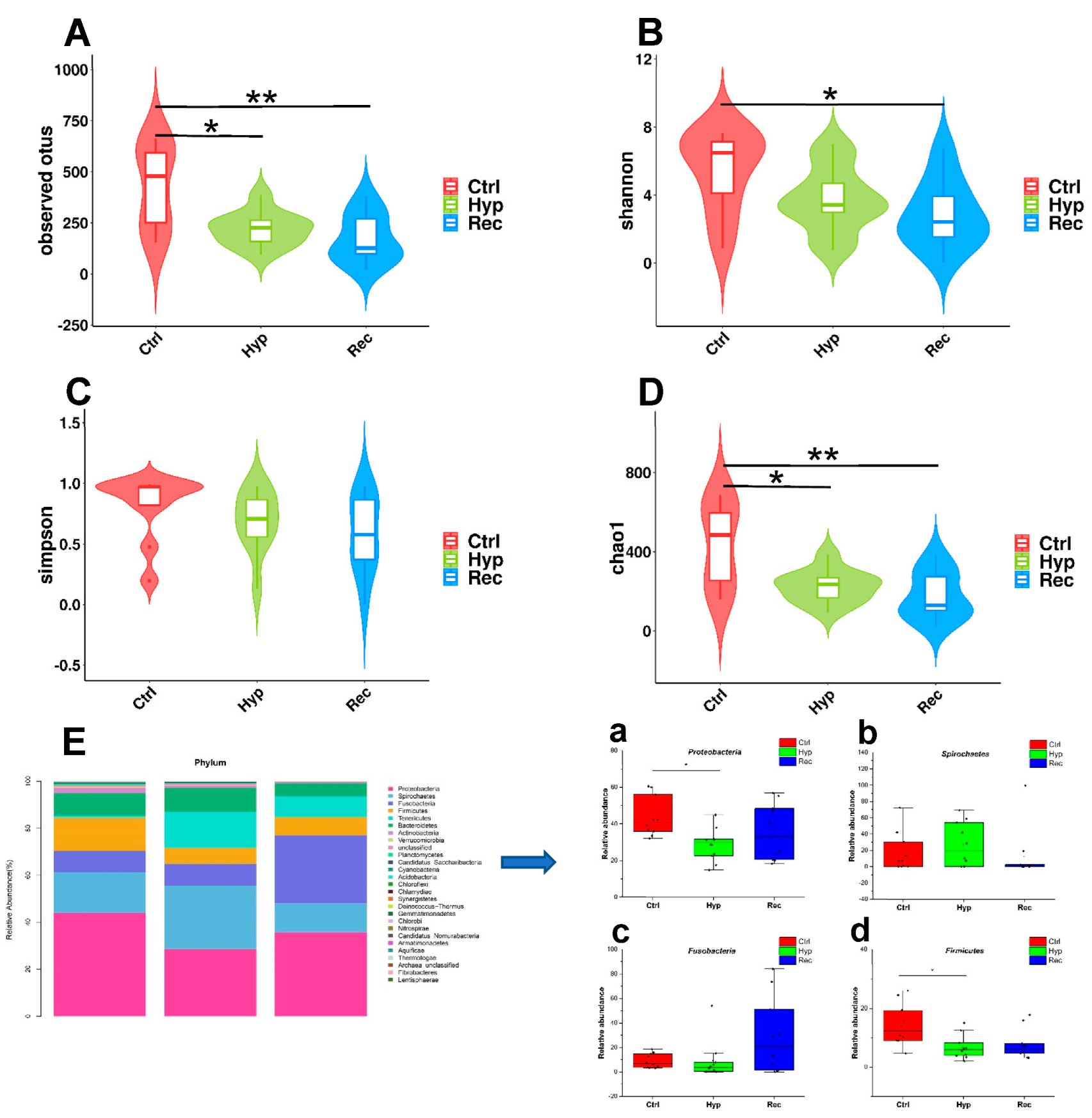
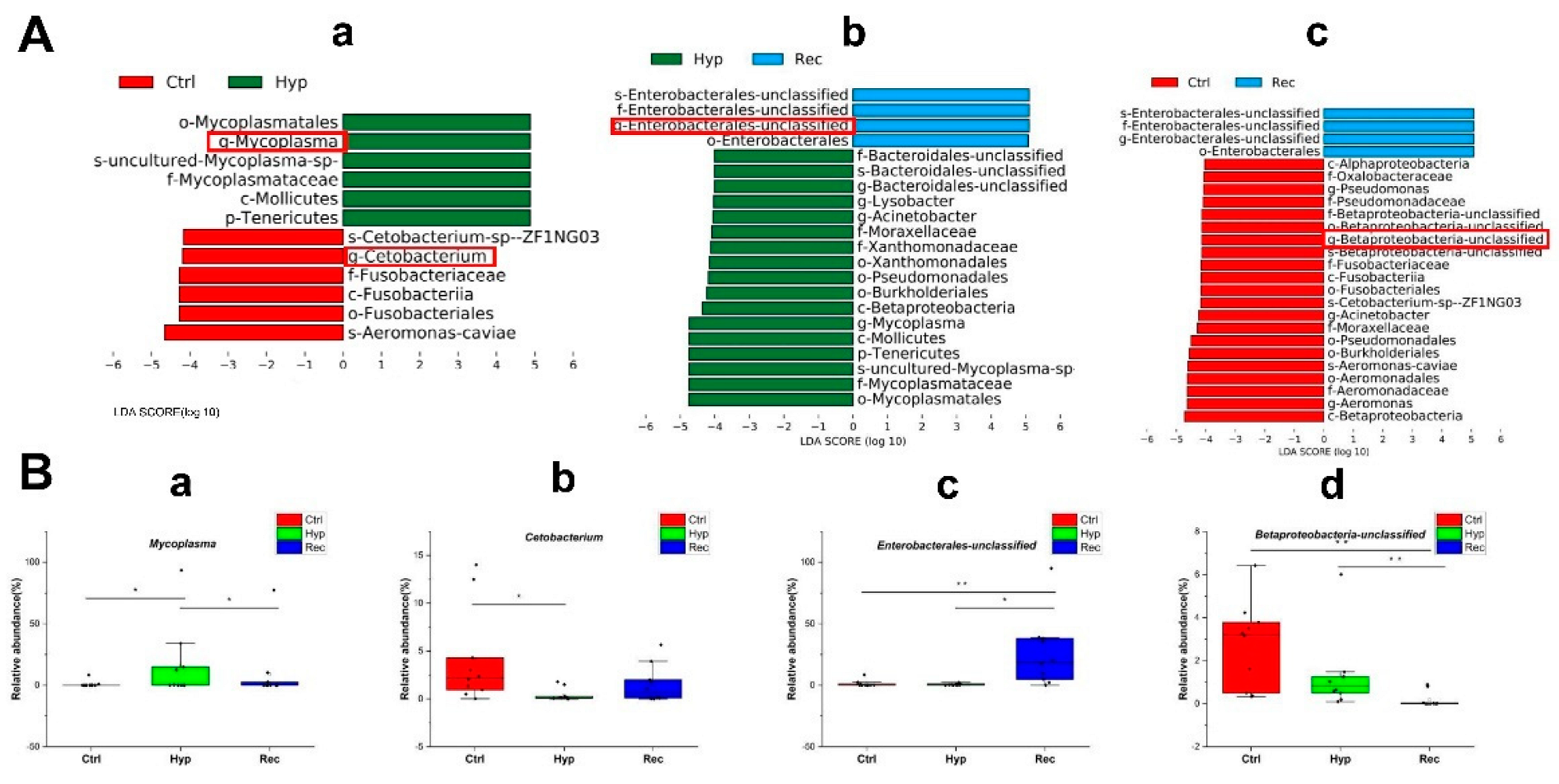
3.6. Sequencing and Analysis of Intestinal 16S rRNA of Largemouth Bass under Hypoxic Stress and Reoxygenation
3.7. Correlations between Intestinal Microorganisms and DEGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Tawwab, M.; Monier, M.N.; Hoseinifar, S.H.; Faggio, C. Fish response to hypoxia stress: Growth, physiological, and immunological biomarkers. Fish Physiol. Biochem. 2019, 45, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Cui, C.; Liu, C.; Sun, J.L.; He, K.; Adam, A.A.; Luo, J.; Li, Z.Q.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Mattiasen, E.G.; Kashef, N.S.; Stafford, D.M.; Logan, C.A.; Sogard, S.M.; Bjorkstedt, E.P.; Hamilton, S.L. Effects of hypoxia on the behavior and physiology of kelp forest fishes. Glob. Change Biol. 2020, 26, 3498–3511. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Liu, C.; Ding, J.; Gao, X.M.; Wang, J.Q.; Zhang, Y.B.; Du, C.; Hou, C.C.; Zhu, J.Q.; Lou, B.; et al. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021, 42, 592–605. [Google Scholar] [CrossRef]
- Pei, X.Y.; Chu, M.X.; Tang, P.; Zhang, H.Y.; Zhang, X.Y.; Zheng, X.; Li, J.; Mei, J.; Wang, T.; Yin, S.W. Effects of acute hypoxia and reoxygenation on oxygen sensors, respiratory metabolism, oxidative stress, and apoptosis in hybrid yellow catfish “Huangyou-1”. Fish Physiol. Biochem. 2021, 47, 1429–1448. [Google Scholar] [CrossRef]
- Varghese, T.; Mishal, P.; Gupta, G.; Kumar, M.; Pal, A.K.; Dasgupta, S. Temporal changes in behavioural responses and serum metabolites of Cirrhinus mrigala exposed to acute hypoxia. J. Environ. Biol. 2019, 40, 641–647. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Lushchak, O.V.; Storey, J.M.; Storey, K.B. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int. J. Biochem. Cell Biol. 2005, 37, 1319–1330. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Taylor, C.T. Determinants of hypoxia-inducible factor activity in the intestinal mucosa. J. Appl. Physiol. (1985) 2017, 123, 1328–1334. [Google Scholar] [CrossRef]
- Colgan, S.P. Targeting hypoxia in inflammatory bowel disease. J. Investig. Med. 2016, 64, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.B.; Sun, Y.X.; Duan, Y.F.; Li, H.; Li, Y.; Liu, Q.S.; Wang, W.H.; Zhang, J.S. The effect of teprenone on the intestinal morphology and microbial community of Chinese sea bass (Lateolabrax maculatus) under intermittent hypoxic stress. Fish Physiol. Biochem. 2020, 5, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Zhang, J.D.; Yang, L.T. Effects of hypoxia stress on digestive enzyme activities, intestinal structure and the expression of tight junction proteins coding genes in juvenile cobia (Rachycentron canadum). Aquac. Res. 2021, 52, 5630–5641. [Google Scholar] [CrossRef]
- Sun, Y.X.; Dong, H.B.; Zhan, A.J.; Wang, W.H.; Duan, Y.F.; Xie, M.J.; Liu, Q.S.; Li, H.; Zhang, J.S. Protection of teprenone against hypoxia and reoxygenation stress in stomach and intestine of Lateolabrax maculatus. Fish Physiol. Biochem. 2020, 46, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Guo, H.H.; Chen, S.J.; Wang, Y.Z.; Lin, Z.H.; Huang, X.D.; Tang, H.J.; He, Y.H.; Sun, J.J.; Gan, L. Transcriptome analysis revealed changes of multiple genes involved in muscle hardness in grass carp (Ctenopharyngodon idellus) fed with faba bean meal. Food Chem. 2020, 314, 126205. [Google Scholar] [CrossRef]
- Yang, Y.J.; Fu, Q.; Wang, X.Z.; Liu, Y.; Zeng, Q.F.; Li, Y.; Gao, S.; Bao, L.S.; Liu, S.K.; Gao, D.Y.; et al. Comparative transcriptome analysis of the swimbladder reveals expression signatures in response to low oxygen stress in channel catfish, Ictalurus punctatus. Physiol. Genom. 2018, 50, 636–647. [Google Scholar] [CrossRef]
- Zhao, S.S.; Su, X.L.; Pan, R.J.; Lu, L.Q.; Zheng, G.D.; Zou, S.M. The transcriptomic responses of blunt snout bream (Megalobrama amblycephala) to acute hypoxia stress alone, and in combination with bortezomib. BMC Genom. 2022, 23, 162. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, D.Y.; Cheng, J.H.; Tang, R.; Chu, M.X.; Chu, P.; Wang, T.; Yin, S.W. Effects of hypoxic stress and recovery on oxidative stress, apoptosis, and intestinal microorganisms in Pelteobagrus vachelli. Aquaculture 2021, 543, 736945. [Google Scholar] [CrossRef]
- Sun, S.M.; Yang, M.; Fu, H.T.; Ge, X.P.; Zou, J. Altered intestinal microbiota induced by chronic hypoxia drives the effects on lipid metabolism and the immune response of oriental river prawn Macrobrachium nipponense. Aquaculture 2020, 526, 735431. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metagenomic cross-talk: The regulatory interplay between immunogenomics and the microbiome. Genome Med. 2015, 7, 120. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Tao, Y.F.; Lu, S.Q.; Qiang, J.; Xu, P. Integrated Transcriptome and 16S rDNA Analyses Reveal That Transport Stress Induces Oxidative Stress and Immune and Metabolic Disorders in the Intestine of Hybrid Yellow Catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂). Antioxidants 2022, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Florindo, L.H.; Leite, C.A.C.; Kalinin, A.L.; Reid, S.G.; Milsom, W.K.; Rantin, F.T. The role of branchial and orobranchial O2 chemoreceptors in the control of aquatic surface respiration in the neotropical fish tambaqui (Colossoma macropomum): Progressive responses to prolonged hypoxia. J. Exp. Biol 2006, 209, 1709–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plante, S.; Chabot, D.; Dutil, J.D. Hypoxia tolerance in Atlantic cod. J. Fish Biol. 1998, 53, 1342–1356. [Google Scholar] [CrossRef]
- Ma, J.L.; Qiang, J.; Tao, Y.F.; Bao, J.W.; Zhu, H.J.; Li, L.G.; Xu, P. Multi-omics analysis reveals the glycolipid metabolism response mechanism in the liver of genetically improved farmed Tilapia (GIFT, Oreochromis niloticus) under hypoxia stress. BMC Genom. 2021, 22, 105. [Google Scholar] [CrossRef]
- Ma, X.Y.; Qiang, J.; He, J. Changes in the physiological parameters, fatty acid metabolism, and SCD activity and expression in juvenile GIFT tilapia (Oreochromis niloticus) reared at three different temperatures. Fish Physiol. Biochem. 2015, 41, 937–950. [Google Scholar] [CrossRef]
- Zheng, T.; Song, Z.; Qiang, J.; Tao, Y.F.; Zhu, H.J.; Ma, J.L.; Xu, P. Transport Stress Induces Skin Innate Immunity Response in Hybrid Yellow Catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂) Through TLR/NLR Signaling Pathways and Regulation of Mucus Secretion. Front. Immunol. 2021, 12, 430. [Google Scholar] [CrossRef]
- Qiang, J.; Bao, J.W.; Tao, Y.F.; He, J.; Li, H.X.; Xu, P.; Sun, L.Y. The expression profiles of miRNA–mRNA of early response in genetically improved farmed tilapia (Oreochromis niloticus) liver by acute heat stress. Sci. Rep. 2017, 7, 8705. [Google Scholar] [CrossRef] [Green Version]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Y.; Leushkin, E.; Liechti, A.; Ovchinnikova, S.; Mößinger, K.; Brüning, T.; Rummel, C.; Grützner, F.; Cardoso-Moreira, M.; Janich, P.; et al. Transcriptome and translatome co-evolution in mammals. Nature 2020, 588, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ni, J.J.; Nie, Z.J.; Gao, J.C.; Sun, Y.; Shao, N.L.; Li, Q.J.; Hu, J.W.; Xu, P.; Xu, G.C. Effects of stocking density on growth, serum parameters, antioxidant status, liver and intestine histology and gene expression of largemouth bass (Micropterus salmoides) farmed in the in-pond raceway system. Aquac. Res. 2020, 51, 5228–5240. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.F.; Qiang, J.; Dagoudo, M.; Zhu, H.J.; Bao, J.W.; Ma, J.L.; Li, M.X.; Xu, P. Transcriptome profiling reveals differential expression of immune-related genes in gills of hybrid yellow catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂) under hypoxic stress: Potential NLR-mediated immune response. Fish Shellfish Immunol. 2021, 119, 409–419. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; He, K.; Yan, T.; Zhou, J.; Zhao, L.L.; Sun, J.L.; Lian, W.Q.; Zhang, D.M.; Du, Z.J.; et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Sci. Total Environ. 2019, 666, 1071–1079. [Google Scholar] [CrossRef]
- Wu, L.Y.; Xu, W.J.; Li, H.Y.; Dong, B.; Geng, H.C.; Jin, J.Y.; Han, D.; Liu, H.K.; Zhu, X.M.; Yang, Y.X.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Jimenez, A.G.; Braun, E.; Tobin, K. How does chronic temperature exposure affect hypoxia tolerance in sheepshead minnows’ (Cyprinodon variegatus variegatus) ability to tolerate oxidative stress? Fish Physiol. Biochem. 2019, 45, 499–510. [Google Scholar] [CrossRef]
- Liu, B.H.; Wen, H.S.; Li, X.H.; Yang, J.; Li, G.L.; Zhang, M.Z.; Li, J.F.; He, F. Acute hypoxia effects on Keap1/Nrf2 (Mafs)-GST pathway related oxidative metabolism in muscle of Japanese flounder (Paralichthys olivaceus). Sci. Total Environ. 2021, 795, 148646. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Kong, Y.D.; Li, M.; Shan, X.F.; Wang, G.Q.; Han, G.H. Effects of deltamethrin subacute exposure in snakehead fish, Channa argus: Biochemicals, antioxidants and immune responses. Ecotoxicol. Environ. Saf. 2021, 209, 111821. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, K.; Waltmire, C.N.; Payne, C.M.; Tome, M.E.; Briehl, M.M.; Dorr, R.T. Induction of mitochondrial changes in myeloma cells by imexon. Blood 2001, 97, 3544–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.L.; Zhong, L.; Kang, Q.; Liu, S.; Feng, Y.; Geng, Y.; Chen, D.F.; OuYang, P.; Yang, S.Y.; Yin, L.Z.; et al. A High Starch Diet Alters the Composition of the Intestinal Microbiota of Largemouth Bass Micropterus salmoides, Which May Be Associated with the Development of Enteritis. Front. Microbiol. 2021, 12, 696588. [Google Scholar] [CrossRef]
- Sharma, R.K.; Oliveira, A.C.; Yang, T.; Karas, M.M.; Li, J.; Lobaton, G.O.; Aquino, V.P.; Robles-Vera, I.; Kloet, A.D.D.; Krause, E.G.; et al. Gut Pathology and Its Rescue by ACE2 (Angiotensin-Converting Enzyme 2) in Hypoxia-Induced Pulmonary Hypertension. Hypertension 2020, 76, 206–216. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sun, R.M.; Wang, G.Z.; Chen, Z.; Li, Y.; Zhao, Y.; Liu, D.S.; Zhao, H.Y.; Zhang, F.; Yao, J.H.; et al. SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020, 28, 101343. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Khamis, O.M.A.; Jiang, H.J.; Cao, Z.M.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W. Effects of dietary supplementation with apple peel powder on the growth, blood and liver parameters, and transcriptome of genetically improved farmed tilapia (GIFT, Oreochromis niloticus). PLoS ONE 2019, 14, e0224995. [Google Scholar] [CrossRef] [PubMed]
- Runchel, C.; Matsuzawa, A.; Ichijo, H. Mitogen-Activated Protein Kinases in Mammalian Oxidative Stress Responses. Antioxid. Redox Signal. 2011, 15, 25–218. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Huang, J.; Zhang, Y.W.; Liu, Y.Y.; Zuo, S.; Li, R. MAP2K4 interacts with Vimentin to activate the PI3K/AKT pathway and promotes breast cancer pathogenesis. Aging 2019, 11, 10697–10710. [Google Scholar] [CrossRef]
- Besnard, A.; Galan-Rodriguez, B.; Vanhoutte, P.; Caboche, J. Elk-1 a Transcription Factor with Multiple Facets in the Brain. Front. Neurosci. 2011, 5, 35. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, B.J.; Giugliano, F.P.; Baan, B.; Meer, J.H.M.V.D.; Meisner, S.; Roest, M.V.; Koelink, P.J.; Boer, R.J.D.; Jones, N.; Breitwieser, W.; et al. ATF2 and ATF7 Are Critical Mediators of Intestinal Epithelial Repair. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 23–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Z.; Zhang, Z.X.; Han, G.L. MiR-29a-3p Enhances the Viability of Rat Neuronal Cells that Injured by Oxygen-Glucose Deprivation/Reoxygenation Treatment Through Targeting TNFRSF1A and Regulating NF-κB Signaling Pathway. J. Stroke Cerebrovasc. Dis. 2020, 29, 105210. [Google Scholar] [CrossRef] [PubMed]
- Habibian, J.S.; Jefic, M.; Bagchi, R.A.; Lane, R.H.; McKnight, R.A.; McKinsey, T.A.; Morrison, R.F.; Ferguson, B.S. DUSP5 functions as a feedback regulator of TNFα-induced ERK1/2 dephosphorylation and inflammatory gene expression in adipocytes. Sci. Rep. 2017, 7, 12879. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef] [Green Version]
- Talwar, C.; Nagar, S.; Lal, R.; Negi, R.K. Fish Gut Microbiome: Current Approaches and Future Perspectives. Indian J. Microbiol. 2018, 58, 397–414. [Google Scholar] [CrossRef]
- Li, F.L.; Tang, Y.; Wei, L.X.; Yang, M.X.; Lu, Z.J.; Shi, F.; Zhan, F.B.; Li, Y.N.; Liao, W.C.; Lin, L.; et al. Alginate oligosaccharide modulates immune response, fat metabolism, and the gut bacterial community in grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2022, 130, 103–113. [Google Scholar] [CrossRef]
- Marciano, F.; Vajro, P. Oxidative Stress and Gut Microbiota; Academic Press: Cambridge, MA, USA, 2017; pp. 113–123. [Google Scholar]
- Fan, S.; Li, H.; Zhao, R. Effects of normoxic and hypoxic conditions on the immune response and gut microbiota of Bostrichthys sinensis. Aquaculture 2020, 525, 735336. [Google Scholar] [CrossRef]
- Gatesoupe, F.; Huelvan, C.; Bayon, N.L.; Delliou, H.L.; Madec, L.; Mouchel, O.; Quazuguel, P.; Mazurais, D.; Zambonino-Infante, J. The highly variable microbiota associated to intestinal mucosa correlates with growth and hypoxia resistance of sea bass, Dicentrarchus labrax, submitted to different nutritional histories. BMC Microbiol. 2016, 16, 266. [Google Scholar]
- Khan, F.U.; Shang, Y.Y.; Chang, X.Q.; Kong, H.; Zuberi, A.; Fang, J.K.H.; Liu, W.; Peng, J.X.; Zhang, X.Z.; Hu, M.H.; et al. Effects of Ocean Acidification, Hypoxia, and Warming on the Gut Microbiota of the Thick Shell Mussel Mytilus coruscus Through 16S rRNA Gene Sequencing. Front. Mar. Sci. 2021, 7, 736338. [Google Scholar] [CrossRef]
- Yang, H.T.; Yang, M.C.; Sun, J.J.; Guo, F.; Lan, J.F.; Wang, X.W.; Zhao, X.F.; Wang, J.X. Catalase eliminates reactive oxygen species and influences the intestinal microbiota of shrimp. Fish Shellfish Immunol. 2015, 47, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Theissig, F.; Engelhardt, H.; Bengmark, S.; Koch, S.; Lochs, H.; Dörffel, Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 2007, 56, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, X.; Li, L.Y.; Qiao, F.; Zhang, M.L.; Du, Z.Y. High protein intake promotes the adaptation to chronic hypoxia in zebrafish (Danio rerio). Aquaculture 2021, 535, 736356. [Google Scholar] [CrossRef]
- Qiao, R.X.; Sheng, C.; Lu, Y.F.; Zhang, Y.; Ren, H.Q.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Ringø, E.; Birkbeck, T.H.; Munro, P.O.; Vadstein, O.; Hjelmeland, K. The effect of early exposure to Vibrio pelagius on the aerobic bacterial flora of turbot, Scophthalmus maximus (L.) larvae. J. Appl. Bacteriol. 1996, 81, 207–211. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.D.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.D.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Zakrzewski, A.J.; Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. A Comparison of Methods for Identifying Enterobacterales Isolates from Fish and Prawns. Pathogens 2022, 11, 410. [Google Scholar] [CrossRef]
- Shimizu, T. Analysis of pathogenic factors of Mycoplasma. Nihon Saikingaku Zasshi 2015, 70, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, S.A.; Lee, J.Y.; Velazquez, E.M.; Foegeding, N.J.; Shelton, C.D.; Tiffany, C.R.; Parry, B.H.; Stull-Lane, A.R.; Olsan, E.E.; Savage, H.P.; et al. 5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-gamma Signaling in the Intestinal Epithelium. mBio 2021, 12, e03227. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Zhang, Z.; Ding, Q.W.; Yang, Y.L.; Bindelle, J.; Ran, C.; Zhou, Z.G. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xie, M.X.; Xie, Y.D.; Liang, H.; Li, M.; Ran, C.; Zhou, Z.G. Effect of dietary supplementation of Cetobacterium somerae XMX-1 fermentation product on gut and liver health and resistance against bacterial infection of the genetically improved farmed tilapia (GIFT, Oreochromis niloticus). Fish Shellfish Immunol. 2022, 124, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Chang, X.Y.; Wu, H.Z.; Xiao, J.F.; Gao, Y.; Zhang, Y.X. Role of intestinal inflammation in predisposition of Edwardsiella tarda infection in zebrafish (Danio rerio). Fish Shellfish Immunol. 2014, 41, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Sorbara, M.T.; Kim, S.G.; Littmann, E.L.; Dong, Q.W.; Walsh, G.; Wright, R.; Amoretti, L.; Fontana, E.; Hohl, T.M.; et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe 2021, 29, 378–393.e5. [Google Scholar] [CrossRef]
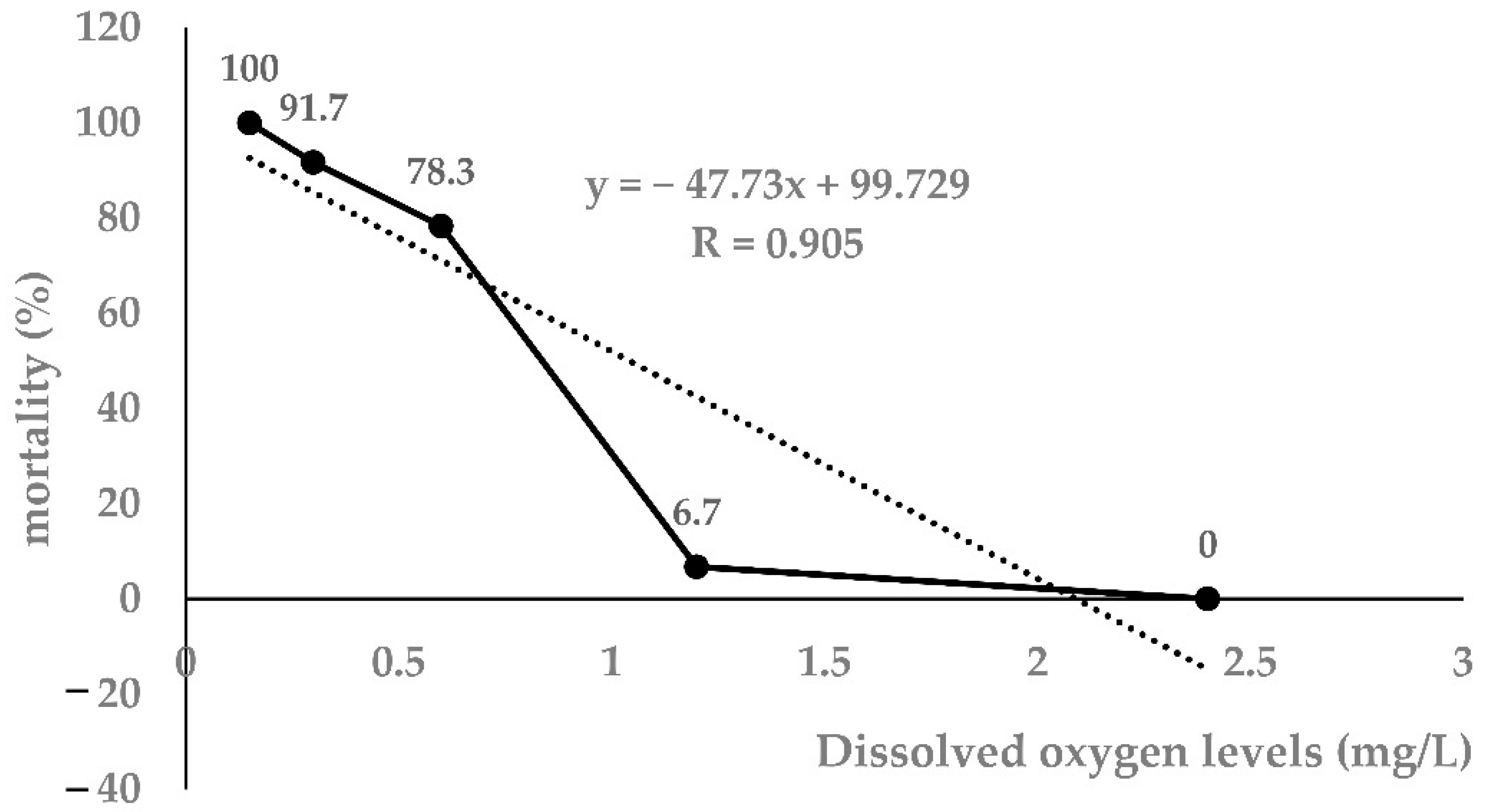



| Time (h) | Dissolved Oxygen Levels (mg/L) | ||||
|---|---|---|---|---|---|
| 2.4 | 1.2 | 0.6 | 0.3 | 0.15 | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 0 | 0 | 20 | 31.7 | 50 |
| 48 | 0 | 0 | 46.7 | 58.3 | 73.3 |
| 72 | 0 | 0 | 68.3 | 78.3 | 100 |
| 96 | 0 | 6.7 | 78.3 | 91.7 | 100 |
| Hyp_0 h | Hyp_6 h | Hyp_24 h | Hyp_96 h | Rec_96 h | |
|---|---|---|---|---|---|
| Villi Length (μm) | 654.85 ± 54.48 | 630.65 ± 42.79 | 452.70 ± 52.72 | 498.94 ± 61.55 | 518.87 ± 94.61 |
| Villi Thickness (μm) | 93.37 ± 6.48 | 89.14 ± 7.11 | 75.05 ± 2.36 | 76.77 ± 1.45 | 78.80 ± 5.6 |
| Muscle Layer Thickness (μm) | 83.34 ± 3.37 a | 67.09 ± 7.46 ab | 53.87 ± 4.90 bc | 42.01 ± 3.91 c | 64.04 ± 4.62 abc |
| Hyp_0 h | Hyp_6 h | Hyp_24 h | Hyp_96 h | Rec_96 h | |
|---|---|---|---|---|---|
| Number of Apoptotic Cells | 18.42 ± 4.09 a | 75.33 ± 12.45 a | 291.58 ± 35.16 b | 978.08 ± 237.68 c | 333.08 ± 31.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Ye, W.; Tao, Y.; Zheng, T.; Qiang, J.; Li, Y.; Liu, W.; Xu, P. Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora. Antioxidants 2023, 12, 1. https://doi.org/10.3390/antiox12010001
Song Z, Ye W, Tao Y, Zheng T, Qiang J, Li Y, Liu W, Xu P. Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora. Antioxidants. 2023; 12(1):1. https://doi.org/10.3390/antiox12010001
Chicago/Turabian StyleSong, Zhuo, Wei Ye, Yifan Tao, Tao Zheng, Jun Qiang, Yan Li, Wenting Liu, and Pao Xu. 2023. "Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora" Antioxidants 12, no. 1: 1. https://doi.org/10.3390/antiox12010001





