Sustainable Extraction Methods Affect Metabolomics and Oxidative Stability of Myrtle Seed Oils Obtained from Myrtle Liqueur By-Products: An Electron Paramagnetic Resonance and Mass Spectrometry Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Myrtle Oil Extraction
2.4. Myrtle Oils Characterization
2.4.1. Fatty Acids Composition
2.4.2. Determination of Peroxide Value
2.5. Analysis of Phenolic Compounds and Antioxidant Activity
2.5.1. Extraction of Phenolic Compounds
2.5.2. Total Phenolic Concentration
2.5.3. LC-MS Identification of Phenolic Compounds
Sample Preparation
LC-ESI-Orbitrap-MS and LC-ESI-Orbitrap-MS/MS Analysis
Determination of Myricetin and Its Derivatives by UPLC–ESI-QTrap-MS/MS Analyses in MRM (Multiple Reaction Monitoring) Modality
2.5.4. Radical Scavenging Activity of Myrtle Oils
2.6. Thermal Treatment and EPR Spin Trapping Analysis of Myrtle Seed Oils: EPR Settings and Spectra Acquisition
2.7. Statistical Analysis
3. Results and Discussion
3.1. Oil Yield and Peroxide Values of Myrtle Oils
3.2. Fatty Acids Composition of the Oils
3.3. Oils Phenolic Compounds and Antioxidant Activity
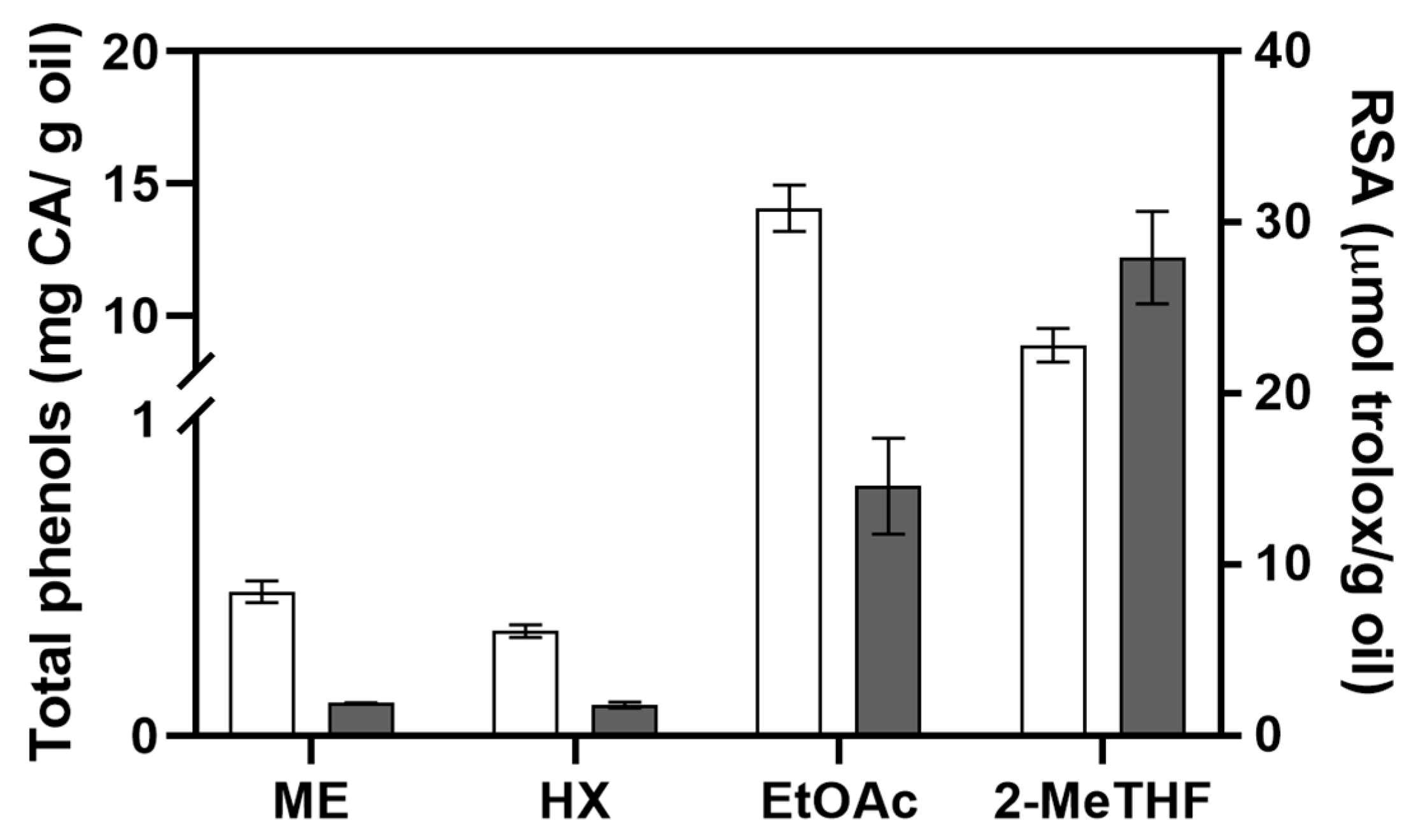
3.3.1. Oil Total Phenols and Antioxidant Activity
3.3.2. LC-ESI-Orbitrap-MS and LC-ESI-Orbitrap-MS/MS Analysis
3.3.3. Quantitative Analysis of Myricetin and Its Derivatives
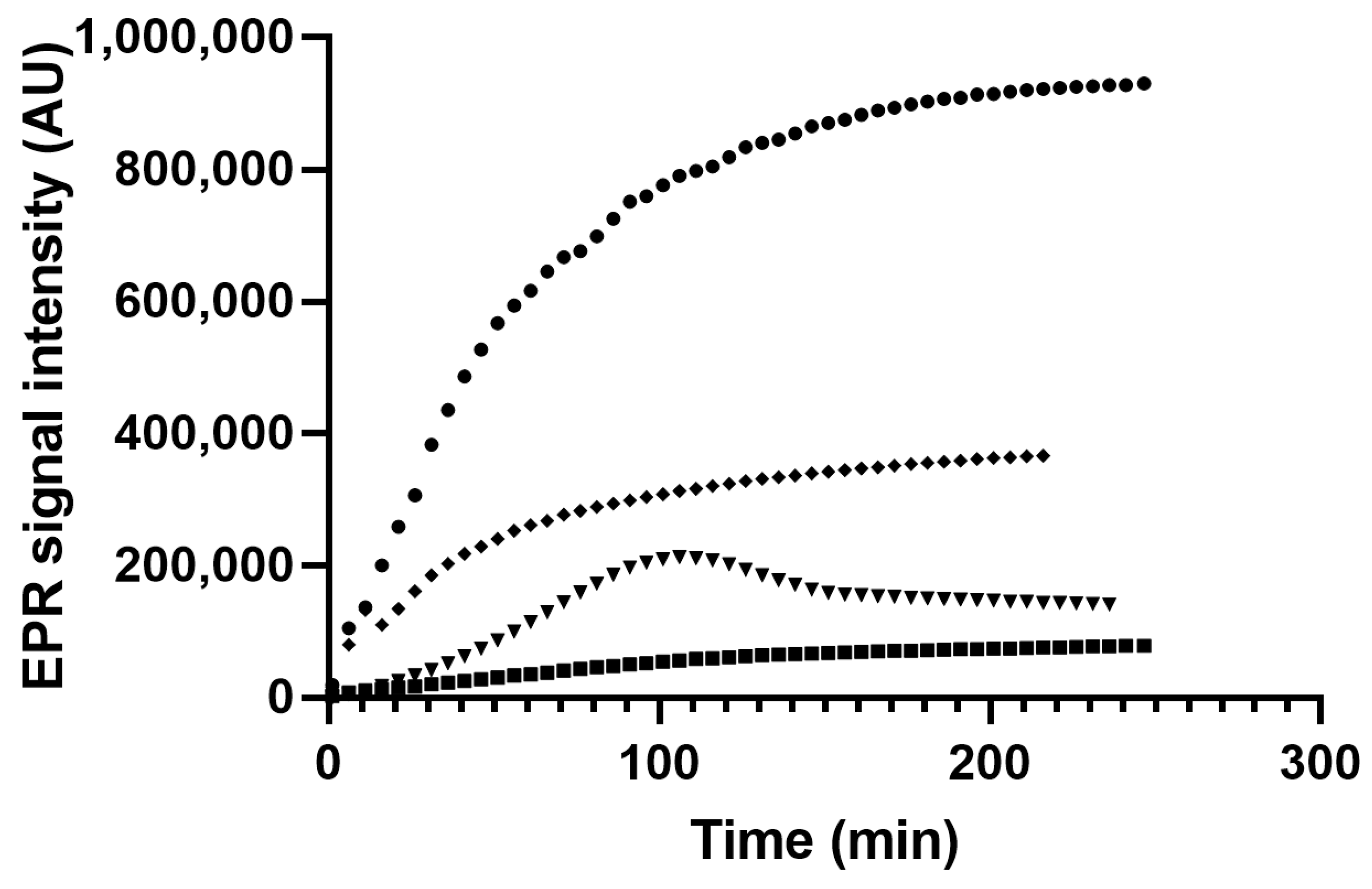
3.4. EPR Spin Trapping of Myrtle Seed Oils Subjected to Thermal Treatment
3.4.1. Optimization of the Assay Temperature
3.4.2. Thermal Treatment of Myrtle Oil
3.4.3. Thermal Treatment of Myrtle Oils Obtained with Mechanical and Chemical Methods
3.4.4. Relationship between EPR Intensity and Composition of Oils Subjected to Thermal Treatment
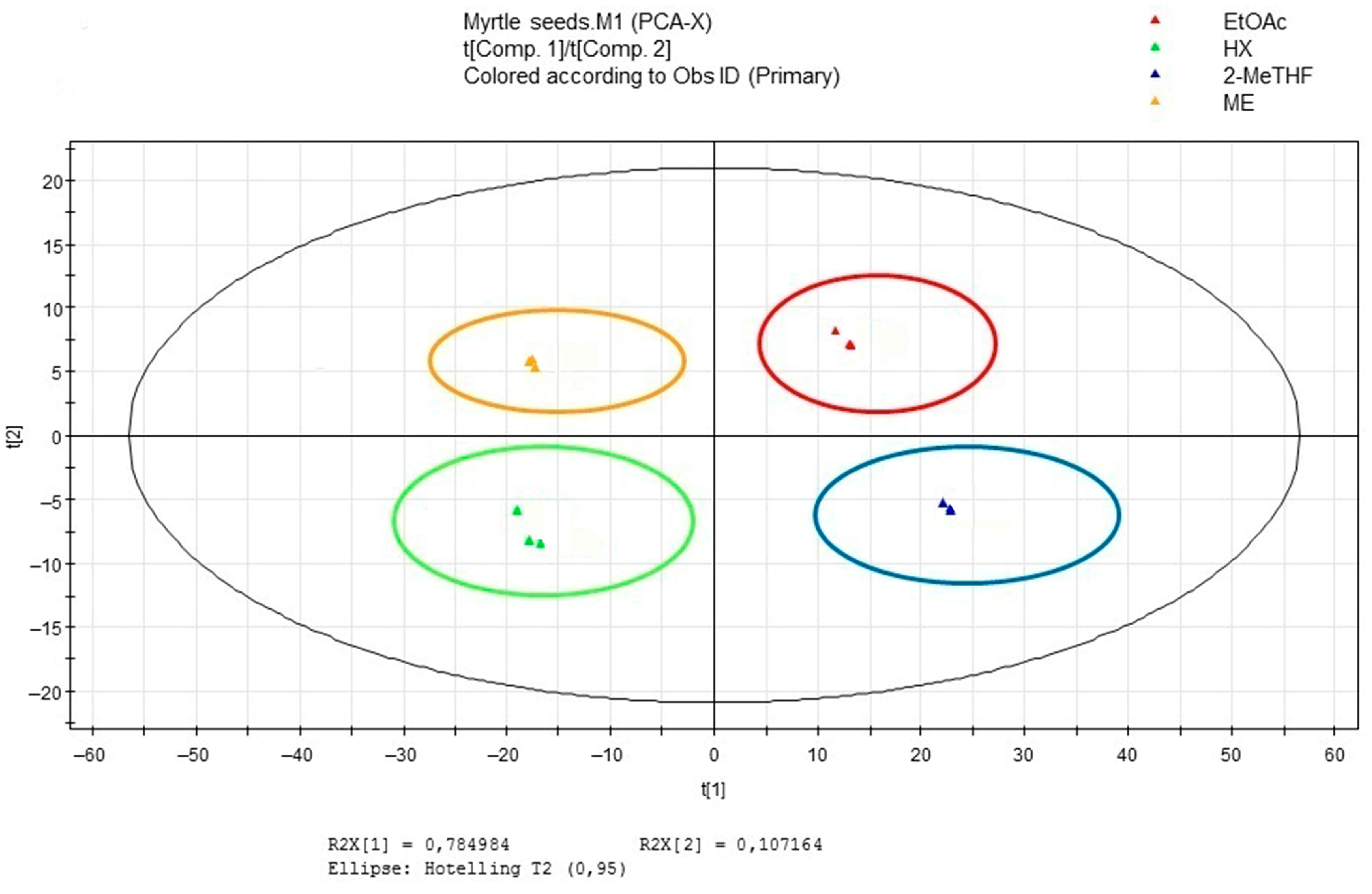
3.5. Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Sicaire, A.-G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative Bio-Based Solvents for Extraction of Fat and Oils: Solubility Prediction, Global Yield, Extraction Kinetics, Chemical Composition and Cost of Manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- DeSimone, J.M. Practical approaches to green solvents. Science 2002, 297, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Lohani, U.C.; Fallahi, P.; Muthukumarappan, K. Comparison of Ethyl Acetate with Hexane for Oil Extraction from Various Oilseeds. J. Am. Oil Chem. Soc. 2015, 92, 743–754. [Google Scholar] [CrossRef]
- Oladipo, B.; Betiku, E. Process optimization of solvent extraction of seed oil from Moringa oleifera: An appraisal of quantitative and qualitative process variables on oil quality using D-optimal design. Biocatal. Agric. Biotechnol. 2019, 20, 101187. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Dakhlaoui, S.; Msaada, K.; Saidani Tounsi, M.; Ksouri, R.; Fauconnier, M.-L.; Hamrouni-Sellami, I. Green extraction of oil from Carum carvi seeds using bio-based solvent and supercritical fluid: Evaluation of its antioxidant and anti-inflammatory activities. Phytochem. Anal. 2020, 31, 37–45. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content, and antioxidant capacity. LWT 2019, 110, 346–352. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Ben Kaab, S.; Hammami, M.; Dakhlaoui, S.; Sawsen, S.; Msaada, K.; Isoda, H.; Ksouri, R.; Fauconnier, M.-L. Green Solvent to Substitute Hexane for Bioactive Lipids Extraction from Black Cumin and Basil Seeds. Foods 2021, 10, 1493. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Akca, S.; Akpinar, A. The Effects of Grape, pomegranate, Sesame Seed Powder and Their Oils on Probiotic Ice Cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Deng, Z.; Pan, X.; Xie, X.; Peng, C. Effective utilization of food wastes: Bioactivity of grape seed extraction and its application in food industry. J. Funct. Foods 2020, 73, 104113. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Cruciani, S.; Santaniello, S.; Garroni, G.; Fadda, A.; Balzano, F.; Bellu, E.; Sarais, G.; Fais, G.; Mulas, M.; Maioli, M. Myrtus Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules 2019, 24, 1515. [Google Scholar] [CrossRef] [Green Version]
- Fadda, A.; Sarais, G.; Lai, C.; Sale, L.; Mulas, M. Control of postharvest diseases caused by Penicillium spp. with myrtle leaf phenolic extracts: In vitro and in vivo study on mandarin fruit during storage. J. Sci. Food Agric. 2021, 101, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.M.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Vrhovsek, U.; Mattivi, F.; Manca, G. Myrtle Seeds (Myrtus communis L.) as a Rich Source of the Bioactive Ellagitannins Oenothein B and Eugeniflorin D2. ACS Omega 2019, 4, 15966–15974. [Google Scholar] [CrossRef] [Green Version]
- Sanna, D.; Mulas, M.; Molinu, M.G.; Fadda, A. Oxidative stability of plant hydroalcoholic extracts assessed by EPR spin trapping under forced ageing conditions: A myrtle case study. Food Chem. 2019, 271, 753–761. [Google Scholar] [CrossRef]
- Wannes, W.A.; Marzouk, B. Characterization of myrtle seed (Myrtus communis var. baetica) as a source of lipids, phenolics, and antioxidant activities. J. Food Drug Anal. 2016, 24, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Correddu, F.; Maldini, M.; Addis, R.; Petretto, G.L.; Palomba, M.; Battacone, G.; Pulina, G.; Nudda, A.; Pintore, G. Myrtus Communis Liquor Byproduct as a Source of Bioactive Compounds. Foods 2019, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, S.; Santaniello, S.; Fadda, A.; Sale, L.; Sarais, G.; Sanna, D.; Mulas, M.; Ginesu, G.C.; Cossu, M.L.; Serra, P.A.; et al. Extracts from Myrtle Liqueur Processing Waste Modulate Stem Cells Pluripotency under Stressing Conditions. BioMed Res. Int. 2019, 2019, 5641034. [Google Scholar] [CrossRef] [Green Version]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, Y.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the oxidative stability of edible oil by electron spin resonance spectroscopy. Food Chem. 2020, 309, 125714. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Merkx, D.W.H.; Plankensteiner, L.; Yu, Y.; Wierenga, P.A.; Hennebelle, M.; Van Duynhoven, J.P.M. Evaluation of PBN spin-trapped radicals as early markers of lipid oxidation in mayonnaise. Food Chem. 2021, 334, 127578. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Andersen, M.L.; Skibsted, L.H. ESR spin trapping for in situ detection of radicals involved in the early stages of lipid oxidation of dried microencapsulated oils. Food Chem. 2021, 341, 128227. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, M.; Szterk, A.; Zawada, K.; Zabkowski, T. New Opportunities of the Application of Natural Herb and Spice Extracts in Plant Oils: Application of Electron Paramagnetic Resonance in Examining the Oxidative Stability. J. Food Sci. 2012, 77, C994–C999. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, M.; Zawada, K. Evaluation of oxidative stability of vegetable oils enriched with herb extracts by EPR spectroscopy. Chem. Pap. 2015, 69, 950–957. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Zaccheria, F.; Psaro, R.; Ravasio, N.; Bondioli, P. Standardization of vegetable oils composition to be used as oleochemistry feedstock through a selective hydrogenation process. Eur. J. Lipid Sci. Technol. 2012, 114, 24–30. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Hrncirik, K.; Fritsche, S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive oil. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Pardo, J.E.; Alvarruiz, A. Storage stability and composition changes of three cold-pressed nut oils under refrigeration and room temperature conditions. Food Chem. 2018, 259, 31–35. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Sarais, G.; Lai, C.; Pizza, C.; Montoro, P. LC-MS based metabolomics study of different parts of myrtle berry from Sardinia (Italy). J. Berry Res. 2017, 7, 217–229. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 2 (R1)Validation of Analytical Procedures:Text and Methodology; ICH Secretariat: Geneva, Switzerland, 1995. [Google Scholar]
- Fadda, A.; Serra, M.; Molinu, M.G.; Azara, E.; Barberis, A.; Sanna, D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014, 35, 112–119. [Google Scholar] [CrossRef]
- D’Urso, G.; d’Aquino, L.; Pizza, C.; Montoro, P. Integrated mass spectrometric and multivariate data analysis approaches for the discrimination of organic and conventional strawberry (Fragaria ananassa Duch.) crops. Food Res. Int. 2015, 77, 264–272. [Google Scholar] [CrossRef]
- Dursun Capar, T.; Dedebas, T.; Yalcin, H.; Ekici, L. Extraction method affects seed oil yield, composition, and antioxidant properties of European cranberrybush (Viburnum opulus). Ind. Crops Prod. 2021, 168, 113632. [Google Scholar] [CrossRef]
- Fadda, A.; Molinu, M.G.; Deiana, P.; Sanna, D. Electron Paramagnetic Resonance Spin Trapping of Sunflower and Olive Oils Subjected to Thermal Treatment: Optimization of Experimental and Fitting Parameters. ACS Food Sci. Technol. 2021, 1, 1294–1303. [Google Scholar] [CrossRef]
- Pal, U.S.; Patra, R.K.; Sahoo, N.R.; Bakhara, C.K.; Panda, M.K. Effect of refining on quality and composition of sunflower oil. J. Food Sci. Technol. 2015, 52, 4613–4618. [Google Scholar] [CrossRef] [Green Version]
- Castejón, N.; Luna, P.; Señoráns, F.J. Alternative oil extraction methods from Echium plantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018, 244, 75–82. [Google Scholar] [CrossRef]
- Messaoud, C.; Laabidi, A.; Boussaid, M. Myrtus communis L. Infusions: The Effect of Infusion Time on Phytochemical Composition, Antioxidant, and Antimicrobial Activities. J. Food Sci. 2012, 77, C941–C947. [Google Scholar] [CrossRef]
- Rached, W.; Bennaceur, M.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Carvalho, A.M.; Marouf, A.; Ferreira, I.C.F.R. Detailed phytochemical characterization and bioactive properties of Myrtus nivelii Batt & Trab. Food Funct. 2017, 8, 3111–3119. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT-Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- D’Urso, G.; Napolitano, A.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Okra fruit: LC-ESI/LTQOrbitrap/MS/MSn based deep insight on polar lipids and specialized metabolites with evaluation of anti-oxidant and anti-hyperglycemic activity. Food Funct. 2020, 11, 7856–7865. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.O.; Rodríguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef] [PubMed]
- De Leon Izeppi, G.A.; Dubois, J.-L.; Balle, A.; Soutelo-Maria, A. Economic risk assessment using Monte Carlo simulation for the production of azelaic acid and pelargonic acid from vegetable oils. Ind. Crops Prod. 2020, 150, 112411. [Google Scholar] [CrossRef]
- Khan, Z.S.; Mandal, A.; Maske, S.; Ahammed Shabeer, T.P.; Gaikwad, N.; Shaikh, S.; Banerjee, K. Evaluation of fatty acid profile in seed and oil of Manjari Medika, a novel Indian grape cultivar and its comparison with Cabernet Sauvignon and Sauvignon Blanc. Sustain. Chem. Pharm. 2020, 16, 100253. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.; Wang, X.; Jin, Q.; Xu, X.; Shen, Y.; Xu, G.; Zhang, H. Analysis of the volatile components of tea seed oil (Camellia sinensis O. Ktze) from China using HS-SPME-GC/MS. Int. J. Food Sci. Technol. 2016, 51, 2591–2602. [Google Scholar] [CrossRef]
- Silva, S.I.; Oliveira, A.F.M.; Negri, G.; Salatino, A. Seed oils of Euphorbiaceae from the Caatinga, a Brazilian tropical dry forest. Biomass Bioenergy 2014, 69, 124–134. [Google Scholar] [CrossRef]
- Appendino, G.; Maxia, L.; Bettoni, P.; Locatelli, M.; Valdivia, C.; Ballero, M.; Stavri, M.; Gibbons, S.; Sterner, O. Antibacterial Galloylated Alkylphloroglucinol Glucosides from Myrtle (Myrtus communis). J. Nat. Prod. 2006, 69, 251–254. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure–antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Dias, F.F.G.; Augusto-Obara, T.R.; Hennebelle, M.; Chantieng, S.; Ozturk, G.; Taha, A.Y.; Vieira, T.M.F.d.S.; Leite Nobrega de Moura Bell, J.M. Effects of industrial heat treatments on bovine milk oxylipins and conventional markers of lipid oxidation. Prostaglandins Leukot. Essent. Fat. Acids 2020, 152, 102040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef]
- Caño-Ochoa, S.d.; Ruiz-Aracama, A.; Guillén, M.D. Alpha-Tocopherol, a Powerful Molecule, Leads to the Formation of Oxylipins in Polyunsaturated Oils Differently to the Temperature Increase: A Detailed Study by Proton Nuclear Magnetic Resonance of Walnut Oil Oxidation. Antioxidants 2022, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Ugurlu, P.; Celik, C.; Sen, N.B.; Helvacıoglu, S.; Charehsaz, M.; Erdogan, M.; Ockun, M.A.; Kırmızıbekmez, H.; Aydın, A.; et al. Myrtus communis L. (Myrtle) plant parts: Comparative assessment of their chemical compositions and antioxidant, anticancer, and antimutagenic activities. South Afr. J. Bot. 2022, 150, 711–720. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B.; Selamat, J.; Yusoff, M.S.A. Application of arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J. Am. Oil Chem. Soc. 2001, 78, 1133. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Cui, L.; Lahti, P.M.; Decker, E.A. Evaluating Electron Paramagnetic Resonance (EPR) to Measure Lipid Oxidation Lag Phase for Shelf-Life Determination of Oils. J. Am. Oil Chem. Soc. 2017, 94, 89–97. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Pasqualone, A.; Summo, C.; Caponio, F. An “Omics” Approach for Lipid Oxidation in Foods: The Case of Free Fatty Acids in Bulk Purified Olive Oil. Eur. J. Lipid Sci. Technol. 2018, 120, 1800102. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS Metabolomic Analysis of Fennel Waste (Foeniculum vulgare Mill.) as a Byproduct Rich in Bioactive Compounds. Foods 2021, 10, 1893. [Google Scholar] [CrossRef]
- Mari, A.; Lyon, D.; Fragner, L.; Montoro, P.; Piacente, S.; Wienkoop, S.; Egelhofer, V.; Weckwerth, W. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics 2013, 9, 599–607. [Google Scholar] [CrossRef]
- Ruan, L.; Lu, L.; Zhao, X.; Xiong, W.; Xu, H.; Wu, S. Effects of natural antioxidants on the oxidative stability of Eucommia ulmoides seed oil: Experimental and molecular simulation investigations. Food Chem. 2022, 383, 132640. [Google Scholar] [CrossRef] [PubMed]
| Oil Extraction Methods | ||||
|---|---|---|---|---|
| ME | HX | 2-MeTHF | EtOAc | |
| Chemical and spectroscopic parameters | ||||
| Yield y | 3.21 ± 0.27 b | 9.37 ± 0.62 a | 10.55 ± 2.82 a | 5.41 ± 0.42 b |
| PV z | 99.91 ± 4.47 b | 99.51 ± 5.53 b | 121.38 ± 6.45 a | 126.96 ± 3.89 a |
| AUC | 2.9 × 107 ± 5.5 × 106 c | 1.5 × 108 ± 1.3 × 107 a | 6.8 × 107 ± 1.3 × 107 b | 9.4 × 106 ± 4.4 × 106 c |
| Fatty acids (%) | ||||
| C16:0 | 7.5 ± 0.1 a | 7.5 ± 0.2 a | 7.5 ± 0.3 a | 7.4 ± 0.3 a |
| C18:0 | 3.3 ± 0.1 a | 3.3 ± 0.1 a | 3.3 ± 0.1 a | 3.2 ± 0.2 a |
| C18:1 | 9.2 ± 0.2 a | 8.8 ± 0.3 a | 8.9 ± 0.5 a | 9.0 ± 0.1 a |
| C18:2 | 78.0 ± 0.1 a | 77.3 ± 0.4 ab | 76.7 ± 0.6 b | 77.8 ± 0.2 a |
| FFA | 1.2 ± 0.1 c | 3.1 ± 0.9 b | 6.5 ± 0.5 a | 7.0 ± 0.6 a |
| Peak N° | RT | [M-H]− | MS/MS | Molecular Formula | Δppm | Identity | EtOAc | HX | 2-MeTHF | ME | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.95 | 331.0667 | 313/271/169 | C13H16O10 | 2.22 | monogalloyl-hexose | x | x | x | x | [32] |
| 2 | 2.04 | 481.0617 | 301/275 | C20H18O14 | 1.01 | HHDP-glucose | x | x | x | x | [32] |
| 3 | 3.01 | 633.0726 | 301/421/615 | C34H22O22 | 1.1 | strictinin (DigalloylHHDP glucose) | x | x | x | x | [32] |
| 4 | 3.71 | 343.0667 | 191/169 | C14H16O10 | 1.9 | galloylquinic acid | x | x | x | x | [32] |
| 5 | 4.37 | 305.0660 | 174/270 | C15H14O7 | 1.9 | epigallocatechin | x | - | - | - | [32] |
| 6 | 7.54 | 341.0881 | 161/179 | C15H17O9 | 4.16 | caffeoylhexose | - | - | x | - | [32] |
| 7 | 8.4 | 785.0836 | 301/433/766 | C34H26O22 | 0.21 | tellimagrandin I | x | - | x | - | [32] |
| 8 | 12.3 | 463.0511 | 301 | C20H16O13 | 1.2 | ellagic acid hexoside | x | - | x | - | [32] |
| 9 | 13.96 | 197.0455 | 167.89 | C9H10O5 | 5.7 | syringic acid | x | - | x | x | [40] |
| 10 | 14.6 | 631.0948 | 479/317 | C28H24O17 | 2.9 | myricetin galloyl hexoside | x | - | x | - | [32] |
| 11 | 15.35 | 479.0835 | 317 | C21H20O13 | 3.2 | myricetin-3-O-gal | - | - | x | - | [32] |
| 12 | 16.65 | 469.0056 | 425 | C21H10O13 | 4.01 | valoneic acid dilactone | x | x | [41] | ||
| 13 | 17.99 | 449.0730 | 317 | C20H18O12 | 3.6 | myricetin pentoside | x | - | x | - | [32] |
| 14 | 18.2 | 615.0985 | 463/301 | C28H24O16 | 0.53 | quercetingalloyl-hexoside | x | x | x | x | [32] |
| 15 | 18.6 | 463.0875 | 301 | C21H20O12 | 0.9 | quercetinhexoside | x | x | x | x | [32] |
| 16 | 18.7 | 463.0875 | 317 | C21H20O12 | 0.9 | myricitrin | x | x | x | x | [32] |
| 17 | 20.35 | 300.9990 | 257.00/228.99/185.07 | C14H6O8 | 3.7 | ellagic acid | x | x | - | [32] | |
| 18 | 21.6 | 447.0926 | 301 | C21H20O11 | 1.4 | quercetindeoxy-hexoside | x | x | x | x | [32] |
| 19 | 21.68 | 187.0977 | 125.03 | C9 H16 O4 | 6.6 | azelaic acid | x | x | x | x | [42] |
| 20 | 23.2 | 615.0984 | 317/463 | C28H24O16 | 0.7 | myricetingalloyl-deoxyhexose | x | x | x | x | [32] |
| 21 | 23.53 | 317.0302 | 178.95/151.04 | C15 H10 O8 | 3.2 | myricetin | x | - | x | - | [32] |
| 22 | 23.99 | 497.0363 | 301.05 | C23 H14 O13 | 2.5 | ethylflavogallonate | x | - | x | - | [32] |
| 23 | 24.68 | 567.2082 | 271.03/313.11/211.12/169.03 | C27 H36 O13 | 1.8 | gallomyrtucommulone C | x | x | x | - | [32] |
| 24 | 27.05 | 301.0354 | 178.86/150.88 | C15 H9 O7 | 3.9 | quercetin | x | x | x | x | [32] |
| 25 | 30.24 | 285.0403 | 150.95 | C15H10O6 | 3.4 | kaempferol | x | x | x | x | [32] |
| 26 | 31.02 | 503.3374 | C30H48O6 | 1.36 | terminolic acid (triterpene acid) | x | x | x | x | [32] | |
| 27 | 36.6 | 313.2382 | 295/277/195/ 183/129 | C18H34O4 | 2.8 | 9,10-DiHOME | x | x | x | x | [43] |
| 28 | 37.9 | 311.2225 | 293/275/211/ 201/183/171 | C18H32O4 | 2.7 | 9,10-DiHODE | x | x | x | x | [43] |
| 29 | 40.82 | 295.2275 | 277/195/179/ 171 | C18H31O3 | 2.6 | 9-HODE (10,12) | x | x | x | x | [43] |
| Ref. | Compounds | EtOAc (mg·g−1 ± SD) | HX (mg·g−1 ± SD) | 2-MeTHF (mg·g−1 ± SD) | ME (mg·g−1 ± SD) |
|---|---|---|---|---|---|
| 11 | Myricetin-3-O-gal | 0.12 ± 0.02 | 0.01 ± 0.00 | 1.82 ± 0.02 | 0.11 ± 0.05 |
| 16 | Myricitrin | 4.16 ± 0.17 | 4.67 ± 0.15 | 4.59 ± 0.05 | 4.67 ± 0.19 |
| 21 | Myricetin | 1.65± 0.00 | 0.06 ± 0.02 | 1.46± 0.06 | 0.13 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadda, A.; Montoro, P.; D’Urso, G.; Ravasio, N.; Zaccheria, F.; Sanna, D. Sustainable Extraction Methods Affect Metabolomics and Oxidative Stability of Myrtle Seed Oils Obtained from Myrtle Liqueur By-Products: An Electron Paramagnetic Resonance and Mass Spectrometry Approach. Antioxidants 2023, 12, 154. https://doi.org/10.3390/antiox12010154
Fadda A, Montoro P, D’Urso G, Ravasio N, Zaccheria F, Sanna D. Sustainable Extraction Methods Affect Metabolomics and Oxidative Stability of Myrtle Seed Oils Obtained from Myrtle Liqueur By-Products: An Electron Paramagnetic Resonance and Mass Spectrometry Approach. Antioxidants. 2023; 12(1):154. https://doi.org/10.3390/antiox12010154
Chicago/Turabian StyleFadda, Angela, Paola Montoro, Gilda D’Urso, Nicoletta Ravasio, Federica Zaccheria, and Daniele Sanna. 2023. "Sustainable Extraction Methods Affect Metabolomics and Oxidative Stability of Myrtle Seed Oils Obtained from Myrtle Liqueur By-Products: An Electron Paramagnetic Resonance and Mass Spectrometry Approach" Antioxidants 12, no. 1: 154. https://doi.org/10.3390/antiox12010154






