Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality
Abstract
1. Introduction
2. Oxidative Stress and Fetal Programming
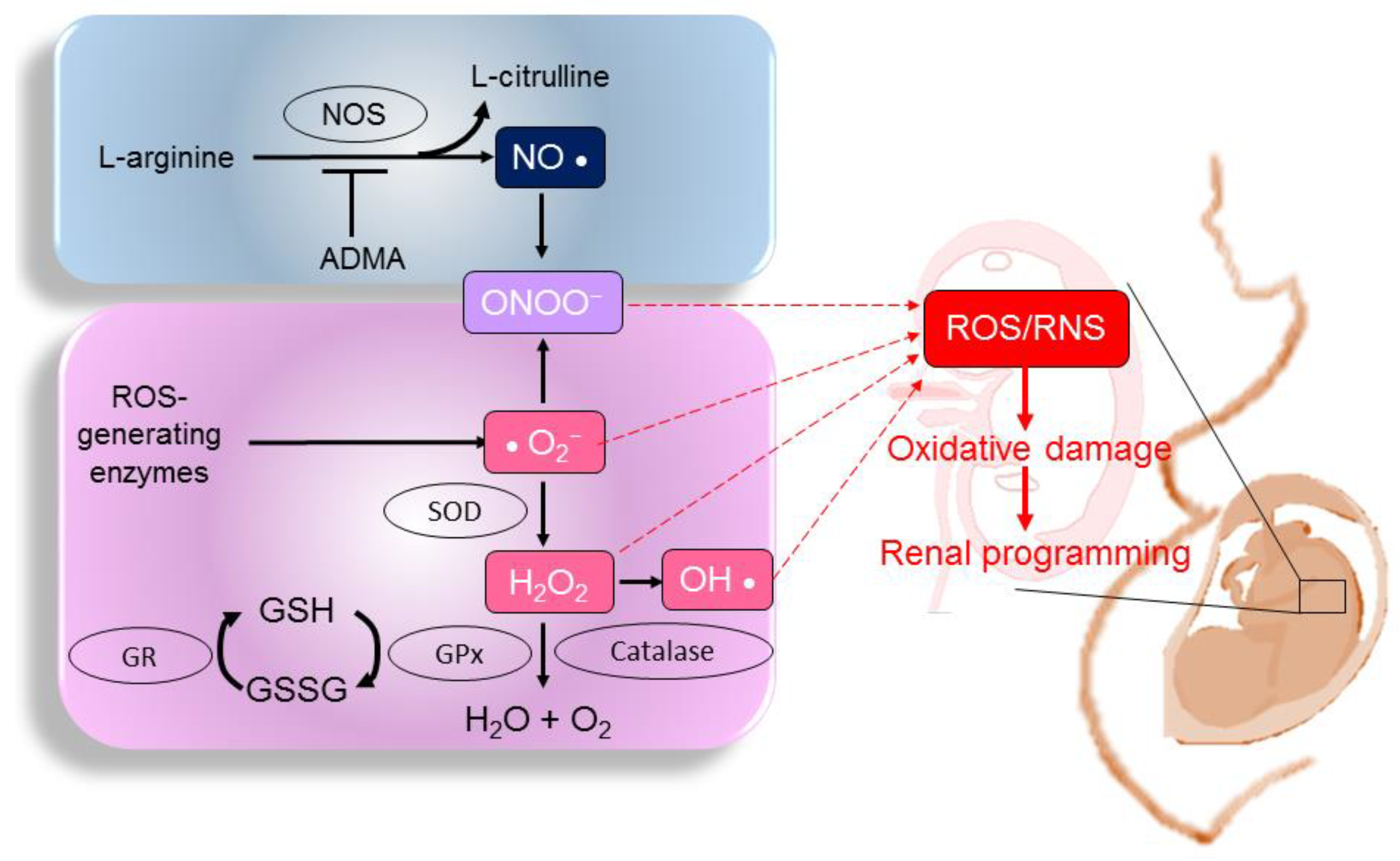
2.1. ROS, RNS, and NO
2.2. Studies in Humans: Oxidative Stress in Fetuses and Neonates
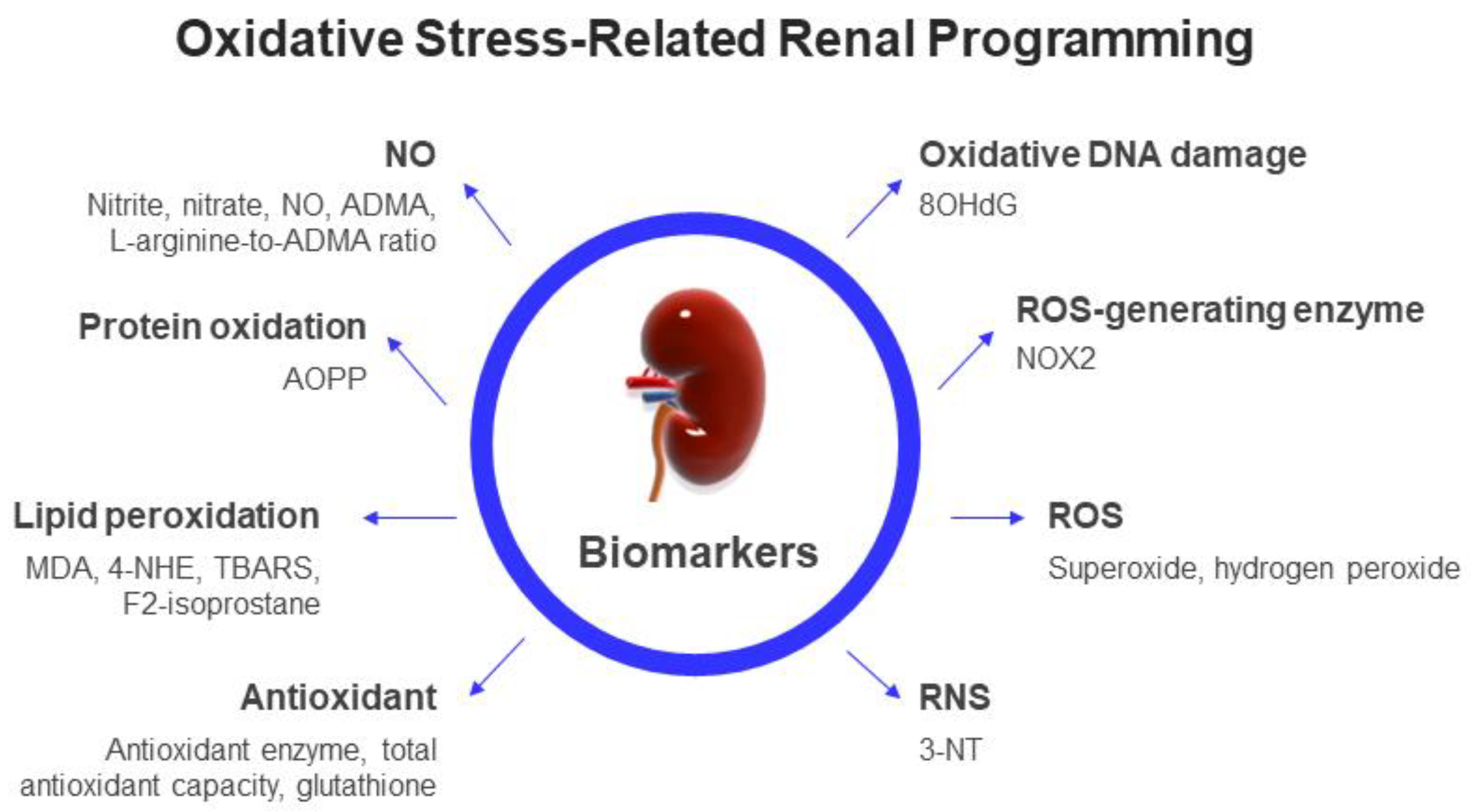
2.3. Biomarkers of Oxidative Stress in Clinical Practice
2.4. What Is Missing from Human Studies?
3. Animal Models of Oxidative-Stress-Related Renal Programming
3.1. Maternal Insults
3.2. Oxidative-Stress-Mediated Mechanisms
4. Antioxidant Strategies for Kidney Health
4.1. Vitamins
4.2. Amino Acids
4.3. Melatonin
4.4. Polyphenols
4.5. N-Acetylcysteine
4.6. Synthetic Antioxidants
5. The Gap between Animal Models and Clinical Reality
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Zullino, S.; Buzzella, F.; Simoncini, T. Nitric oxide and the biology of pregnancy. Vascul. Pharmacol. 2018, 110, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Moore, T.A.; Ahmad, I.M.; Zimmerman, M.C. Oxidative stress and preterm birth: An integrative review. Biol. Res. Nurs. 2018, 20, 497–512. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Developmental Origins of Kidney Disease: Why Oxidative Stress Matters? Antioxidants 2021, 10, 33. [Google Scholar] [CrossRef]
- Little, M.H.; McMahon, A.P. Mammalian kidney development: Principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012, 4, a008300. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. The clinical importance of nephron mass. J. Am. Soc. Nephrol. 2010, 21, 898–910. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. 2020, 15, 723–731. [Google Scholar] [CrossRef]
- Hartman, H.A.; Lai, H.L.; Patterson, L.T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007, 310, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Early-Life Programming and Reprogramming of Adult Kidney Disease and Hypertension: The Interplay between Maternal Nutrition and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3572. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, S.H.H.; Chan, J.Y.H. Biochemical basis for pharmacological intervention as a reprogramming strategy against hypertension and kidney disease of developmental origin. Biochem. Pharmacol. 2018, 153, 82–90. [Google Scholar] [CrossRef]
- Wilcox, C.S. Reactive oxygen species: Roles in blood pressure and kidney function. Curr. Hypertens. Rep. 2002, 4, 160–166. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Jenkins, C.; Wilson, R.; Roberts, J.; Miller, H.; McKillop, J.H.; Walker, J.J. Antioxidants: Their role in pregnancy and miscarriage. Antioxid. Redox Signal. 2000, 2, 623–628. [Google Scholar] [CrossRef]
- Kone, B.C. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin. Nephrol. 2004, 24, 299–315. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Carter, A.M. Placental oxygen consumption. Part I. In vivo studies—A review. Placenta 2000, 21, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Ye, C.; Connelly, P.W.; Hanley, A.J.; Sermer, M.; Zinman, B.; Retnakaran, R. Asymmetric dimethylarginine and arginine metabolites in women with and without a history of gestational diabetes. J. Diabetes Complicat. 2017, 31, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Hedner, T.; Milsom, I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998, 77, 808–813. [Google Scholar] [PubMed]
- de Almeida, V.O.; Pereira, R.A.; Amantéa, S.L.; Rhoden, C.R.; Colvero, M.O. Neonatal diseases and oxidative stress in premature infants: An integrative review. J. Pediatr. (Rio. J.) 2022, 98, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Negro, S.; Boutsikou, T.; Briana, D.D.; Tataranno, M.L.; Longini, M.; Proietti, F.; Bazzini, F.; Dani, C.; Malamitsi-Puchner, A.; Buonocore, G.; et al. Maternal obesity and perinatal oxidative stress: The strength of the association. J. Biol. Regul. Homeost. Agents 2017, 31, 221–227. [Google Scholar]
- Longini, M.; Perrone, S.; Vezzosi, P.; Marzocchi, B.; Kenanidis, A.; Centini, G.; Rosignoli, L.; Buonocore, G. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 2007, 40, 793–797. [Google Scholar] [CrossRef]
- Kamath, U.; Rao, G.; Kamath, S.U.; Rai, L. Maternal and fetal indicators of oxidative stress during intrauterine growth retardation (IUGR). Indian J. Clin. Biochem. 2006, 21, 111–115. [Google Scholar] [CrossRef]
- Bracci, R.; Buonocore, G. The antioxidant status of erythrocytes in preterm and term infants. Semin. Neonatol. 1998, 3, 191–197. [Google Scholar] [CrossRef]
- Perrone, S.; Laschi, E.; Buonocore, G. Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic. Biol. Med. 2019, 142, 23–31. [Google Scholar] [CrossRef]
- Longini, M.; Perrone, S.; Kenanidis, A.; Vezzosi, P.; Marzocchi, B.; Petraglia, F. Isoprostanes in amniotic fluid: A predictive marker for fetal growth restriction in pregnancy. Free Radic. Biol. Med. 2005, 38, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Belvisi, E.; Proietti, F.; Bazzini, F.; Buonocore, G.; Perrone, S. Oxidative Stress Biomarkers: Establishment of Reference Values for Isoprostanes, AOPP, and NPBI in Cord Blood. Mediat. Inflamm. 2017, 2017, 1758432. [Google Scholar] [CrossRef] [PubMed]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Kyozuka, H.; Fukuda, T.; Endo, Y.; Kanno, A.; Yasuda, S.; Yamaguchi, A.; Sato, A.; Ogata, Y.; Shinoki, K.; et al. Japan Environment and Children’s Study (JECS) Group. Urinary 8-hydroxy-2′-deoxyguanosine levels and small-for-gestational age infants: A prospective cohort study from the Japan Environment and Children’s Study. BMJ Open 2021, 11, e054156. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef]
- Dizdar, E.A.; Uras, N.; Oguz, S.; Erdeve, O.; Sari, F.N.; Aydemir, C.; Dilmen, U. Total antioxidant capacity and total oxidant status after surfactant treatment in preterm infants with respiratory distress syndrome. Ann. Clin. Biochem. 2011, 48, 462–467. [Google Scholar] [CrossRef]
- Gücüyener, K.; Ergenekon, E.; Demiryürek, T.; Erbaş, D.; Oztürk, G.; Koç, E.; Atalay, Y. Cerebrospinal fluid levels of nitric oxide and nitrotyrosine in neonates with mild hypoxic-ischemic encephalopathy. J. Child Neurol. 2002, 17, 815–818. [Google Scholar] [CrossRef]
- Banks, B.A.; Ischiropoulos, H.; McClelland, M.; Ballard, P.L.; Ballard, R.A. Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics 1998, 101, 870–874. [Google Scholar] [CrossRef]
- Tsukahara, H.; Ohta, N.; Tokuriki, S.; Nishijima, K.; Kotsuji, F.; Kawakami, H.; Ohta, N.; Sekine, K.; Nagasaka, H.; Mayumi, M. Determination of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, in umbilical blood. Metabolism 2008, 57, 215–220. [Google Scholar] [CrossRef]
- Lücke, T.; Kanzelmeyer, N.; Kemper, M.J.; Tsikas, D.; Das, A.M. Developmental changes in the L-arginine/nitric oxide pathway from infancy to adulthood: Plasma asymmetric dimethylarginine levels decrease with age. Clin. Chem. Lab Med. 2007, 45, 1525–2530. [Google Scholar] [CrossRef]
- Tashie, W.; Fondjo, L.A.; Owiredu, W.K.B.A.; Ephraim, R.K.D.; Asare, L.; Adu-Gyamfi, E.A.; Seidu, L. Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia. BioMed Res. Int. 2020, 2020, 3251956. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat. Rev. Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Nenov, V.D.; Taal, M.W.; Sakharova, O.V.; Brenner, B.M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 2000, 9, 85–97. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The First Thousand Days: Kidney Health and Beyond. Healthcare 2021, 9, 1332. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014, 2014, 283180. [Google Scholar] [CrossRef]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016, 38, 86–92. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 30, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chen, W.H.; Su, C.H.; Yu, H.R.; Tain, Y.L.; Huang, L.T.; Sheen, J.M. Maternal Iron Deficiency Programs Rat Offspring Hypertension in Relation to Renin-Angiotensin System and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 8294. [Google Scholar] [CrossRef]
- Do Nascimento, L.C.P.; Neto, J.P.R.C.; de Andrade Braga, V.; Lagranha, C.J.; de Brito Alves, J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020, 261, 118367. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Tsai, C.C.; Huang, L.T.; Hsu, C.N. High Fat Diets Sex-Specifically Affect the Renal Transcriptome and Program Obesity, Kidney Injury, and Hypertension in the Offspring. Nutrients 2017, 9, 357. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, C.N.; Tain, Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients 2020, 12, 448. [Google Scholar] [CrossRef]
- do Nascimento, L.C.P.; de Souza, E.L.; de Luna Freire, M.O.; de Andrade Braga, V.; de Albuqeurque, T.M.R.; Lagranha, C.J.; de Brito Alves, J.L. Limosilactobacillus fermentum prevent gut-kidney oxidative damage and the rise in blood pressure in male rat offspring exposed to a maternal high-fat diet. J. Dev. Orig. Health Dis. 2022, 19, 1–8. [Google Scholar]
- Nguyen, L.T.; Mak, C.H.; Chen, H.; Zaky, A.A.; Wong, M.G.; Pollock, C.A.; Saad, S. SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet. Nutrients 2019, 11, 146. [Google Scholar] [CrossRef]
- Larkin, B.P.; Saad, S.; Glastras, S.J.; Nguyen, L.T.; Hou, M.; Chen, H.; Wang, R.; Pollock, C.A. Low-dose hydralazine during gestation reduces renal fibrosis in rodent offspring exposed to maternal high fat diet. PLoS ONE 2021, 16, e0248854. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N oxide. J. Nutr. Biochem. 2021, 93, 108630. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Martínez Gascón, L.E.; Ortiz, M.C.; Galindo, M.; Sanchez, J.M.; Sancho-Rodriguez, N.; Albaladejo Otón, M.D.; Rodriguez Mulero, M.D.; Rodriguez, F. Role of heme oxygenase in the regulation of the renal hemodynamics in a model of sex dependent programmed hypertension by maternal diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R181–R191. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Lee, C.T.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine prevents programmed hypertension in male rat offspring born to suramin-treated mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef]
- Svitok, P.; Okuliarova, M.; Varga, I.; Zeman, M. Renal impairment induced by prenatal exposure to angiotensin II in male rat offspring. Exp. Biol. Med. 2019, 244, 923–931. [Google Scholar] [CrossRef]
- Vieira, L.D.; Farias, J.S.; de Queiroz, D.B.; Cabral, E.V.; Lima-Filho, M.M.; Sant’Helena, B.R.M.; Aires, R.S.; Ribeiro, V.S.; SantosRocha, J.; Xavier, F.E.; et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: Prevention by early treatment with α-tocopherol. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3577–3587. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef] [PubMed]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Nguyen, L.T.; Anwer, A.G.; Zaky, A.A.; Santiyanont, R.; Oliver, B.G.; Goldys, E.; Pollock, C.A.; et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018, 8, 6631. [Google Scholar] [CrossRef]
- Jeje, S.O.; Akindele, O.O.; Ushie, G.; Rajil, Y. Changes in kidney function and oxidative stress biomarkers in offspring from dams treated with dexamethasone during lactation in Wistar rats. Afr. J. Med. Med. Sci. 2016, 45, 237–242. [Google Scholar] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Shaltout, H.A.; Rose, J.C.; Diz, D.I.; Chappell, M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 2011, 57, 620–626. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Tain, Y.L.; Hsu, C.N. Developmental Origins of Chronic Kidney Disease: Should We Focus on Early Life? Int. J. Mol. Sci. 2017, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.V.; Hora, S.; Pal, A.; Jha, S.; Taneja, R. Stressing the (Epi) Genome: Dealing with Reactive Oxygen Species in Cancer. Antioxid. Redox Signal. 2018, 29, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Bovee, R.C.; Thomas, D.D. Nitric oxide, the new architect of epigenetic landscapes. Nitric Oxide 2016, 59, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Hsieh, C.S.; Chang, K.A.; Tain, Y.L. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int. J. Mol. Sci. 2012, 13, 14606–14622. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Lunkenbein, S.; Ströhle, A.; Hahn, A. Antioxidants in food: Mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 2012, 52, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Nexo, E. Gastrointestinal Handling of Water-Soluble Vitamins. Compr. Physiol. 2018, 8, 1291–1311. [Google Scholar] [PubMed]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation-Induced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef]
- Farias, J.S.; Santos, K.M.; Lima, N.K.S.; Cabral, E.V.; Aires, R.S.; Veras, A.C.; Paixão, A.D.; Vieira, L.D. Maternal endotoxemia induces renal collagen deposition in adult offspring: Role of NADPH oxidase/TGF-β1/MMP-2 signaling pathway. Arch. Biochem. Biophys. 2020, 684, 108306. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 3, CD007176. [Google Scholar] [CrossRef]
- Azaïs-Braesco, V.; Pascal, G. Vitamin A in pregnancy: Requirements and safety limits. Am. J. Clin. Nutr. 2000, 71, 1325S–1333S. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.; Rodushkin, I.; Genuis, S.J. Heavy metal contamination of prenatal vitamins. Toxicol. Rep. 2018, 5, 390–395. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Amino Acid Metabolism in the Kidneys: Nutritional and Physiological Significance. Adv. Exp. Med. Biol. 2020, 1265, 71–95. [Google Scholar] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early Origins of Hypertension: Should Prevention Start Before Birth Using Natural Antioxidants? Antioxidants 2020, 9, 1034. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tain, Y.L.; Sheen, J.M.; Huang, L.T. Melatonin utility in neonates and children. J. Formos. Med. Assoc. 2012, 111, 57–66. [Google Scholar] [CrossRef]
- Aversa, S.; Pellegrino, S.; Barberi, I.; Reiter, R.J.; Gitto, E. Potential utility of melatonin as an antioxidant during pregnancy and in the perinatal period. J. Matern. Fetal Neonatal Med. 2012, 25, 207–221. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Reiter, R.J.; Gitto, E. Potential utility of melatonin in preeclampsia, intrauterine fetal growth retardation, and perinatal asphyxia. Reprod. Sci. 2016, 23, 970–977. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18, 426. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise over-view on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Bao, H.; Peng, A. The Green Tea Polyphenol (-)-epigallocatechin-3-gallate and its beneficial roles in chronic kidney disease. J. Transl. Int. Med. 2016, 4, 99–103. [Google Scholar] [CrossRef]
- Guerreiro, Í.; Ferreira-Pêgo, C.; Carregosa, D.; Santos, C.N.; Menezes, R.; Fernandes, A.S.; Costa, J.G. Polyphenols and Their Metabolites in Renal Diseases: An Overview. Foods 2022, 11, 1060. [Google Scholar] [CrossRef]
- Roumes, H.; Goudeneche, P.; Pellerin, L.; Bouzier-Sore, A.K. Resveratrol and Some of Its Derivatives as Promising Prophylactic Treatments for Neonatal Hypoxia-Ischemia. Nutrients 2022, 14, 3793. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, J.; Xu, H.; Lyv, Y.; Feng, X.; Fang, Y.; Xu, Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014, 53, 1669–1683. [Google Scholar] [CrossRef]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from 926 clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol 957 in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Salamon, S.; Kramar, B.; Marolt, T.P.; Poljšak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014, 41, 4–10. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pavlenko, T.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Silachev, D.N. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 2017, 284, 3069–3078. [Google Scholar] [CrossRef]
- Johnson, S.T.; Bigam, D.L.; Emara, M.; Obaid, L.; Slack, G.; Korbutt, G. N-acetylcysteine improves the hemodynamics and oxidative stress in hypoxic newborn pigs reoxygenated with 100% oxygen. Shock 2007, 28, 484–490. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Smith, R.A.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tintoré, M.; Sastre-Garriga, J. Multiple sclerosis: Dimethyl fumarate is coming of age. Nat. Rev. Neurol. 2016, 12, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Yu, H.R.; Lin, I.C.; Sheen, J.M.; Huang, L.T.; Tain, Y.L. Protection of Male Rat Offspring against Hypertension Programmed by Prenatal Dexamethasone Administration and Postnatal High-Fat Diet with the Nrf2 Activator Dimethyl Fumarate during Pregnancy. Int. J. Mol. Sci. 2019, 20, 3957. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Koeners, M.P.; Braam, B.; Joles, J.A. Perinatal inhibition of NF-kappaB has long-term antihypertensive effects in spontaneously hypertensive rats. J. Hypertens. 2011, 29, 1160–1166. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Bozikas, A.; Eleftheriadis, T.; Dounousi, E. Antioxidant Supplementation in Renal Replacement Therapy Patients: Is There Evidence? Oxidative Med. Cell. Longev. 2019, 2019, 9109473. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef]
- Yuksel, S.; Yigit, A.A.; Cinar, M.; Atmaca, N.; Onaran, Y. Oxidant and Antioxidant Status of Human Breast Milk during Lactation Period. Dairy Sci. Technol. 2015, 95, 295–302. [Google Scholar] [CrossRef]
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, D.L.; Pritchard, D.E.; Singh, J.; Owens, B.M.; Blankenship, L.J.; Orenstein, J.M.; Patierno, S.R. Apoptosis and P53 induction in human lung fibroblasts exposed to chromium (VI): Effect of ascorbate and tocopherol. Toxicol. Sci. 2000, 55, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Okamura, D.M.; Himmelfarb, J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr. Nephrol. 2009, 24, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Cox, S.N. Biomarkers and Precision Medicine in IgA Nephropathy. Semin. Nephrol. 2018, 38, 521–530. [Google Scholar] [CrossRef]
- Jin, C.; Wu, P.; Li, L.; Xu, W.; Qian, H. Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases. Stem Cells Int. 2021, 2021, 7844455. [Google Scholar] [CrossRef]

| Animal Models | Species/ Gender | Age at Evaluation | Mechanisms of Oxidative Stress | Renal Outcomes | Ref. |
|---|---|---|---|---|---|
| Maternal nutritional insults | |||||
| Maternal caloric restriction diet | SD rat/M | 12 weeks | ↑ ADMA, ↓ NO, ↑ renal 8-OHdG expression | ↓nephron number, glomerular hypertrophy, ↑ tubulointerstitial injury, hypertension | [47,48] |
| Maternal protein restriction diet | Wistar rat/M | 12 weeks | ↑ F2-isoprostane, ↓ glutathione | ↑BP | [49] |
| Maternal high-fructose diet | SD rat/M | 12 weeks | ↓ NO, ↑renal 8-OHdG expression | ↑BP | [50] |
| Maternal plus post-weaning high-fructose diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [51] |
| Maternal methyl-deficient diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [52] |
| Maternal high-methyl-donor diet | SD rat/M | 12 weeks | ↑ renal 8-OHdG expression | ↑BP | [52] |
| Maternal iron deficiency | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression | ↑BP | [53] |
| Maternal high-fat and high-cholesterol diet | SD rat/M and F | 90 days | ↓ SOD activity in M, ↑ renal MDA level in F | ↑BP | [54] |
| Maternal plus post-weaning high-fat diet | SD rat/M and F | 16 weeks | ↓ NO, ↑renal 8-OHdG expression | ↑BP, ↑kidney injury in M | [55,56] |
| Maternal high-fat and high-cholesterol diet | SD rat/M | 18 weeks | ↑renal MDA, ↓antioxidant enzymatic activity | hypertension, impaired renal function | [57] |
| Maternal high-fat diet | C57BL/6 mice/M | 9 weeks | ↑renal 8-OHdG expression | ↑renal hypertrophy, ↑albuminuria | [58] |
| Maternal high-fat diet | C57BL/6 mice/M | 32 weeks | ↑ renal 3-NT, ↑ renal NOX2 expression | ↑renal global DNA methylation, ↑albuminuria, ↑glomerulosclerosis | [59] |
| Maternal disorders | |||||
| Maternal L-NAME administration | SD rat/M | 12 weeks | ↑ renal F2-isoprostane | ↑BP | [60] |
| Maternal ADMA administration | SD rat/M | 12 weeks | ↓ NO | ↑BP | [61] |
| Streptozotocin-induced diabetes | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA | ↓nephron number,↑ tuburointerstitial injury | [62] |
| Streptozotocin-induced diabetes | SD rat/M | 12 weeks | ↑ renal TBARS, ↑3-NT | ↑BP, discurbed acute renal hemodynamics | [63] |
| Maternal suramin administration | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA | ↑BP | [64] |
| Maternal adenine-induced CKD | SD rat/M | 12 weeks | ↓ NO, ↑ ADMA, ↑ renal 8-OHdG expression, | ↑BP, ↑renal hypertrophy | [65,66] |
| Reduced uterine perfusion | SD rat/M | 16 weeks | ↑ urinary F2-isoprostane level and renal NADPH-oxidase-dependent superoxide | ↑BP | [67] |
| Maternal angiotensin II administration | Wistar rat/M | 18 week | ↑ renal ROS | ↑BP, ↑tuburointerstitial injury | [68] |
| Prenatal LPS Exposure | Wistar rat/M | 28 weeks | ↑ renal MDA | ↑BP | [69] |
| Toxins | |||||
| Prenatal bisphenol A exposure plus high-fat diet | SD rat/M | 16 weeks | ↑ ADMA, ↓ NO, ↑renal 8-OHdG expression | ↑BP | [70] |
| Prenatal dexamethasone plus TCDD exposure | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression, ↑ ADMA | ↑BP | [71] |
| Maternal di-n-butyl phthalate exposure | SD rat/M and F | 18 months | ↑ renal ROS | Renal dysplasia,↑ tuburointerstitial injury | [72] |
| Matenal smoking exposure | Balb/c mice/M | 13 weeks | ↑ renal ROS | ↓nephron number,↑albuminuria | [73] |
| Medication and Drugs | |||||
| Dexamethasone administration during lactation | Wistar rat/M and F | 12 weeks | ↑renal MDA level, ↓SOD and catalase activity | ↑Tubular necrosis, renal dysfunction | [74] |
| Prenatal dexamethasone exposure | SD rat/M | 16 weeks | ↓ renal NO | ↑BP | [75] |
| Prenatal dexamethasone exposure plus postnatal high-fat intake | SD rat/M | 16 weeks | ↑ renal 8-OHdG expression, ↓ NO | ↑BP | [76] |
| Prenatal betamethasone exposure | Sheep/M and F | 18 months | ↓ NO,↑ ROS | ↑BP | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality. Antioxidants 2023, 12, 13. https://doi.org/10.3390/antiox12010013
Tain Y-L, Hsu C-N. Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality. Antioxidants. 2023; 12(1):13. https://doi.org/10.3390/antiox12010013
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2023. "Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality" Antioxidants 12, no. 1: 13. https://doi.org/10.3390/antiox12010013
APA StyleTain, Y.-L., & Hsu, C.-N. (2023). Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality. Antioxidants, 12(1), 13. https://doi.org/10.3390/antiox12010013







