Evaluation of Tunisian Olive Leaf Extracts to Reduce the Bioavailability of Acrylamide in Californian-Style Black Olives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
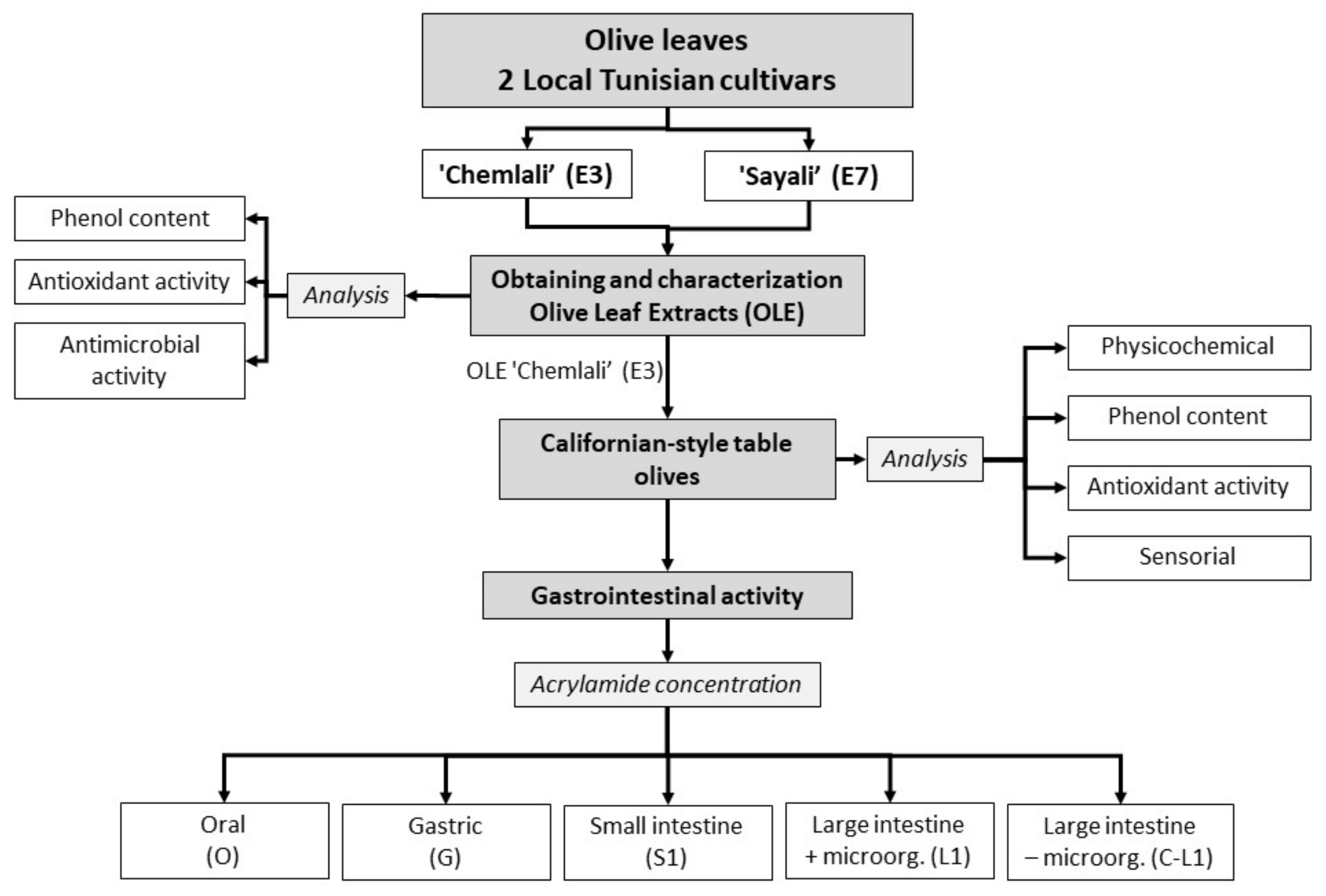
2.2. Experimental Design of the Study
2.3. Obtaining and Characterization of Olive Leaf Extract (OLE)
2.3.1. Determination of Phenolic Profile
2.3.2. Antioxidant Activity
2.3.3. Antimicrobial Activity
2.4. Californian-Style Black Table Olives Elaboration Process
2.5. Experimental Treatments of OLE Addition to Californian-Style Black Olives
2.5.1. Physicochemical Analysis
2.5.2. Sensory Analysis
2.5.3. Gastrointestinal Activity of Table Olives with OLE Addition on Acrylamide Concentration
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of OLE Obtained of Tunisian Olive Leaves
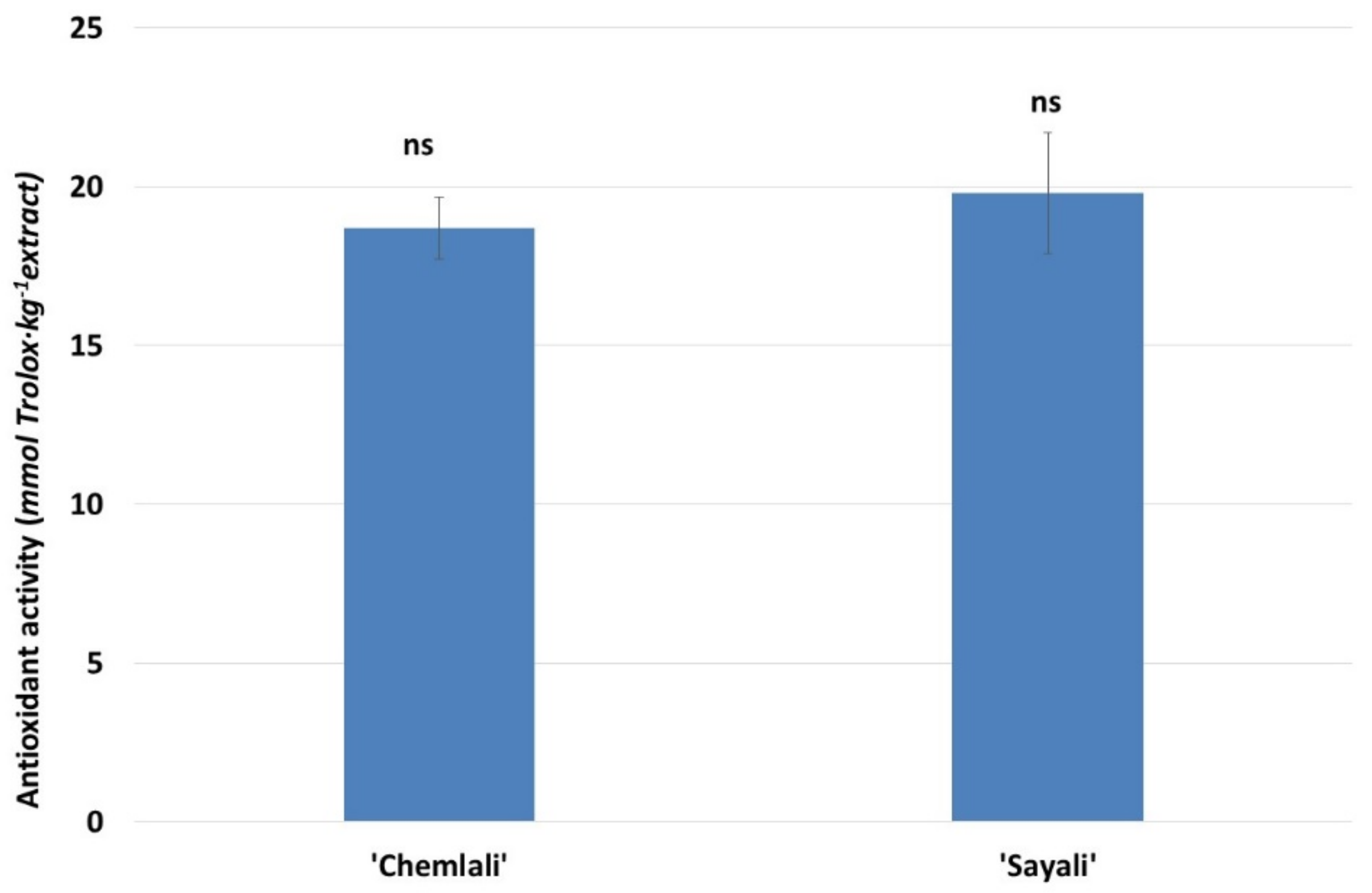
3.1.1. Phenol and Antioxidant Activity of Tunisian OLE
3.1.2. Antimicrobial Activity of Tunisian OLE
3.2. Influence of OLE Addition in Californian-Style Black Olives
3.2.1. Physicochemical Characteristics of Californian-Style Black Olives
3.2.2. Phenol and Antioxidant Activity of Californian-Style Black Olives
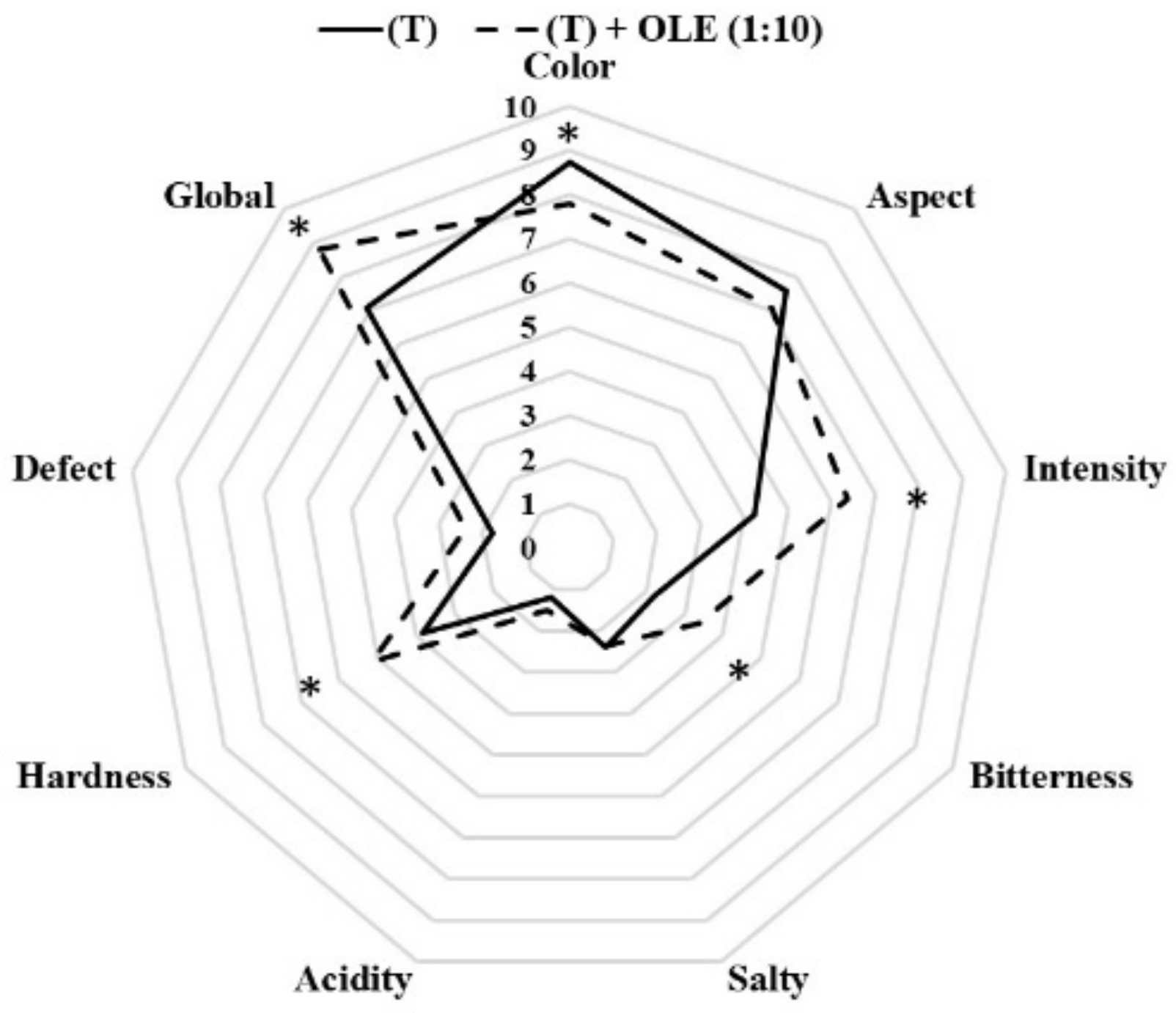
3.2.3. Sensory Characteristics of Californian-Style Black Olives
3.2.4. Effect of Gastrointestinal Activity on Acrylamide Content in Californian-Style Black Olives with OLE
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. Production. 2020. Available online: https://www.fao.org/faostat/es/#data (accessed on 17 May 2022).
- García Martín, J.F.; Cuevas, M.; Feng, C.H.; Mateos, P.Á.; García, M.T.; Sánchez, S. Energetic valorisation of olive biomass: Olive-tree pruning, olive stones and pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- de Brogniez, D.; Ballabio, C.; Stevens, A.; Jones, R.J.A.; Montanarella, L.; van Wesemael, B. A map of the topsoil organic carbon content of Europe generated by a generalized additive model. Eur. J. Soil Sci. 2015, 66, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Volpi, I.; Marchi, S.; Petacchi, R.; Hoxha, K.; Guidotti, D. Detecting olive grove abandonment with Sentinel-2 and machine learning: The development of a web-based tool for land management. Smart Agric. Technol. 2023, 3, 100068. [Google Scholar] [CrossRef]
- IOC. World Table Olive and Olive Oil Production. 2020. Available online: http://www.internationaloliveoil.org (accessed on 10 May 2022).
- Abdelhamid, S.; Grati-Kamoun, N.; Marra, F.; Caruso, T. Genetic similarity among Tunisian cultivated olive estimated through SSR markers. Genet. Plant Breed. 2013, 70, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef] [Green Version]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Lemaire, A.; Limbourg, S. How can food loss and waste management achieve sustainable development goals? J. Clean. Prod. 2019, 234, 1221–1234. [Google Scholar] [CrossRef]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef]
- Delgado-Adámez, J.; Baltasar, M.N.F.; Yuste, M.C.A.; Martín-Vertedor, D. Oxidative stability, phenolic compounds and antioxidant potential of a virgin olive oil enriched with natural bioactive compounds. J. Oleo Sci. 2014, 63, 55–65. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Garrido, M.; Pariente, J.A.; Espino, J.; Delgado-Adámez, J. Bioavailability of bioactive molecules from olive leaf extracts and its functional value. Phytother Res. 2016, 30, 1172–1179. [Google Scholar] [CrossRef]
- Magrone, T.; Spagnoletta, A.; Salvatore, R.; Magrone, M.; Dentamaro, F.; Russo, M.A.; Jirillo, E. Olive leaf extracts act as modulators of the human immune response. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 85–93. [Google Scholar] [CrossRef]
- Nath, P.; Majumder, D.; Debnath, R.; Debnath, M.; Sekhawat, S.S.; Maiti, D. Immunotherapeutic potential of ethanolic olive leaves extract (EOLE) and IL-28B combination therapy in ENU induced animal model of leukemia. Cytokine 2022, 156, 155913. [Google Scholar] [CrossRef]
- IOC. International Olive Council World Table Olive Figures. 2020. Available online: https://www.internationaloliveoil.org/estaticos/view/132-world-table-olive-figures/ (accessed on 1 May 2022).
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Martín-Tornero, E.; Sánchez, R.; Lozano, J.; Martínez, M.; Arroyo, P.; Martín-Vertedor, D. Characterization of polyphenol and volatile fractions of Californian-style black olives and innovative application of e-nose for acrylamide determination. Foods 2021, 10, 2973. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef] [Green Version]
- Martín-Vertedor, D.; Fernández, A.; Mesías, M.; Martínez, M.; Martín-Tornero, E. Identification of mitigation strategies to reduce acrylamide levels during the production of black olives. J. Food Compost. Anal. 2021, 102, 104009. [Google Scholar] [CrossRef]
- Mechi, D.; Fernández, A.; Baccouri, B.; Abaza, L.; Martín-Vertedor, D. Addition of ‘Chetoui’olive leaf extract to reduce acrylamide in Californian-style black olive. Food Biosci. 2022, 50, 102080. [Google Scholar] [CrossRef]
- Baccouri, B.; Mechi, D.; Rajhi, I.; Martín-Vertedor, D. Tunisian wild olive leaves: Phenolic compounds and antioxidant activity as an important step toward their valorization. In Food Analytical Methods; Springer: Berlin, Germany, 2022. [Google Scholar]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ordiales, E.; Ruiz-Moyano, S.; Córdoba, M.G. Antioxidant and antimicrobial activity of natural phenolic extract from defatted soybean flour by-product for stone fruit postharvest application. J. Sci. Food Agric. 2016, 96, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2019, 116, 1306–1317. [Google Scholar] [CrossRef]
- Olmo-García, L.; Kessler, N.H.; Neuweger, K.; Wendt, J.M.; Olmo-Peinado, A.; Fernández-Gutierrez, C.; Baessmann, A. Unravelling the distribution of secondary metabolites in Olea europaea L.: Exhaustive characterization of eight olive-tree derived matrices by complementary platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 2018, 23, 2419. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Bañegil, M.; Schaide, T.; Manzano, R.; Delgado-Adámez, J.; Durán-Merás, I.; Martín-Vertedor, D. Optimization and validation of a rapid liquid chromatography method for determination of the main polyphenolic compounds in table olives and in olive paste. Food Chem. 2017, 233, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vertedor, D.; Schaide, T.; Boselli, E.; Martínez, M.; García-Parra, J.; Pérez-Nevado, F. Effect of high hydrostatic pressure in the storage of Spanish-style table olive fermented with olive leaf extract and Saccharomyces cerevisiae. Molecules 2022, 27, 2028. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Talaverano, M.I.; Pérez-Nevado, F.; Boselli, E.; Cordeiro, A.M.; Martillanes, S.; Foligni, R.; Martín-Vertedor, D. Evaluation of phenolics and acrylamide and their bioavailability in high hydrostatic pressure treated and fried table olives. J. Food Process. Preserv. 2020, 44, e14384. [Google Scholar] [CrossRef]
- Sánchez, R.; Pérez-Nevado, F.; Montero-Fernández, I.; Lozano, J.; Meléndez, F.; Martín-Vertedor, D. Application of electronic nose to discriminate species of mold strains in synthetic brines. Front. Microbiol. 2022, 13, 1657. [Google Scholar] [CrossRef]
- Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 1 December 2022).
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- González-Mulero, L.; Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Assessment of the acrylamide bioaccessibility in cereal and potato-based foods after in vitro digestion. Food Res. Int. 2022, 161, 111820. [Google Scholar] [CrossRef]
- Sansano, M.; Heredia, A.; Peinado, I.; Andrés, A. Dietary acrylamide: What happens during digestion. Food Chem. 2017, 237, 58–64. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Fernández, A.; Mesías, M.; Martínez, M.; Díaz, M.; Martín-Tornero, E. Industrial strategies to reduce acrylamide formation in Californian-style green ripe olives. Foods 2020, 9, 1202. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]

| Lactic Acid Bacteria | Pathogenic Bacteria |
|---|---|
| Lactobacillus sakei CECT5766 | Bacillus cereus CECT 131 |
| Lactobacilus curvatus CECT 904 | Escherichia coli CECT 4267 |
| Pediococcus acidilactici MS200 | Salmonella cholerasuis CECT 4395 |
| Staphylococcus aureus CECT 59 | |
| Enterococcus faecalis CECT 36 | |
| Listeria inocua CECT 910 |
| Variety | ||
|---|---|---|
| ‘Chemlali’ | ‘Sayali’ | |
| Phenolic Profile (mg·100 g−1) | ||
| Hydroxytyrosol | 4190.4 ± 5.7 j A | 9278.0 ± 14.4 i B |
| Tyrosol | 177.6 ± 0.9 d A | 258.3 ± 6.2 d B |
| PB1 | 198.1 ± 1.2 e NS | 120.5 ± 7.9 c |
| Epicatechin | 70.9 ± 6.5 c A | 115.3 ± 0.0 c B |
| Verbascoside | 652.7 ± 9.7 h A | 782.7 ± 12.9 f B |
| Quercetin 3 rutinoside | 558.5 ± 9.9 g A | 709.4 ± 3.8 f B |
| Luteolin-7-O-glucoside | 1855.4 ± 67.6 i A | 2007.9 ± 7.3 h B |
| Oleuropein | 8485.6 ± 20.8 k A | 15,781.7 ± 84.7 j B |
| Quercetin | 2015.6 ± 200.1 i B | 1120.8 ± 22.7 g A |
| Gallic acid | 2.1 ± 0.2 a NS | 2.7 ± 0.2 a |
| Vanillic acid | 0.6 ± 0.1 a NS | 1.0 ± 0.1 a |
| Cafeic acid | 257.4 ± 11.1 f A | 311.3 ± 9.8 e B |
| p-Coumaric | 1.2 ± 0.0 a NS | 1.5 ± 0.0 a |
| Chlorogenic acid | 45.8 ± 10.5 b A | 60.4 ± 10.3 b B |
| Σ phenols | 18,511.9 ± 344.3 a A | 30,551.5 ± 180.3 b B |
| Bacteria Strain | ‘Chemlali’ (E3) | ‘Sayali’ (E7) | ||||
|---|---|---|---|---|---|---|
| 1% | 0.1% | 0.05% | 1% | 0.1% | 0.05% | |
| Lactic acid bacteria | ||||||
| L. sakei CECT5766 | 11.3 ± 1.6 dD | 7.7 ± 1.5 cD | 6.8 ± 1.2 bC | 10.5 ± 2.5 dD | 7.5 ± 1.1 cD | 5.7 ± 1.1 aC |
| L. curvatus CECT 904 | 21.4 ± 3.5 eE | 7.0 ± 3.9 cC | 7.2 ± 3.6 cD | 14.8 ± 3.9 dE | 4.4 ± 2.7 aC | 6.2 ± 2.4 bD |
| P. acidilactici MS200 | 10.0 ± 2.1 cC | 0.52 ± 1.1 aA | 0.4 ± 1.1 aA | 8.8 ± 2.4 bC | 0.2 ± 1.8 aA | 0.8 ± 1.1 aA |
| Pathogenic bacteria | ||||||
| S. cholerasuis CECT 4395 | 64.1 ± 3.0 eG | 63.7 ± 2.8 dF | 42.6 ± 3.7 aF | 64.3 ± 2.7 eI | 62.8 ± 2.3 cG | 53.7 ± 2.7 bF |
| E. coli CECT 4267 | 2.8 ± 1.6 cA | 0.8 ± 0.5 aA | 0.3 ± 0.5 aA | 1.2 ± 1.5 bB | 1.7 ± 1.5 bB | 0.4 ± 0.9 aA |
| E. faecalis CECT 36 | 9.3 ± 1.0 cB | 0.3 ± 1.5 aA | 0.6 ± 1.9 aA | 0.3 ± 1.3 aA | 1.4 ± 1.5 bB | 0.7 ± 1.9 aA |
| B. cereus CECT 131 | 21.9 ± 2.4 cE | 21.1 ± 1.4 cE | 18.4 ± 1.5 bE | 34.5 ± 2.8 dF | 21.9 ± 1.4 cE | 1.1 ± 2.7 aB |
| S. aureus CECT 59 | 29.9 ± 1.2 dF | 3.6 ± 2.9 aB | 4.6 ± 1.8 aB | 41.7 ± 1.7 eG | 20.4 ± 2.4 cE | 9.3 ± 2.4 bE |
| L. inocua CECT 910 | 8.8 ± 3.5 bB | 3.8 ± 2.2 aB | 3.1 ± 2.7 aB | 56.6 ± 2.9 cH | 55.2 ± 3.5 cF | 4.2 ± 2.0 aC |
| Oxidazed Black Olive (T) | (T) + OLE (1:10) | |
|---|---|---|
| Firmness (N) | 3.7 ± 0.3 a | 5.2 ± 0.4 b |
| pH | 7.1 ± 0.1 ns | 7.0 ± 0.1 ns |
| Acidity (g∙100 mL−1) | 0.1 ± 0.0 ns | 0.1 ± 0.0 ns |
| Chlorides (g∙100 g−1) | 2.0 ± 0.1 ns | 2.0 ± 0.1 ns |
| ‘Hojiblanca’ | ||
|---|---|---|
| Oxidazed Black Olive (T) | (T) + OLE (1:10) | |
| Phenolic Profile (mg·100 g−1) | ||
| Hydroxytyrosol | 201.9 ± 26.9 a F | 1809.6 ± 25.3 b I |
| Tyrosol | 11.0 ± 3.0 a D | 68.4 ± 7.4 b E |
| PB1 | 8.0 ± 2.9 a C | 42.7 ± 3.6 b D |
| Epicatechin | 1.2 ± 1.1 a A | 35.6 ± 7.6 b D |
| Verbascoside | 1.7 ± 0.3 a A | 219.2 ± 0.3 b G |
| Quercetin 3 rutinoside | 1.0 ± 0.8 a A | 250.1 ± 10.0 b G |
| Luteolin-7-O-glucoside | 1.1 ± 0.9 a A | 438.4 ± 38.0 b H |
| Oleuropein | 47.9 ± 9.7 a E | 2662.2 ± 38.0 b J |
| Quercetin | nq | 246.4 ± 27.1 G |
| Gallic acid | nq | 2.2 ± 0.1 b |
| Vanillic acid | 1.3 ± 0.2 a A | 2.4 ± 0.4 b A |
| Cafeic acid | nq | 96.1 ± 8.6 F |
| p-coumaric | 3.6 ± 0.5 a B | 5.7 ± 1.1 b B |
| Chlorogenic acid | nq | 20.9 ± 3.0 C |
| Σ phenols | 278.6 ± 4.5 a G | 6186.1 ± 13.0 b K |
| DPPH radical scavenging activity | ||
| DPPH (mmol Trolox·kg−1 extract) | 0.8 ± 0.2 a | 7.1 ± 1.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechi, D.; Pérez-Nevado, F.; Montero-Fernández, I.; Baccouri, B.; Abaza, L.; Martín-Vertedor, D. Evaluation of Tunisian Olive Leaf Extracts to Reduce the Bioavailability of Acrylamide in Californian-Style Black Olives. Antioxidants 2023, 12, 117. https://doi.org/10.3390/antiox12010117
Mechi D, Pérez-Nevado F, Montero-Fernández I, Baccouri B, Abaza L, Martín-Vertedor D. Evaluation of Tunisian Olive Leaf Extracts to Reduce the Bioavailability of Acrylamide in Californian-Style Black Olives. Antioxidants. 2023; 12(1):117. https://doi.org/10.3390/antiox12010117
Chicago/Turabian StyleMechi, Dalel, Francisco Pérez-Nevado, Ismael Montero-Fernández, Bechir Baccouri, Leila Abaza, and Daniel Martín-Vertedor. 2023. "Evaluation of Tunisian Olive Leaf Extracts to Reduce the Bioavailability of Acrylamide in Californian-Style Black Olives" Antioxidants 12, no. 1: 117. https://doi.org/10.3390/antiox12010117








