In Mild and Moderate Acute Ischemic Stroke, Increased Lipid Peroxidation and Lowered Antioxidant Defenses Are Strongly Associated with Disabilities and Final Stroke Core Volume
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessments
2.3. Assays
2.4. Statistics
3. Results
3.1. Demographic and Clinical Data
3.2. Associations between OS/ANTIOX Status and the Diagnostic Groups
3.3. Effects of Time on the OS/ANTIOX Data
3.4. Intercorrelations
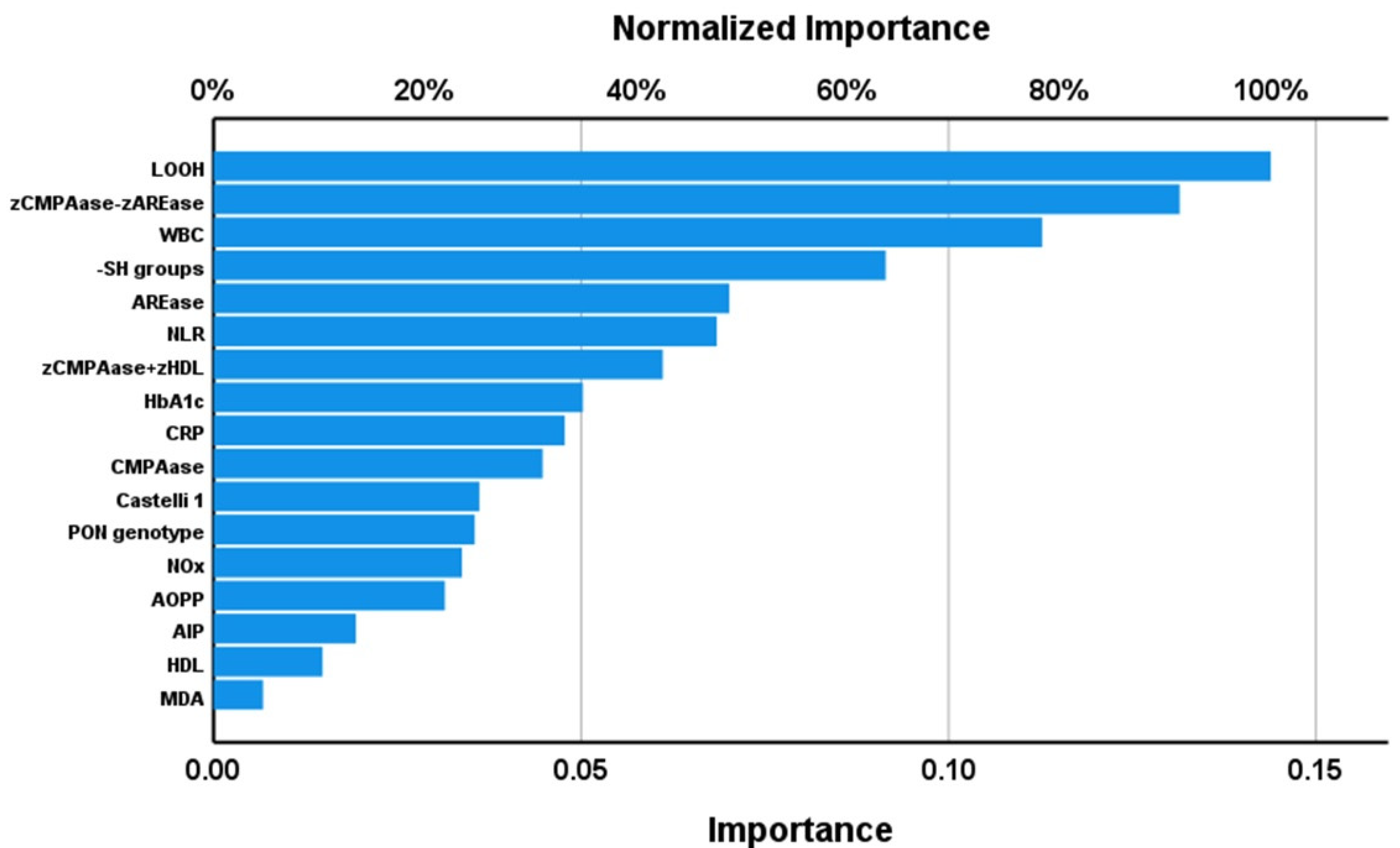
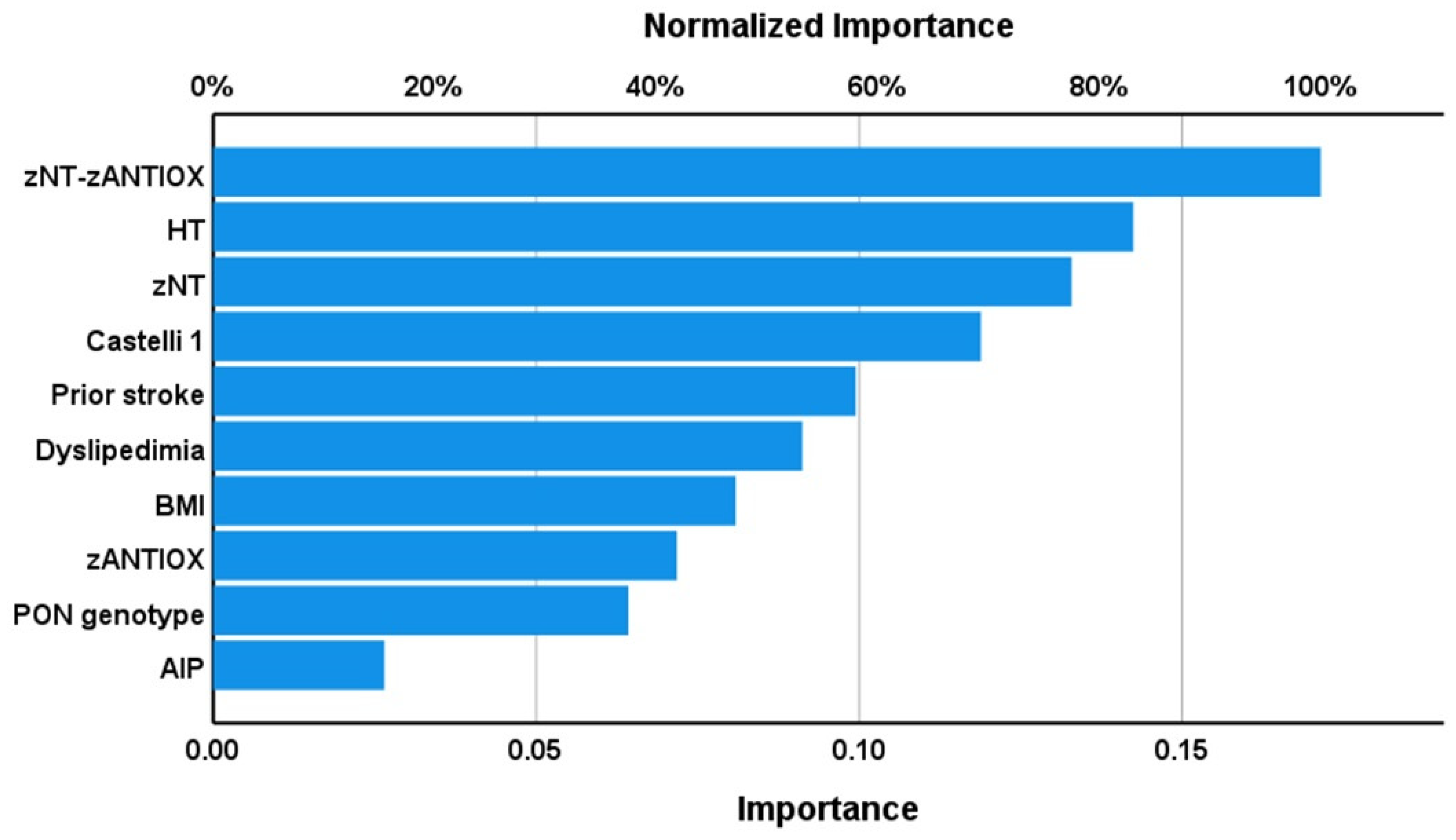
3.5. Results of Multiple Regression Analyses
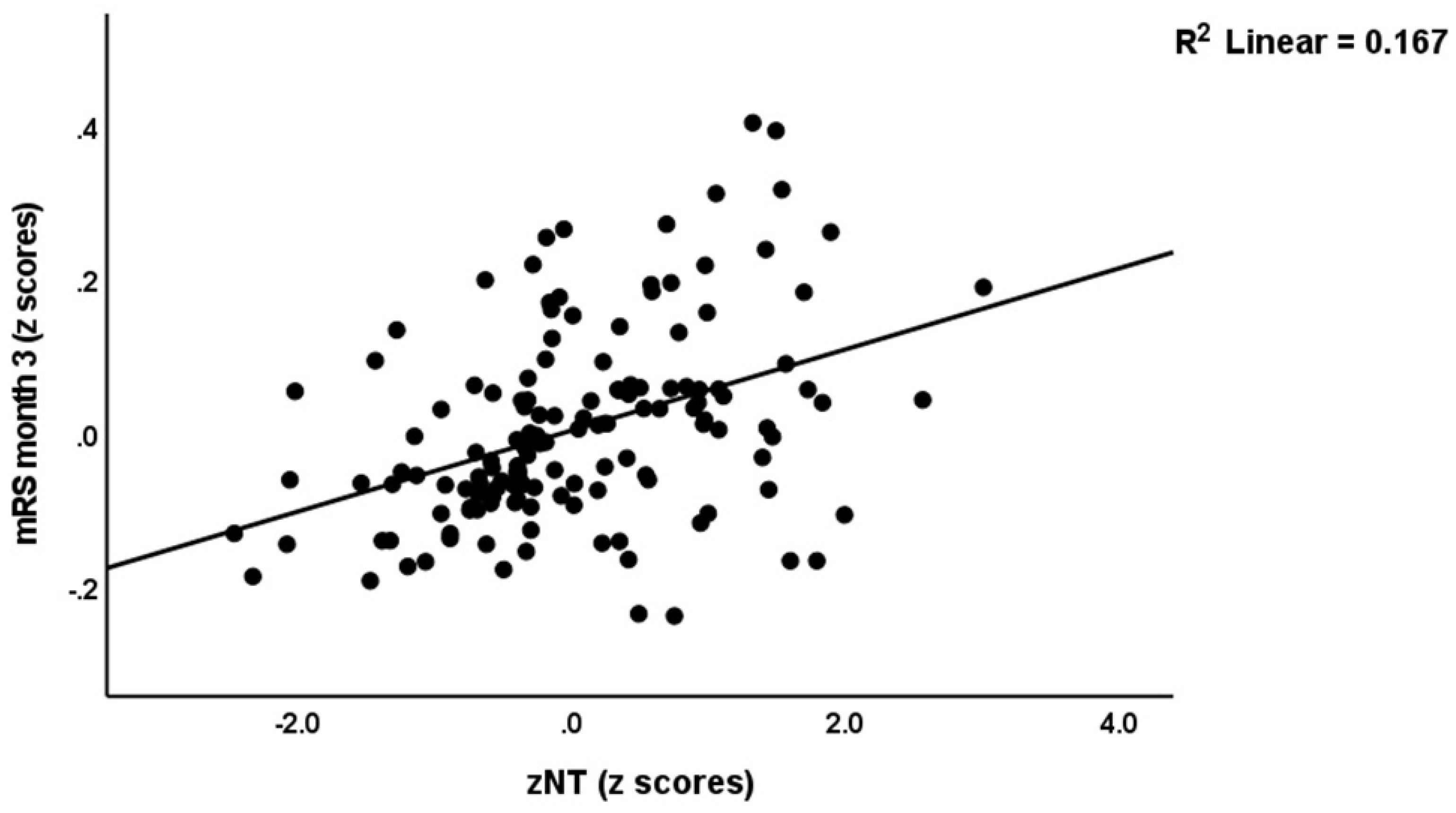
3.6. O&NS and MRI Measurements
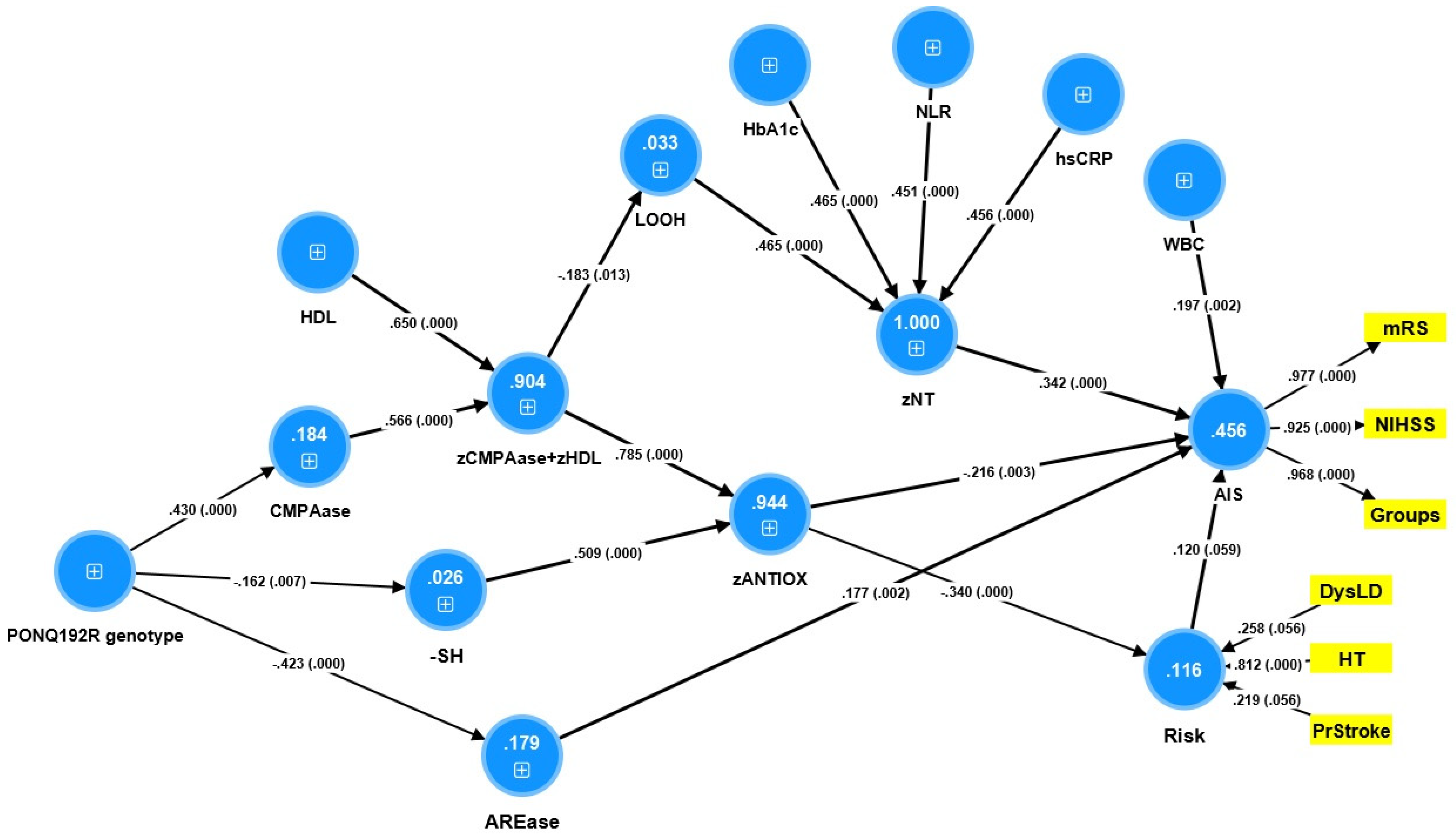
3.7. Results of PLS Analysis
4. Discussion
4.1. Oxidative Stress in AIS
4.2. Antioxidants in AIS
4.3. Oxidative and Antioxidant Markers Predict AIS Outcome
4.4. Oxidative/Antioxidant Markers and Brain Imaging
4.5. PON1 Gene and AIS
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- World Stroke Organization Annual Report. Available online: www.world-stroke.org/news-and-blog/news/wso-annual-report-for-2021 (accessed on 22 November 2022).
- GBD. Neurology Collaborators. 2019. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Benson, R.T.; Kargman, D.E.; Boden-Albala, B.; Tuck, C.; Lin, I.-F.; Cheng, J.F.; Paik, M.C.; Shea, S.; Berglund, L. High-Density Lipoprotein Cholesterol and Ischemic Stroke in the Elderly: The Northern Manhattan Stroke Study. JAMA 2001, 285, 2729–2735. [Google Scholar] [CrossRef]
- Lehmann, A.L.C.F.; Alfieri, D.F.; de Araújo, M.C.M.; Trevisani, E.R.; Nagao, M.R.; Pesente, F.S.; Gelinski, J.R.; de Freitas, L.B.; Flauzino, T.; Lehmann, M.F.; et al. Carotid intima media thickness measurements coupled with stroke severity strongly predict short-term outcome in patients with acute ischemic stroke: A machine learning study. Metab. Brain Dis. 2021, 36, 1747–1761. [Google Scholar] [CrossRef]
- Gorelick, P.B. The global burden of stroke: Persistent and disabling. Lancet Neurol. 2019, 18, 417–418. [Google Scholar] [CrossRef]
- Piper, M.A.; Evans, C.V.; Burda, B.U.; Margolis, K.L.; O’Connor, E.; Smith, N.; Webber, E.; Perdue, L.A.; Bigler, K.D.; Whitlock, E.P. Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Report No.: 13-05194-EF-1; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014.
- Maes, M.; Nikiforov, N.G.; Plaimas, K.; Suratanee, A.; Alfieri, D.F.; Reiche, E.M.V. New Drug Targets to Prevent Death Due to Stroke: A Review Based on Results of Protein-Protein Interaction Network, Enrichment, and Annotation Analyses. Int. J. Mol. Sci. 2021, 22, 12108. [Google Scholar] [CrossRef]
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and Inflammation after Ischemic Stroke: What is Known and Where We Go from Here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.J.; Buckwalter, M. Stroke, Inflammation and the Immune Response: Dawn of a New Era. Neurotherapeutics 2016, 13, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, M.L.; Bochev, P.G. Oxidative stress during the chronic phase after stroke. Free Radic. Biol. Med. 2005, 39, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Chapman, K.Z.; Dale, V.Q.; Dénes, A.; Bennett, G.; Rothwell, N.J.; Allan, S.M.; McColl, B.W. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J. Cereb. Blood Flow Metab. 2009, 29, 1764–1768. [Google Scholar] [CrossRef]
- Lehmann, A.L.C.F.; Alfieri, D.F.; de Araújo, M.C.M.; Trevisani, E.R.; Nagao, M.R.; Pesente, F.S.; Gelinski, J.R.; de Freitas, L.B.; Flauzino, T.; Lehmann, M.F.; et al. Immune-inflammatory, coagulation, adhesion, and imaging biomarkers combined in machine learning models improve the prediction of death 1 year after ischemic stroke. Clin. Exp. Med. 2021, 22, 111–123. [Google Scholar] [CrossRef]
- Alfieri, D.F.; Lehmann, M.F.; Flauzino, T.; De Araújo, M.C.M.; Pivoto, N.; Tirolla, R.M.; Simão, A.N.C.; Maes, M.; Reiche, E.M.V. Immune-Inflammatory, Metabolic, Oxidative, and Nitrosative Stress Biomarkers Predict Acute Ischemic Stroke and Short-Term Outcome. Neurotox. Res. 2020, 38, 330–343. [Google Scholar] [CrossRef]
- Reiche, E.M.V.; Gelinksi, J.R.; Alfieri, D.F.; Flauzino, T.; Lehmann, M.F.; de Araújo, M.C.M.; Lozovoy, M.A.B.; Simão, A.N.C.; de Almeida, E.R.D.; Maes, M. Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab. Brain Dis. 2019, 34, 789–804. [Google Scholar] [CrossRef]
- Brinholi, F.F.; Michelin, A.-P.; Matsumoto, A.K.; Semeão, L.; Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Barbosa, D.S.; Maes, M. Paraoxonase 1 status is a major Janus-faced component of mild and moderate acute ischemic stroke and consequent disabilities. medRxiv 2022, 08.12.22278728. [Google Scholar] [CrossRef]
- Matsuo, R.; Ago, T.; Hata, J.; Wakisaka, Y.; Kuroda, J.; Kuwashiro, T.; Kitazono, T.; Kamouchi, M.; on behalf of the Fukuoka Stroke Registry Investigators. Plasma C-Reactive Protein and Clinical Outcomes after Acute Ischemic Stroke: A Prospective Observational Study. PLoS ONE 2016, 11, e0156790. [Google Scholar] [CrossRef]
- Poole, K.E.S.; Loveridge, N.; Barker, P.J.; Halsall, D.J.; Rose, C.; Reeve, J.; Warburton, E.A. Reduced Vitamin D in Acute Stroke. Stroke 2006, 37, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Lansberg, M.G.; Straka, M.; Kemp, S.; Mlynash, M.; Wechsler, L.R.; Jovin, T.G.; Wilder, M.J.; Lutsep, H.L.; Czartoski, T.J.; Bernstein, R.A.; et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012, 11, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mlynash, M.; Lansberg, M.G.; De Silva, D.A.; Lee, J.; Christensen, S.; Straka, M.; Campbell, B.C.; Bammer, R.; Olivot, J.-M.; Desmond, P.; et al. Refining the Definition of the Malignant Profile: Insights from the DEFUSE-EPITHET pooled data set. Stroke 2011, 42, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.G.; Latour, L.L.; Todd, J.W.; Luby, M.; Schellinger, P.D.; Kang, D.-W.; Warach, S. Lesion Volume Change After Treatment with Tissue Plasminogen Activator Can Discriminate Clinical Responders from Nonresponders. Stroke 2007, 38, 2919–2923. [Google Scholar] [CrossRef]
- Osa García, A.; Brambati, S.M.; Desautels, A.; Marcotte, K. Timing stroke: A review on stroke pathophysiology and its influence over time on diffusion measures. J. Neurol. Sci. 2022, 441, 120377. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.; Johnston, K.C.; Homer, D.; Wityk, R.; Koroshetz, W.; Truskowski, L.L.; Haley, E.C. Infarct Volume as a Surrogate or Auxiliary Outcome Measure in Ischemic Stroke Clinical Trials. The RANTTAS Investigators. Stroke 1999, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Peng, M.; Geng, W.; Chen, H.; Su, H.; Zhao, B.; Chen, Y.-C.; Yin, X. FLAIR hyperintensities-DWI mismatch in acute stroke: Associations with DWI volume and functional outcome. Brain Imaging Behav. 2020, 14, 1230–1237. [Google Scholar] [CrossRef]
- Zheng, M.-Z.; Yang, Q.-Y.; Lu, X.-D.; Hu, S.-L.; Chai, C.; Shen, W.; Chang, B.-G.; Wang, Z.-Y.; Xia, S. Middle cerebral artery thrombus susceptibility-weighted imaging mapping predicts prognosis. Quant. Imaging Med. Surg. 2019, 9, 1556–1565. [Google Scholar] [CrossRef]
- Srinivasan, A.; Goyal, M.; Al Azri, F.; Lum, C. State-of-the-Art Imaging of Acute Stroke. Radiographics 2006, 26, S75–S95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, Y.-C.; Zhang, H.; Peng, M.; Chen, H.; Geng, W.; Xu, Q.; Yin, X.; Ma, Y. FLAIR vascular hyperintensity in acute stroke is associated with collateralization and functional outcome. Eur. Radiol. 2019, 29, 4879–4888. [Google Scholar] [CrossRef]
- Maeda, M.; Koshimoto, Y.; Uematsu, H.; Yamada, H.; Kimura, H.; Kawamura, Y.; Itoh, H.; Sakuma, H.; Takeda, K. Time course of arterial hyperintensity with fast fluid-attenuated inversion-recovery imaging in acute and subacute middle cerebral arterial infarction. J. Magn. Reson. Imaging 2001, 13, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.; Dupont, P.; Norrving, B.; Laage, R.; Thomalla, G.; Albers, G.W.; Thijs, V.; Lemmens, R. Prediction of Stroke Onset Is Improved by Relative Fluid-Attenuated Inversion Recovery and Perfusion Imaging Compared to the Visual Diffusion-Weighted Imaging/Fluid-Attenuated Inversion Recovery Mismatch. Stroke 2016, 47, 2559–2564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giese, A.-K.; Schirmer, M.D.; Dalca, A.V.; Sridharan, R.; Donahue, K.L.; Nardin, M.; Irie, R.; McIntosh, E.C.; Mocking, S.J.; Xu, H.; et al. White matter hyperintensity burden in acute stroke patients differs by ischemic stroke subtype. Neurology 2020, 95, e79–e88. [Google Scholar] [CrossRef]
- He, J.-R.; Zhang, Y.; Lu, W.-J.; Liang, H.-B.; Tu, X.-Q.; Ma, F.-Y.; Yang, G.-Y.; Zeng, L.-L. Age-Related Frontal Periventricular White Matter Hyperintensities and miR-92a-3p Are Associated with Early-Onset Post-Stroke Depression. Front. Aging Neurosci. 2017, 9, 328. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1588. [CrossRef] [PubMed]
- Alajbegovic, A.; DjelilovicVranic, J.; Alajbegovic, S.; Nakicevic, A.; Todorovic, L.; Tiric-Campara, M. Post Stroke Depression. Med. Arch. 2014, 68, 47–50. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A literature review and synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef]
- Park, J.-H.; Ovbiagele, B. Relationship of functional disability after a recent stroke with recurrent stroke risk. Eur. J. Neurol. 2016, 23, 361–367. [Google Scholar] [CrossRef]
- Flecha, B.G.; Llesuy, S.; Boveris, A. Hydroperoxide-initiated chemiluminescence: An assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic. Biol. Med. 1991, 10, 93–100. [Google Scholar] [CrossRef]
- Panis, C.; Herrera, A.C.S.A.; Victorino, V.J.; Campos, F.C.; Freitas, L.F.; De Rossi, T.; Simão, A.N.C.; Cecchini, A.L.; Cecchini, R. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res. Treat. 2012, 133, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.S.; Loureiro, A.P.D.M.; de Oliveira, T.F.; Corbi, S.C.T.; Caminaga, R.M.S.; Júnior, C.R.; Orrico, S.R. Quantitation of malondialdehyde in gingival crevicular fluid by a high-performance liquid chromatography-based method. Anal. Biochem. 2012, 423, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hanasand, M.; Omdal, R.; Norheim, K.B.; Gøransson, L.G.; Brede, C.; Jonsson, G. Improved detection of advanced oxidation protein products in plasma. Clin. Chim. Acta 2012, 413, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- A Navarro-Gonzálvez, J.; García-Benayas, C.; Arenas, J. Semiautomated Measurement of Nitrate in Biological Fluids. Clin. Chem. 1998, 44, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L. [41] Measurement of protein thiol groups and glutathione in plasma. Methods Enzym. 1994, 233, 380–385. [Google Scholar] [CrossRef]
- Taylan, E.; Resmi, H. The analytical performance of a microplatemethod for total sulfhydryl measurement in biological samples. Turk. J. Biochem. 2010, 35, 275–278. [Google Scholar]
- Moreira, E.G.; Correia, D.G.; Bonifácio, K.L.; de Moraes, J.B.; Cavicchioli, F.L.; Nunes, C.S.; Nunes, S.O.V.; Vargas, H.O.; Barbosa, D.S.; Maes, M. Lowered PON1 activities are strongly associated with depression and bipolar disorder, recurrence of (hypo)mania and depression, increased disability and lowered quality of life. World J. Biol. Psychiatry 2017, 20, 368–380. [Google Scholar] [CrossRef]
- Moreira, E.G.; Boll, K.M.; Correia, D.G.; Soares, J.F.; Rigobello, C.; Maes, M. Why Should Psychiatrists and Neuroscientists Worry about Paraoxonase 1? Curr. Neuropharmacol. 2019, 17, 1004–1020. [Google Scholar] [CrossRef]
- Maes, M.; Barbosa, D.S.; Almulla, A.F.; Kanchanatawan, B. A Novel Pathway Phenotype of Temporal Lobe Epilepsy and Comorbid Psychiatric Disorders: Results of Precision Nomothetic Medicine. Antioxidants 2022, 11, 803. [Google Scholar] [CrossRef]
- Matsumoto, A.K.; Maes, M.; Supasitthumrong, T.; Maes, A.; Michelin, A.P.; Semeão, L.D.O.; Pedrão, J.V.D.L.; Moreira, E.G.; Kanchanatawan, B.; Barbosa, D.S. Deficit schizophrenia and its features are associated with PON1 Q192R genotypes and lowered paraoxonase 1 (PON1) enzymatic activity: Effects on bacterial translocation. CNS Spectrums 2021, 26, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ringle, C.M.; Sarstedt, M.; Straub, D.W. A critical look at the use of PLS-SEM in “MIS Quarterly”. MIS Q. 2012, 36, iii–xiv. [Google Scholar] [CrossRef]
- Hair, J.F.; Risher, J.J.; Sarstedt, M.; Ringle, C.M. When to use and how to report the results of PLS-SEM. Eur. Bus. Rev. 2019, 31, 2–24. [Google Scholar] [CrossRef]
- El Kossi, M.M.H.; Zakhary, M.M. Oxidative Stress in the Context of Acute Cerebrovascular Stroke. Stroke 2000, 31, 1889–1892. [Google Scholar] [CrossRef]
- Domínguez, C.; Delgado, P.; Vilches, A.; Martín-Gallán, P.; Ribo, M.; Santamarina, E.; Molina, C.; Corbeto, N.; Rodríguez-Sureda, V.; Rosell, A.; et al. Oxidative Stress After Thrombolysis-Induced Reperfusion in Human Stroke. Stroke 2010, 41, 653–660. [Google Scholar] [CrossRef]
- Dogan, O.; Kisa, U.; Erdemoglu, A.K.; Kacmaz, M.; Caglayan, O.; Kurku, H. Oxidative and nitrosative stress in patients with ischemic stroke. LaboratoriumsMedizin 2018, 42, 195–200. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Kumar, R. Evaluating the Role of Oxidative Stress in Acute Ischemic Stroke. J. Neurosci. Rural. Pract. 2020, 11, 156–159. [Google Scholar] [CrossRef]
- Sharpe, P.C.; Mulholland, C.; Trinick, T. Ascorbate and malondialdehyde in stroke patients. Ir. J. Med. Sci. 1994, 163, 488–490. [Google Scholar] [CrossRef]
- Maes, M.; Bonifacio, K.L.; Morelli, N.R.; Vargas, H.O.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways Between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol. Neurobiol. 2018, 56, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vojdani, A.; Sirivichayakul, S.; Barbosa, D.S.; Kanchanatawan, B. Inflammatory and Oxidative Pathways Are New Drug Targets in Multiple Episode Schizophrenia and Leaky Gut, Klebsiella pneumoniae, and C1q Immune Complexes Are Additional Drug Targets in First Episode Schizophrenia. Mol. Neurobiol. 2021, 58, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Rama, R.; Dávalos, A. Nitric Oxide–Related Brain Damage in Acute Ischemic Stroke. Stroke 2000, 31, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Gumanova, N.G.; Teplova, N.V.; Ryabchenko, A.U.; Denisov, E.N. Serum nitrate and nitrite levels in patients with hypertension and ischemic stroke depend on diet: A multicenter study. Clin. Biochem. 2015, 48, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Akyol, A.; Yenisey, C.; Arpaci, E.; Kiylioglu, N.; Tataroglu, C. Oxidative stress in acute ischemic stroke. J. Clin. Neurosci. 2007, 14, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, L.; Taffi, R.; Vignini, A.; Moroni, C.; Raffaelli, F.; Bacchetti, T.; Silvestrini, M.; Provinciali, L.; Mazzanti, L. Reactive oxygen species plasmatic levels in ischemic stroke. Mol. Cell. Biochem. 2007, 303, 19–25. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Chen, Z.-Q.; Mou, R.-T.; Feng, D.-X. The role of nitric oxide in stroke. Med. Gas Res. 2017, 7, 194–203. [Google Scholar] [CrossRef]
- Mohammadi, M.T. Overproduction of nitric oxide intensifies brain infarction and cerebrovascular damage through reduction of claudin-5 and ZO-1 expression in striatum of ischemic brain. Pathol. Res. Pract. 2016, 212, 959–964. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, J.; Wang, J.; Zhou, C.; Zhang, W. Effects and Mechanism of Action of Inducible Nitric Oxide Synthase on Apoptosis in a Rat Model of Cerebral Ischemia-Reperfusion Injury. Anat. Rec. 2015, 299, 246–255. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Klein, H.; Walder, K.; Galecki, P.; Maes, M. Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome. Mol. Neurobiol. 2016, 54, 4271–4291. [Google Scholar] [CrossRef]
- Thisayakorn, P.; Thipakorn, Y.; Tantavisut, S.; Sirivichayakul, S.; Maes, M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry 2022, 22, 369. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, U.; Ozturk, O.; Nergiz, S.; Tamam, Y.; Varol, S. Relationship Between Paraoxonase-1 Activity and Pulse Pressure Index in Patients with a Acute Ischemic Stroke. Dicle Med. J. Dicle Tip Derg. 2020, 47, 820–827. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, K.; Wang, Q.; Ma, Y.; Liu, X. The Antioxidant Enzyme PON1: A Potential Prognostic Predictor of Acute Ischemic Stroke. Oxidative Med. Cell. Longev. 2021, 2021, 6677111. [Google Scholar] [CrossRef] [PubMed]
- Shenhar-Tsarfaty, S.; Waiskopf, N.; Ofek, K.; Shopin, L.; Usher, S.; Berliner, S.; Shapira, I.; Bornstein, N.M.; Ritov, Y.; Soreq, H.; et al. Atherosclerosis and arteriosclerosis parameters in stroke patients associate with paraoxonase polymorphism and esterase activities. Eur. J. Neurol. 2013, 20, 891–898. [Google Scholar] [CrossRef]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Varol, S.; Uzar, E.; Akil, E.; Cevik, M.; Arikanoglu, A.; Yucel, Y.; Tasdemir, N.; Evliyaoglu, O.; Ekici, F. Evaluation of serum oxidant/antioxidant balance in patients with acute stroke. J. Pak. Med. Assoc. 2013, 63, 590–593. [Google Scholar]
- Michalak, S.; Kazmierski, R.; Hellmann, A.; Wysocka, E.; Kocialkowska-Adamczewska, D.; Wencel-Warot, A.; Nowinski, W.L. Serum Paraoxonase/Arylesterase Activity Affects Outcome in Ischemic Stroke Patients. Cerebrovasc. Dis. 2011, 32, 124–132. [Google Scholar] [CrossRef]
- Kotur-Stevuljevic, J.; Bogavac-Stanojevic, N.; Jelic-Ivanovic, Z.; Stefanovic, A.; Gojkovic, T.; Joksic, J.; Sopic, M.; Gulan, B.; Janac, J.; Milosevic, S. Oxidative stress and paraoxonase 1 status in acute ischemic stroke patients. Atherosclerosis 2015, 241, 192–198. [Google Scholar] [CrossRef]
- Demarin, V.; Lisak, M.; Morović, S.; Cengić, T. Low high-density lipoprotein cholesterol as the possible risk factor for stroke. Acta Clin. Croat. 2010, 49, 429–439. [Google Scholar]
- Luo, Y.; Li, J.; Zhang, J.; Xu, Y. Low HDL cholesterol is correlated to the acute ischemic stroke with diabetes mellitus. Lipids Health Dis. 2014, 13, 171. [Google Scholar] [CrossRef]
- Acharjee, S.; Boden, W.E.; Hartigan, P.M.; Teo, K.K.; Maron, D.J.; Sedlis, S.P.; Kostuk, W.; Spertus, J.A.; Dada, M.; Chaitman, B.R.; et al. Low Levels of High-Density Lipoprotein Cholesterol and Increased Risk of Cardiovascular Events in Stable Ischemic Heart Disease Patients: A post-hoc analysis from the COURAGE Trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation). J. Am. Coll. Cardiol. 2013, 62, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Lai, M.-C.; Cheng, T.-J.; Lau, M.-T.; Hu, M.-L. Plasma Levels of Antioxidant Vitamins, Selenium, Total Sulfhydryl Groups and Oxidative Products in Ischemic-Stroke Patients as Compared to Matched Controls in Taiwan. Free Radic. Res. 1998, 28, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, W.M.; Abdel-Gawad, E.-H.A.; Mesallam, D.I.A.; El-Serafy, T.S. The relationship between oxidative stress and acute ischemic stroke severity and functional outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 74. [Google Scholar] [CrossRef]
- Vila, N.; Castillo, J.; Dávalos, A.; Chamorro, A. Proinflammatory Cytokines and Early Neurological Worsening in Ischemic Stroke. Stroke 2000, 31, 2325–2329. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Inflammation in Ischemic Stroke Subtypes. Curr. Pharm. Des. 2012, 18, 4289–4310. [Google Scholar] [CrossRef]
- Žitňanová, I.; Šiarnik, P.; Kollár, B.; Chomová, M.; Pazderová, P.; Andrezálová, L.; Ježovičová, M.; Koňariková, K.; Laubertová, L.; Krivošíková, Z.; et al. Oxidative Stress Markers and Their Dynamic Changes in Patients after Acute Ischemic Stroke. Oxidative Med. Cell. Longev. 2016, 2016, 9761697. [Google Scholar] [CrossRef]
- Petrova, J.J.; Manolov, V.E.; Hadjidekova, S.P.; Hadjiev, E.A.; Bogov, B.I.; Marinov, B.M.; Vasilev, V.G.; Tzatchev, K.N. Is There a Link Between Changes in Levels of Hepcidin and Stroke? Clin. Lab. 2015, 61, 1935–1939. [Google Scholar] [CrossRef]
- Geng, H.-H.; Wang, X.-W.; Fu, R.-L.; Jing, M.-J.; Huang, L.-L.; Zhang, Q.; Wang, X.-X.; Wang, P.-X. The Relationship between C-Reactive Protein Level and Discharge Outcome in Patients with Acute Ischemic Stroke. Int. J. Environ. Res. Public Health 2016, 13, 636. [Google Scholar] [CrossRef]
- Raoult, H.; Lassalle, M.; Parat, B.; Rousseau, C.; Eugène, F.; Vannier, S.; Evain, S.; Le Bras, A.; Ronziere, T.; Ferre, J.; et al. DWI-Based Algorithm to Predict Disability in Patients Treated with Thrombectomy for Acute Stroke. Am. J. Neuroradiol. 2020, 41, 274–279. [Google Scholar] [CrossRef]
- Lorenzano, S.; Rost, N.S.; Khan, M.; Li, H.; Lima, F.O.; Maas, M.B.; Green, R.; Thankachan, T.K.; DiPietro, A.J.; Arai, K.; et al. Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke. Stroke 2018, 49, 630–637. [Google Scholar] [CrossRef]
- Kang, H.G.; Cheong, J.S.; Lim, I.H.; Yun, K.H.; Park, H.Y. Antioxidative activity of statins and HDL-PON1 association in lacunar ischemic stroke with and without white matter hyperintensity. Signa Vitae 2021, 17, 104–110. [Google Scholar]
- Zhang, M.; Wang, Z.; Wang, C.; Wu, Y.; Li, Z.; Liu, Z. Visualizing Oxidative Stress Level for Timely Assessment of Ischemic Stroke via a Ratiometric Near-Infrared-II Luminescent Nanoprobe. ACS Nano 2021, 15, 11940–11952. [Google Scholar] [CrossRef]
- Kontos, H.A. Oxygen Radicals in Cerebral Ischemia: The 2001 Willis Lecture. Stroke 2001, 32, 2712–2716. [Google Scholar] [CrossRef]
- Leinonen, J.S.; Ahonen, J.-P.; Lönnrot, K.; Jehkonen, M.; Dastidar, P.; Molnár, G.; Alho, H. Low Plasma Antioxidant Activity Is Associated with High Lesion Volume and Neurological Impairment in Stroke. Stroke 2000, 31, 33–39. [Google Scholar] [CrossRef]
- Topcuoglu, M.A.; Demirkaya, S. Low Plasma Antioxidant Activity Is Associated with High Lesion Volume and Neurological Impairment in Stroke. Stroke 2000, 31, 2266–2278. [Google Scholar] [CrossRef]
- Manzanero, S.; Santro, T.; Arumugam, T.V. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem. Int. 2013, 62, 712–718. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Iolascon, G.; Gimigliano, F.; Tonin, P.; Tozzi Ciancarelli, M.G. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients. 2022, 15, 108. [Google Scholar] [CrossRef]
- Samdani, A.F.; Dawson, T.M.; Dawson, V.L. Nitric oxide synthase in models of focal ischemia. Stroke 1997, 28, 1283–1288. [Google Scholar] [CrossRef]
- Liu, T.H.; Beckman, J.S.; Freeman, B.A.; Hogan, E.L.; Hsu, C.Y. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am. J. Physiol. Circ. Physiol. 1989, 256, H589–H593. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Liu, H.; Xia, P.; Liu, M.; Ji, X.M.; Bin Sun, H.; Tao, L.; Mu, Q.W. PON Gene Polymorphisms and Ischaemic Stroke: A Systematic Review and Meta Analysis. Int. J. Stroke 2013, 8, 111–123. [Google Scholar] [CrossRef]
- Demirdöğen, B.C.; Türkanoğlu, A.; Bek, S.; Sanisoğlu, Y.; Demirkaya, S.; Vural, O.; Arınç, E.; Adalı, O. Paraoxonase/arylesterase ratio, PON1 192Q/R polymorphism and PON1 status are associated with increased risk of ischemic stroke. Clin. Biochem. 2008, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karakus, N.; Basol, N.; Yigit, S.; Cogas, P.; Ceviz, T.; Kablan, A.U.; Demir, O. Importance of Genetic Polymorphisms (Rs662 and Rs854560) of the Paraoxonase-1 Gene as Risk Factor for Acute Ischemic Stroke in Turkish Population. Int. J. Crit Care Emerg. Med. 2019, 5, 94. [Google Scholar]
- Voetsch, B.; Benke, K.S.; Damasceno, B.P.; Siqueira, L.H.; Loscalzo, J. Paraoxonase 192 Gln→Arg Polymorphism: An independent risk factor for nonfatal arterial ischemic stroke among young adults. Stroke 2002, 33, 1459–1464. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.-H.; Yang, Q.-D.; Xiao, B.; Ge, L.; Zhang, N.; Xia, J.; Zhang, L.; Liu, Z.-J. Human serum paraoxonase gene polymorphisms, Q192R and L55M, are not associated with the risk of cerebral infarction in Chinese Han population. Neurol. Res. 2006, 28, 549–554. [Google Scholar] [CrossRef]
- Xiao, Z.J.; Chen, J.; Sun, Y.; Zheng, Z.J. Lack of association between the paraoxonase 1 Q/R192 single nucleotide polymorphism and stroke in a Chinese cohort. Acta Neurol. Belg. 2009, 109, 205–209. [Google Scholar]
- Martínez-Salazar, M.F.; Soriano-Martínez, M.D.L.L.; Juantorena-Ugas, A.; Almenares-López, D.; Yescas, P.; Boll, M.-C.; Monroy-Noyola, A. Paraoxonase-1 polymorphisms and cerebral ischemic stroke: A pilot study in mexican patients. Colomb. Medica 2018, 49, 223–227. [Google Scholar] [CrossRef]
- Sand, P.G. Paraoxonase Genes and the Susceptibilty to Ischemic Stroke. Int. J. Stroke 2013, 8, E39. [Google Scholar] [CrossRef]
- Kim, D.S.; Burt, A.A.; Ranchalis, J.E.; Richter, R.J.; Marshall, J.K.; Eintracht, J.F.; Rosenthal, E.A.; Furlong, C.E.; Jarvik, G.P. Additional Common Polymorphisms in the PON Gene Cluster Predict PON1 Activity but Not Vascular Disease. J. Lipids 2012, 2012, 476316. [Google Scholar] [CrossRef]
- Menini, T.; Gugliucci, A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014, 19, 49–58. [Google Scholar] [CrossRef]
- Dounousi, E.; Bouba, I.; Spoto, B.; Pappas, K.; Tripepi, G.; Georgiou, I.; Tselepis, A.; Elisaf, M.; Tsakiris, D.; Zoccali, C.; et al. A Genetic Biomarker of Oxidative Stress, the Paraoxonase-1 Q192R Gene Variant, Associates with Cardiomyopathy in CKD: A Longitudinal Study. Oxid. Med. Cell. Longev. 2016, 2016, 1507270. [Google Scholar] [CrossRef]
- Liu, J.-L.; Li, J.-P.; Wang, X.-L.; Yang, Y. [Relationship between Q192R polymorphisms in paraoxonase 1 gene and young ischemic stroke]. Zhonghua Yi Xue Za Zhi 2010, 90, 912–916. [Google Scholar]
- Zhao, F.; Yue, Y.; Jiang, H.; Yuan, Y. Shared genetic risk factors for depression and stroke. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 55–70. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Kotańska, M. Aberrations in the Cross-Talks Among Redox, Nuclear Factor-κB, and Wnt/β-Catenin Pathway Signaling Underpin Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Front. Psychiatry 2022, 13, 822382. [Google Scholar] [CrossRef]


| Variables | HC A (n = 40) | Mild AIS B (n = 85) | Moderate AIS C (n = 37) | F/χ2 | df | p |
|---|---|---|---|---|---|---|
| Age (years) | 60.63 ± 9.18 | 59.84 ± 9.01 | 60.65 ± 9.46 | 0.16 | 2/159 | 0.857 |
| Education (years) | 4.58 ± 1.97 | 4.87 ± 1.98 | 4.16 ± 1.98 | 1.71 | 2/159 | 0.185 |
| BMI (kg/m2) | 24.90 ± 3.94 | 24.9114 ± 3.82 | 25.00 ± 3.89 | 0.01 | 2/159 | 0.994 |
| Sex (Male/Female) | 17/22 | 49/36 | 19/18 | 2.16 | 2 | 0.340 |
| TUD (no/yes) | 40/0 | 81/4 | 36/1 | 2.04 | 2 | 0.361 |
| HT (no/yes) | - | 26/59 | 14/23 | 0.62 | 1 | 0.433 |
| T2DM (no/yes) | - | 54/31 | 28/9 | 1.73 | 1 | 0.189 |
| Dyslipidemia (no/yes) | - | 56/29 | 26/11 | 0.23 | 1 | 0.635 |
| Previous stroke (no/yes) | - | 71/14 | 28/9 | 1.04 | 1 | 0.308 |
| TOAST LAC/CEI/LAAS/U | - | 11/4/46/10 | 11/3/18/2 | FFHET | - | 0.146 |
| NIHSS + mRS index (z score) | −1.288 ± 0.00 B,C | 0.129 (0.395) A,C | 1.363 (0.594) A,B | KWT | - | <0.001 |
| NIHSS-basal | 0.00 ± 0.00 B,C | 2.20 ± 1.00 A,C | 5.03 ± 1.94 A,B | KWT | - | <0.001 |
| mRS-basal | 0.00 ± 0.00 B,C | 1.73 ± 0.50 A,C | 3.22 ± 0.42 A,B | KWT | - | <0.001 |
| mRS-3 months | 0.00 ± 0.00 B,C | 0.89 ± 0.88 A,C | 1.65 ± 1.20 A,B | KWT | - | <0.001 |
| mRS-6 months | 0.00 ± 0.00 B,C | 1.08 ± 1.41 A | 1.04 ± 1.55 A | KWT | - | <0.001 |
| hsCRP * (mg/L) | 1.69 ± 1.05 C | 3.87 ± 9.63 C | 8.12 ± 15.35 A,B | 9.59 | 2/159 | 0.019 |
| White blood cell count (K/µL) | 5.34 (0.71) B,C | 7.91 (2.30) A,C | 9.06 (3.18) A,B | 27.93 | 2/159 | <0.001 |
| Neutrophile/Lymphocyte (NLR) * | 1.652 (0.401) C | 2.246 (1.151) C | 3.762 (3.782) A,B | 11.66 | 2/159 | <0.001 |
| zIMMUNE (z score) | −0.744 (0.376) B,C | −0.006 (0.906) A,C | 0.817 (1.049) A,B | 32.60 | 2/159 | <0.001 |
| Basal blood glucose (mg/dL) | 107.6 (29.0) B | 132.1 (57.3) A | 124.7 (52.0) | 3.21 | 2/159 | 0.043 |
| HbA1C (%) | 5.94 (0.64) B | 7.11 (2.16) A | 6.72 (2.08) | 5.35 | 2/159 | 0.006 |
| Total cholesterol * (mg/dL) | 187.9 (23.6) | 187.8 (45.9) | 202.1 (78.4) | 0.32 | 2/159 | 0.726 |
| HDL-cholesterol (mg/dL) | 59.31 (10.24) B,C | 45.70 (12.97) A | 44.00 (12.37) A | 20.40 | 2/159 | <0.001 |
| Triglycerides (mg/dL) | 113.3 (44.3) | 139.9 (89.9) | 137.6 (106.2) | 0.46 | 2/159 | 0.632 |
| zTC-zHDL (z score) | −0.696 (0.517) B,C | 0.153 1.039 A | 0.401 (0.950) A | 16.23 | 2/159 | <0.001 |
| zTG-zHDL (z score) | −0.571 (0.800) B,C | 0.156 1.060 A | 0.259 (0.818) A | 9.75 | 2/159 | <0.001 |
| FLAIR signal intensitity (mm3) * | 4651 (5223) C | 12,653 (12,406) C | 29,569 (23,415) A,B | 11.31 | 2/57 | <0.001 |
| FLAIR signal/brain volume * | 2989 (2.713) B,C | 9988 (10,319) A,C | 25,410 (20,451) A,B | 13.83 | 2/56 | <0.001 |
| Total DWI stroke volume (mm3) | 0.0 C | 1095 (2410) C | 17,997 (21,005) A,B | KWT | - | <0.001 |
| zFLAIR + zDWI (z score) | −0.969 (0.581) B,C | 0.043 (0.808) A,C | 1.088 (0.567) A,B | 30.47 | 2/57 | <0.001 |
| Variable | HC A (n = 40) | Mild AIS B (n = 85) | Moderate AIS C (n = 37) | F/χ2 | df | p |
|---|---|---|---|---|---|---|
| -SH groups basal (µmol/L) | 296.7 (59.0) B,C | 254.7 (63.0) A | 247.7 (66.8) A | 7.58 | 2/159 | <0.001 |
| -SH groups 3 months (µmol/L) | 296.7 (59.0) B,C | 246.6 (71.7) A | 262.2 (55.5) A | 6.94 | 2/113 | 0.001 |
| PON1 Q192R QQ/QR/RR | 6/23/11 | 8/37/38 | 5/18/12 | FFHET | - | 0.343 |
| CMPAase basal (U/mL) | 38.20 (1.73) B,C | 34.65 (1.17) A | 34.17 (1.65) A | 3.12 | 2/153 | 0.047 |
| CMPAase 3 month (U/mL) | 38.75 (1.73) B,C | 31.60 (1.48) A | 28.79 (2.10) A | 7.66 | 2/106 | <0.001 |
| AREase basal (U/mL) | 254.00 (16.58) B,C | 333.82 (12.41) A | 335.80 (17.56) A | 9.52 | 2/153 | <0.001 |
| AREase 3 month (U/mL) | 244.11 (90.25) B,C | 199.43 (67.54) A | 196.67 (95.54) A | 10.33 | 2/153 | <0.001 |
| zCMPAase-zAREase (z score) | 0.852 (0.741) B,C | −0.254 (0.946) A | −0.337 (0.777) A | 26.05 | 2/169 | <0.001 |
| zCMPAase + zHDL (z score) | −0.635 (0.806) B,C | −0.153 (0.964) A | −0.334 (0.993) A | 12.74 | 2/159 | <0.001 |
| LOOH basal (RLU) | 14,452 (3930) B,C | 27,381 (17,969) A | 26,788 (13,781) A | 22.19 | 2/159 | <0.001 |
| LOOH 3 months (RLU) | 14,452 (3930) B,C | 21,203 (11,701) A | 22,655 (10,144) A | 7.74 | 2/112 | <0.001 |
| AOPP basal (µmol/L/eq. cloramin T) | 262.3 (195.1) | 213.7 (128.2) | 196.4 (112.9) | 2.22 | 2/159 | 0.112 |
| AOPP 3 months (µmol/L/eq. cloramin T) | 262.3 (195.1) | 251.8 (175.0) | 179.0 (105.9) | 1.99 | 2/113 | 0.141 |
| MDA basal (µM/mg protein) | 1.526 (0.465) | 1.433 (0.415) | 1.505 (0.423) | 0.76 | 2/1591 | 0.469 |
| MDA 3 months (µM/mg protein) | 1.526 (0.465) | 1.691 (0.764) | 1.403 (0.599) | 1.82 | 2/112 | 0.166 |
| NOx basal (µmol/L) | 7.42 (5.37) B,C | 5.48 (3.02) A | 5.41 (2.23) A | 4.46 | 2/1591 | 0.013 |
| NOx 3 months (µmol/L) | 7.42 (5.37) | 7.61 (4.32) | 7.00 (4.57) | 0.14 | 2/133 | 0.873 |
| NT (z score) | −0.889 (0.442) B,C | 0.136 (1.002) A,C | 0.649 (0.749) A,B | 34.60 | 2/159 | <0.001 |
| ANTIOX (z score) | 0.785 (0.736) B,C | −0.195 (0.916) A | −0.399 (0.994) A | 21.14 | 2/159 | <0.001 |
| NT/ANTIOX (z score) | −0.995 (0588) B,C | −0197 (0.932) A,C | 0.623 (0.693) A,B | 43.89 | 2/159 | <0.001 |
| Models | NN#1 AIS versus HC | NN#2 AIS versus HC | |
|---|---|---|---|
| Input Layer | Number of units | 19 | 12 |
| Rescaling method | Normalized | Normalized | |
| Hidden layers | Number of hidden layers | 2 | 2 |
| Number of units in hidden layer 1 | 3 | 5 | |
| Number of units in hidden layer 2 | 2 | 4 | |
| Activation Function | Hyperbolic tangent | Hyperbolic tangent | |
| Output layer | Dependent variables | AIS versus HC | AIS versus HC |
| Number of units | 2 | 2 | |
| Activation function | Identity | Identity | |
| Error function | Sum of squares | Sum of squares | |
| Training | Sum of squares error term | 2.990 | 3.940 |
| % incorrect or relative error | 5.5% | 4.7% | |
| Prediction (sens, spec) | 98.2%, 83.3% | 93.4%, 100% | |
| Testing | Sum of Squares error | 0.843 | 0.634 |
| % incorrect or relative error | 3.8% | 3.7% | |
| Prediction (sens spec) | 95.0%, 100% | 100%, 66.7% | |
| AUC ROC | 0.991 | 0.984 | |
| Holdout | % incorrect or relative error | 8.5% | 4.3% |
| Prediction (sens, spec) | 93.0%, 87.5% | 97.0%, 92.3% |
| Variable | NIHSS Basal | mRS Basal | mRS 3 Months | FLAIR Lesions | DWI Stroke Volume |
|---|---|---|---|---|---|
| -Sulfhydryl (SH) groups | −0.275 (<0.001) | −0.303 (<0.001) | −0.187 (0.028) | 0.072 (0.589) | −0.290 (0.026) |
| CMPAase | −0.157 (0.046) | −0.203 (0.010) | −0.244 (0.003) | −0.443 (<0.001) | −0.483 (<0.001) |
| AREase | 0.242 (0.002) | 0.236 (0.003) | 0.158 (0.060) | 0.177 (0.188) | 0.057 (0.666) |
| zCMPAase-zAREase | −0.379 (<0.001) | −0.417 (<0.001) | −0.373 (<0.001) | −0.548 (<0.001) | −0.492 (<0001) |
| HDL cholesterol | −0.321 (<0.001) | −0.418 (<0.001) | −0.366 (<0.001) | −0.320 (0.015) | −0.207 (0.112) |
| zCMPAase + zHDL | −0.271 (<0.001) | −0.353 (<0.001) | −0.377 (<0.001) | −0.489 (<0.001) | −0.393 (0.002) |
| Lipid hydroperoxides | 0.292 (<0.001) | 0.404 (<0.001) | 0.294 (<0.001) | 0.099 (0.466) | 0.107 (0.416) |
| AOPP | −0.104 (0.192) | −0.140 (0.079) | 0.033 (0.698) | −0.143 (0.288) | −0.304 (0.019) |
| Malondialdehyde | −0.059 (0.471) | −0.029 (0.721) | −0.025 (0.776) | −0.012 (0.929) | 0.167 (0.211) |
| Nitric oxide metabolites | −0.149 (0.062) | 0.041 (0.158) | −0.093 (0.274) | −0.056 (0.682) | −0.073 (0.581) |
| zNT | 0.416 (<0.001) | 0.538 (<0.001) | 0.437 (<0.001) | 0.347 (0.0085) | 0.439 (<0.001) |
| zANTIOX | −0.362 (<0.001) | −0.477 (<0.001) | −0.393 (<0.001) | −0.506 (<0.001) | −0.474 (<0.001) |
| zNT-zANTIOX | 0.462 (<0.001) | 0.585 (<0.001) | 0.481 (<0.001) | 0.515 (<0.001) | 0.554 (<0.001) |
| zIMMUNE | 0.382 (<0.001) | 0.529 (<0.001) | 0.257 (0.002) | 0.391 (0.003) | 0.497 (<0.001) |
| Dependent Variable | Explanatory Variable | β | t | p | Fmodel | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| NIHSS basal | Model #1 | 11.36 | 6/143 | <0.001 | 0.323 | |||
| HDL cholesterol | −0.164 | −2.17 | 0.032 | HDLc | −0.164 | −2.17 | ||
| AREase | 0.312 | 3.96 | <0.001 | AREase | 0.312 | 3.96 | ||
| LOOH | 0.182 | 2.55 | 0.012 | LOOH | 0.182 | 2.55 | ||
| -SH groups | −0.196 | −2.78 | 0.006 | -SH groups | −0.196 | −2.78 | ||
| NLR | 0.177 | 2.47 | 0.015 | NLR | 0.177 | 2.47 | ||
| CMPAase | −0.198 | −2.44 | 0.016 | CMPAase | −0.198 | −2.44 | ||
| mRS basal | Model #2 | 20.27 | 8/141 | <0.001 | 0.535 | |||
| HT | 0.128 | 1.87 | 0.064 | |||||
| HDLcholesterol | −0.152 | −2.32 | 0.022 | |||||
| LOOH | 0.248 | 4.05 | <0.001 | |||||
| WBC | 0.193 | 2.90 | 0.004 | |||||
| -SH groups | −0.183 | −3.01 | 0.003 | |||||
| NLR | 0.191 | 3.16 | 0.002 | |||||
| AREase | 0.222 | 3.21 | 0.002 | |||||
| CMPAase | −0.180 | −2.63 | 0.009 | |||||
| NIHSS basal | Model #3 | 27.15 | 2/158 | <0.001 | 0.256 | |||
| zNT-zANTIOX | 0.367 | 4.90 | <0.001 | |||||
| zCMPAaase-zAREase | −0.230 | −3.06 | 0.003 | |||||
| mRS basal | Model #4 | 43.00 | 45/153 | <0.001 | 0.592 | |||
| zNT-zANTIOX | 0.278 | 3.52 | <0.001 | |||||
| HT | 0.282 | 4.53 | <0.001 | |||||
| zCMPAase-zAREase | −0.233 | −3.77 | <0.001 | |||||
| zIMMUNE | 0.191 | 2.59 | 0.010 | |||||
| mRS 3 months | Model #5 | 23.92 | 3/95 | <0.001 | 0.430 | |||
| NT | 0.475 | 5.87 | <0.001 | |||||
| zCMPAase-zREase | −0.280 | −3.47 | <0.001 | |||||
| Previous stroke | 0.166 | 2.13 | 0.036 | |||||
| mRS 6 months | Model #6 | 7.03 | 4/85 | <0.001 | 0.249 | |||
| Dyslipidemia | 0.289 | 2.98 | 0.004 | |||||
| zCMPAase-zAREase | −0.227 | −2.34 | 0.022 | |||||
| AOPP basal | −0.417 | −3.014 | 0.003 | |||||
| AOPP 3 months | 0.302 | 2.18 | 0.032 | |||||
| Dependent Variable | Explanatory Variable | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| NIHSS basal | Model #1 | 15.93 | 3/55 | <0.001 | 0.465 | |||
| DWI Left Posterior | 0.423 | 4.11 | <0.001 | |||||
| zIMMUNE | 0.323 | 3.15 | 0.003 | |||||
| zCMPAase-zAREase | −0.228 | −2.16 | 0.035 | |||||
| mRS basal | Model #2 | 24.32 | 4/53 | <0.001 | 0.647 | |||
| zNT-zANTIOX | 0.458 | 4.80 | <0.001 | |||||
| HT | 0.250 | 2.69 | 0.010 | |||||
| DWI Left Posterior | 0.295 | 3.42 | 0.001 | |||||
| DWI Right Anterior | 0.199 | 2.33 | 0.024 | |||||
| mRS 3 months | Model #3 | 12.07 | 4/46 | <0.001 | 0.512 | |||
| zNT-zANTIOX | 0.304 | 2.54 | 0.015 | |||||
| DWI Right Anterior | 0.281 | 2.65 | 0.011 | |||||
| Dyslipidemia | 0.304 | 2.92 | 0.005 | |||||
| FLAIR signal intensity | 0.284 | 2.36 | 0.022 | |||||
| Total DWI | Model #4 | 16.71 | 2/55 | <0.001 | 0.378 | |||
| zNT-zANTIOX | 0.433 | 3.44 | 0.001 | |||||
| zCMPAase-zAREase | −0.263 | −2.09 | 0.042 | |||||
| FLAIR signal intensity | Model #5 | 15.61 | 3/52 | <0.001 | 0.474 | |||
| zCMPAase-zAREase | −0.343 | −2.86 | 0.006 | |||||
| Previous stroke | 0.322 | 3.12 | 0.003 | |||||
| zNT-zANTIOX | 0.272 | 2.26 | 0.028 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maes, M.; Brinholi, F.F.; Michelin, A.P.; Matsumoto, A.K.; de Oliveira Semeão, L.; Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Barbosa, D.S. In Mild and Moderate Acute Ischemic Stroke, Increased Lipid Peroxidation and Lowered Antioxidant Defenses Are Strongly Associated with Disabilities and Final Stroke Core Volume. Antioxidants 2023, 12, 188. https://doi.org/10.3390/antiox12010188
Maes M, Brinholi FF, Michelin AP, Matsumoto AK, de Oliveira Semeão L, Almulla AF, Supasitthumrong T, Tunvirachaisakul C, Barbosa DS. In Mild and Moderate Acute Ischemic Stroke, Increased Lipid Peroxidation and Lowered Antioxidant Defenses Are Strongly Associated with Disabilities and Final Stroke Core Volume. Antioxidants. 2023; 12(1):188. https://doi.org/10.3390/antiox12010188
Chicago/Turabian StyleMaes, Michael, Francis F. Brinholi, Ana Paula Michelin, Andressa K. Matsumoto, Laura de Oliveira Semeão, Abbas F. Almulla, Thitiporn Supasitthumrong, Chavit Tunvirachaisakul, and Decio S. Barbosa. 2023. "In Mild and Moderate Acute Ischemic Stroke, Increased Lipid Peroxidation and Lowered Antioxidant Defenses Are Strongly Associated with Disabilities and Final Stroke Core Volume" Antioxidants 12, no. 1: 188. https://doi.org/10.3390/antiox12010188
APA StyleMaes, M., Brinholi, F. F., Michelin, A. P., Matsumoto, A. K., de Oliveira Semeão, L., Almulla, A. F., Supasitthumrong, T., Tunvirachaisakul, C., & Barbosa, D. S. (2023). In Mild and Moderate Acute Ischemic Stroke, Increased Lipid Peroxidation and Lowered Antioxidant Defenses Are Strongly Associated with Disabilities and Final Stroke Core Volume. Antioxidants, 12(1), 188. https://doi.org/10.3390/antiox12010188







