Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatments
2.2. Neonatal Rat Cardiomyocyte Culture and Treatment
2.3. Measurement of Triglyceride (TG), Glycosylated Hemoglobin (HbA1c) and Fasting Insulin (FINS) Levels
2.4. Echocardiography
2.5. Histological Analysis
2.6. Measurement of Lactate Dehydrogenase (LDH) and Adenosine Triphosphate (ATP)
2.7. Assessment of Oxidative Stress
2.8. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labelling (TUNEL) Assay
2.9. Quantitaive Real-Time PCR
2.10. Western Blotting
2.11. Immunofuorescence
2.12. Statistical Analysis
3. Results
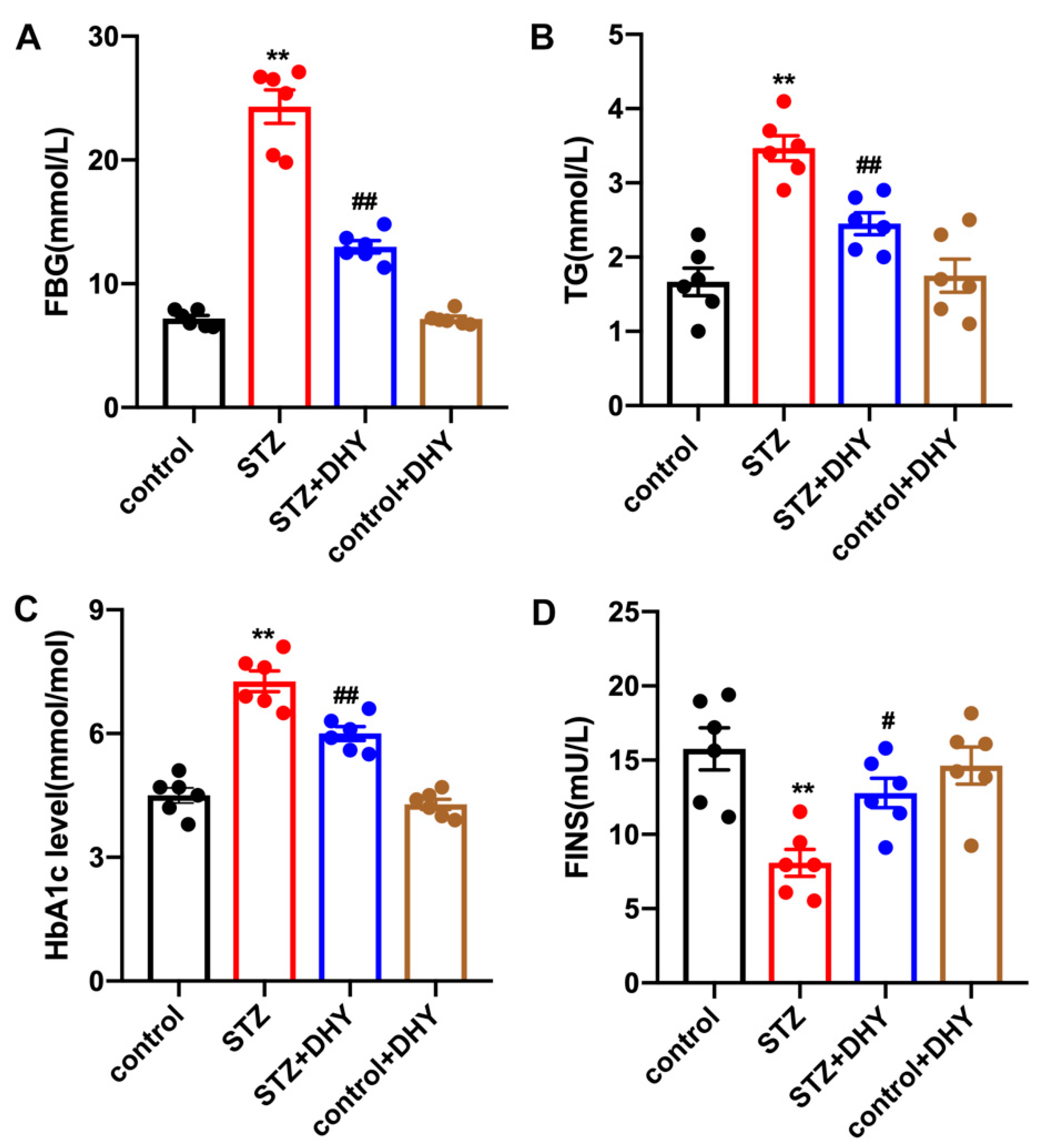
3.1. DHY Alleviated FBG, TG, HbA1c Level and Increased FINS in STZ-Induced Mice
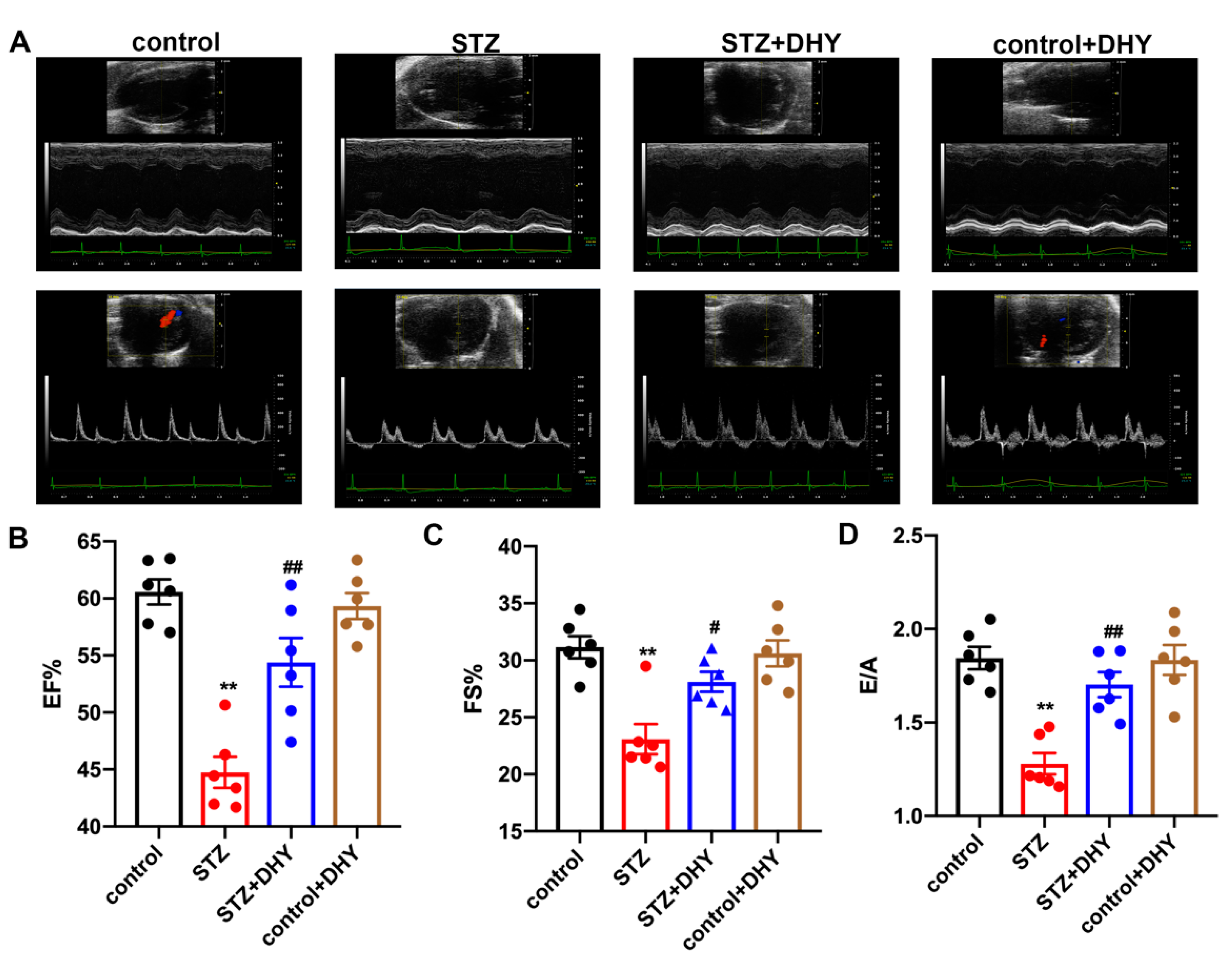
3.2. DHY Improved Cardiac Dysfunction in STZ-Induced Mice
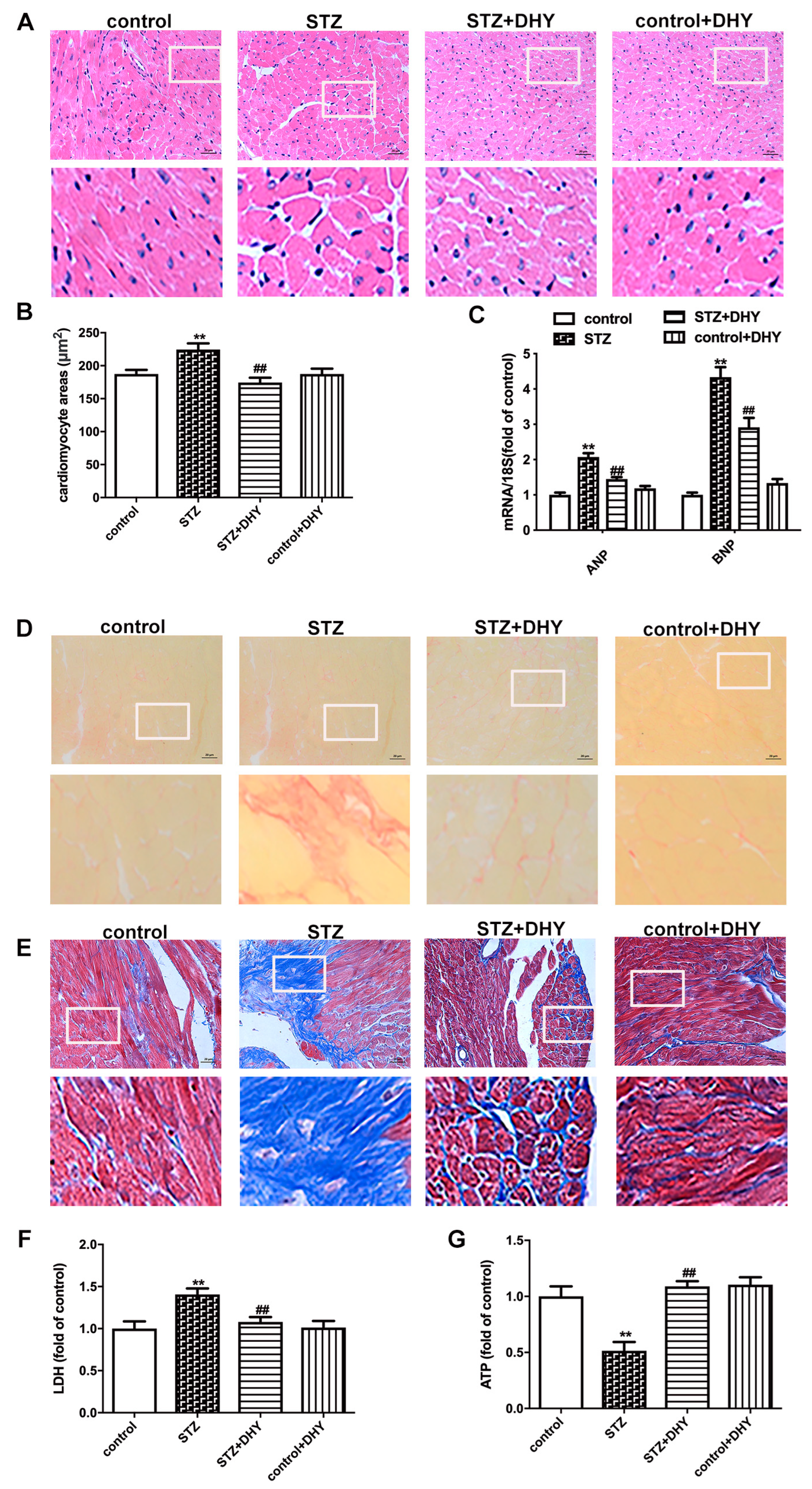
3.3. DHY Ameliorated Myocardial Hypertrophy, Fibrosis and Injury in STZ-Induced Mice
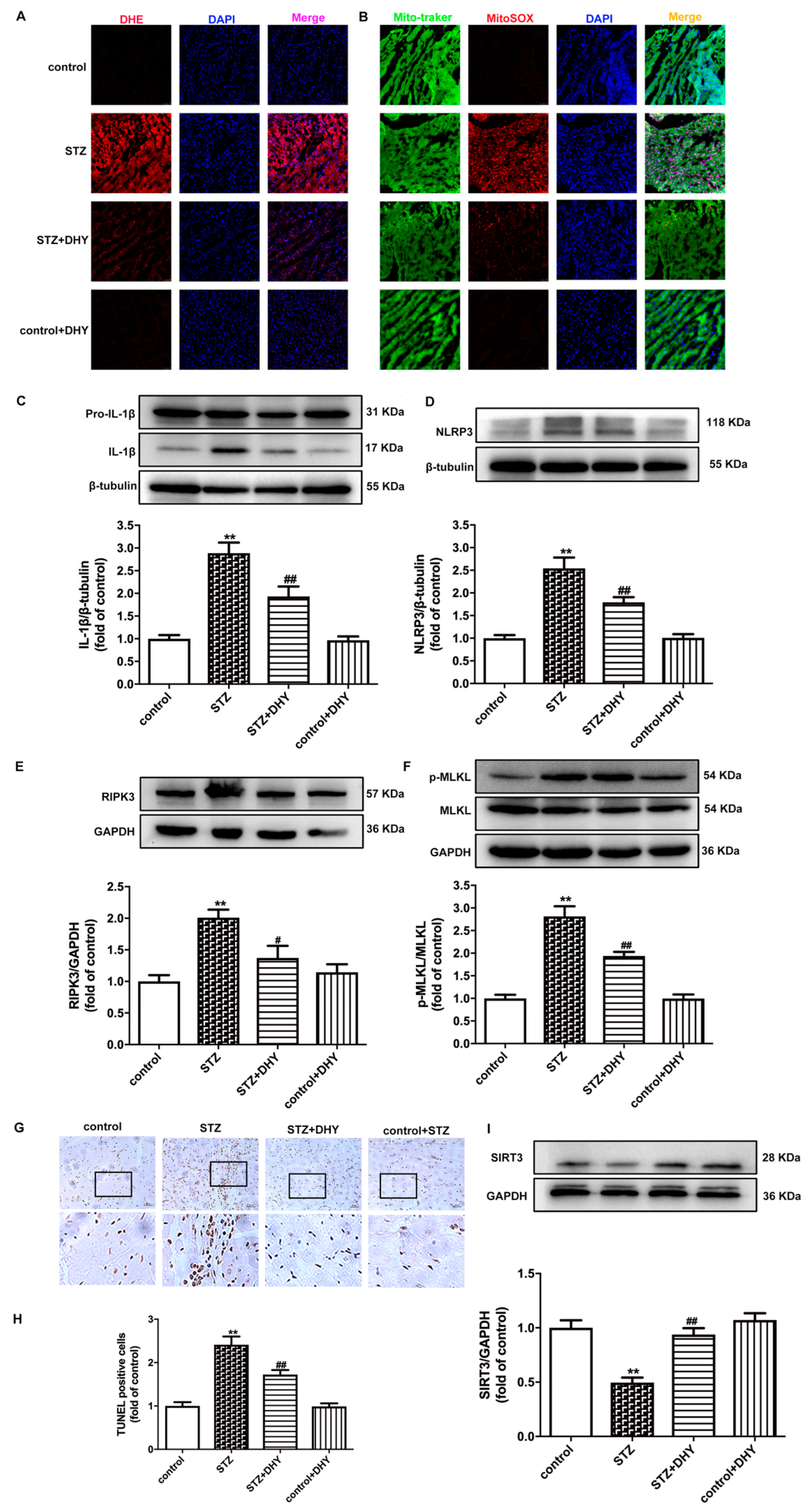
3.4. DHY Suppressed Oxidative Stress, Inflammasome and Necroptosis but Improved SIRT3 Expression in STZ-Induced Mice
3.5. DHY Attenuated Cell Damage but Improved SIRT3 Expression in Cardiomyocytes with High Glucose Stimulation
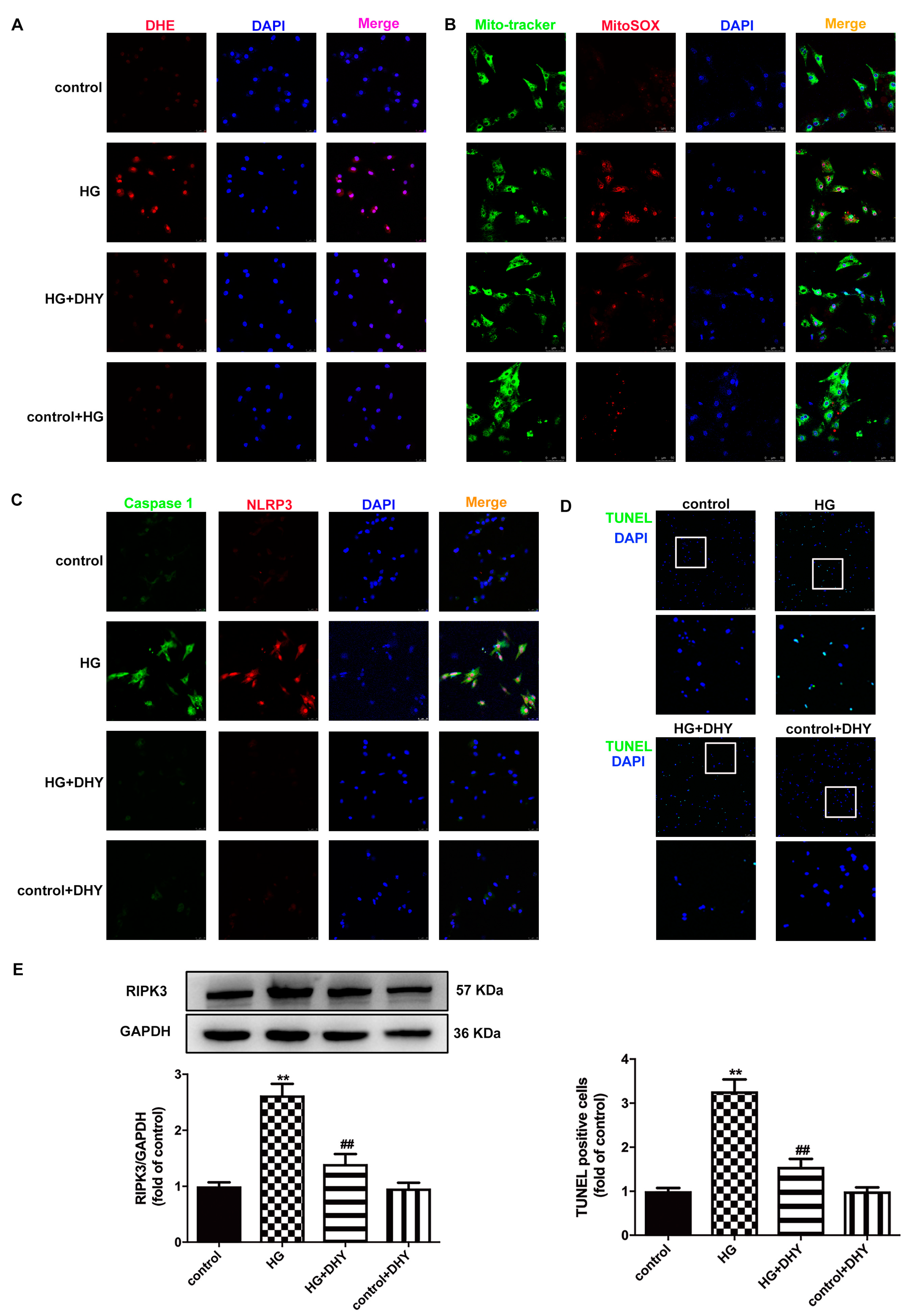
3.6. DHY Inhibited Oxidative Stress, Inflammasome and Necroptosis in Cardiomyocytes with High Glucose Stimulation
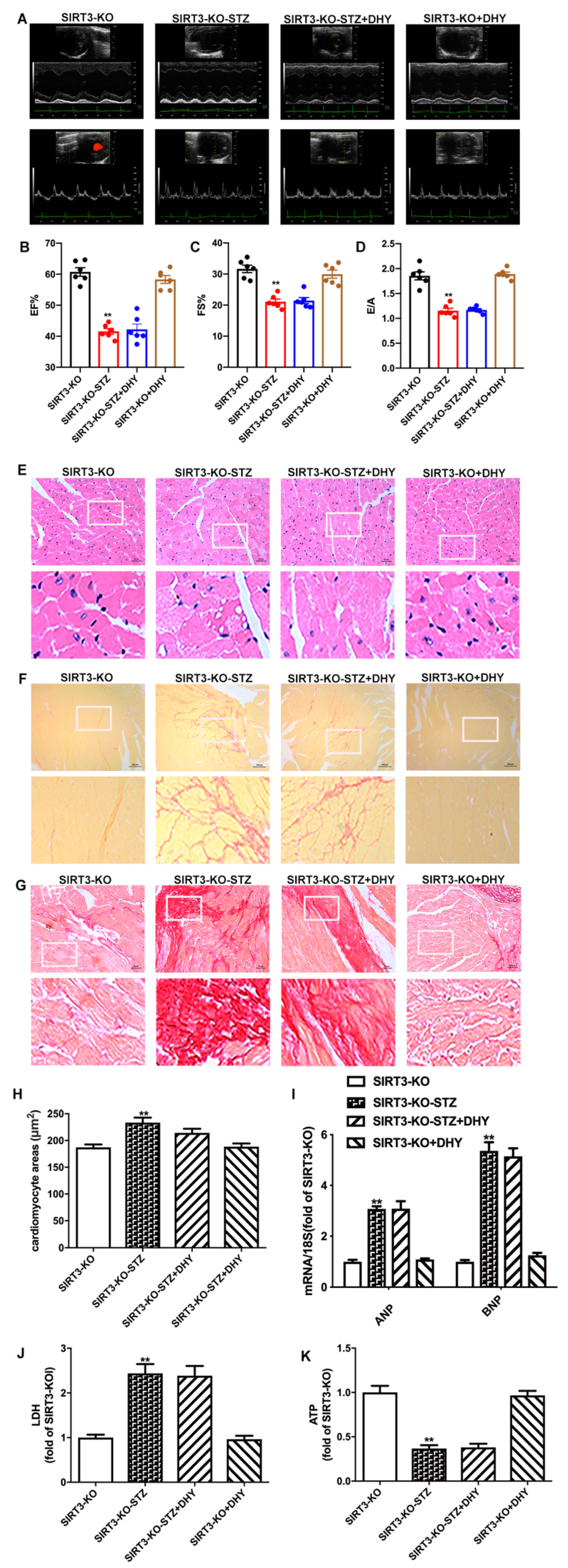
3.7. DHY Failed to Alleviate Diabetic Cardiomyopathy in STZ-Induced SIRT3-KO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Geest, B.; Mishra, M. Role of oxidative stress in diabetic cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lu, W.; Lin, N.; Lin, H.; Zhang, J.; Ni, T.; Meng, L.; Zhang, C.; Guo, H. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem. Pharmacol. 2020, 175, 113888. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, Z.; Chen, L.; Kuang, D.; Zou, Y.; Li, J.; Deng, X.; Luo, S.; Luo, J.; He, J.; et al. Dihydromyricetin increases endothelial nitric oxide production and inhibits atherosclerosis through microRNA-21 in apolipoprotein E-deficient mice. J. Cell. Mol. Med. 2020, 24, 5911–5925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, H.Q.; Sun, L.L.; Xu, M.T.; Yu, J.; Liu, L.L.; Zhang, J.Y.; Wang, Y.Q.; Wang, H.X.; Bao, X.F.; et al. Dihydromyricetin attenuates myocardial hypertrophy induced by transverse aortic constriction via oxidative stress inhibition and SIRT3 pathway enhancement. Int. J. Mol. Sci. 2018, 19, 2592. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Liu, L.; Yu, J.; Zhang, J.; Xu, M.; Sun, L.; Luo, H.; Feng, Z.; Meng, G. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur. J. Pharmacol. 2017, 807, 159–167. [Google Scholar] [CrossRef]
- Meng, G.; Yang, S.; Chen, Y.; Yao, W.; Zhu, H.; Zhang, W. Attenuating effects of dihydromyricetin on angiotensin II-induced rat cardiomyocyte hypertrophy related to antioxidative activity in a NO-dependent manner. Pharm. Biol. 2015, 53, 904–912. [Google Scholar] [CrossRef]
- Hua, Y.Y.; Zhang, Y.; Gong, W.W.; Ding, Y.; Shen, J.R.; Li, H.; Chen, Y.; Meng, G.L. Dihydromyricetin improves endothelial dysfunction in diabetic mice via oxidative stress inhibition in a SIRT3-dependent manner. Int. J. Mol. Sci. 2020, 21, 6699. [Google Scholar] [CrossRef]
- Ding, Y.; Gong, W.; Zhang, S.; Shen, J.; Liu, X.; Wang, Y.; Chen, Y.; Meng, G. Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem. Pharmacol. 2021, 192, 114665. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Zhang, X.; Tong, H.; Sun, X.; Sun, G. Ginsenoside Rb1 protects against diabetic cardiomyopathy by regulating the adipocytokine pathway. J. Inflamm. Res. 2022, 15, 71–83. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Han, J.; Khan, Z.A.; Fang, Q.; Jin, Y.; Chen, X.; Zhang, Y.; Wang, M.; Qian, J.; et al. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat. Commun. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Ge, Q.; Zhao, L.; Ren, X.M.; Ye, P.; Hu, Z.Y. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp. Biol. Med. 2019, 244, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Arif, M.; Varga, Z.V.; Mátyás, C.; Paloczi, J.; Lehocki, A.; Haskó, G.; Pacher, P. Cannabinoid receptor 2 activation alleviates diabetes-induced cardiac dysfunction, inflammation, oxidative stress, and fibrosis. Geroscience 2022, 44, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Al-Khawalde, A.A.A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; et al. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ding, Y.; Dai, G.L.; Zhang, Y.; Xu, M.T.; Shen, J.R.; Chen, T.T.; Chen, Y.; Meng, G.L. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2021, 42, 230–241. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tan, X.; Xian, W.; Geng, J.; Li, H.; Tang, B.; Zhang, H.; Wang, H.; Gao, Q.; Kang, P. Changes of necroptosis in Irbesartan medicated cardioprotection in diabetic rats. Diabetes Metab. Syndr. Obes. 2021, 14, 3851–3863. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Sun, Y.; Berleth, N.; Deitersen, J.; Schlutermann, D.; Stuhldreier, F.; Wallot-Hieke, N.; Jose Mendiburo, M.; Cox, J.; et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy 2021, 17, 3992–4009. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tang, N.; Liu, P.; Sun, Y.; Lu, S.; Liu, W.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy 2022, 18, 1503–1521. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY) 2020, 12, 16224–16237. [Google Scholar] [CrossRef]
- Lin, J.; Du, J.; Wu, X.; Xu, C.; Liu, J.; Jiang, L.; Cheng, X.; Ge, G.; Chen, L.; Pang, Q.; et al. SIRT3 mitigates intervertebral disc degeneration by delaying oxidative stress-induced senescence of nucleus pulposus cells. J. Cell. Physiol. 2021, 236, 6441–6456. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, S.; Chen, Y.; Shen, J.; Zheng, Y.; Liu, X.; Zhu, M.; Meng, G. Protective role of hydrogen sulfide against diabetic cardiomyopathy via alleviating necroptosis. Free Radic. Biol. Med. 2022, 181, 29–42. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Dong, L.; Dang, X.; Wang, L.; Cheng, L.; Huang, Y. Dihydromyricetin attenuates metabolic syndrome and improves insulin sensitivity by upregulating insulin receptor substrate-1 (Y612) tyrosine phosphorylation in db/db Mice. Diabetes Metab. Syndr. Obes. 2019, 12, 2237–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhou, M.; Lang, H.; Zhou, Y.; Mi, M. Dihydromyricetin enhances glucose uptake by inhibition of MEK/ERK pathway and consequent down-regulation of phosphorylation of PPARgamma in 3T3-L1 cells. J. Cell. Mol. Med. 2018, 22, 1247–1256. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin ameliorates inflammation-induced insulin resistance via phospholipase C-CaMKK-AMPK signal pathway. Oxid. Med. Cell. Longev. 2021, 2021, 8542809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wei, Y.; Cui, M.; Li, X.; Ruan, H.; Zhang, L.; Bao, J.; Ren, S.; Gao, D.; Wang, M.; et al. Effect of dihydromyricetin on SARS-CoV-2 viral replication and pulmonary inflammation and fibrosis. Phytomedicine 2021, 91, 153704. [Google Scholar] [CrossRef]
- Jia, W.; Bai, T.; Zeng, J.; Niu, Z.; Fan, D.; Xu, X.; Luo, M.; Wang, P.; Zou, Q.; Dai, X. Combined administration of metformin and atorvastatin attenuates diabetic cardiomyopathy by inhibiting inflammation, apoptosis, and oxidative stress in type 2 diabetic mice. Front. Cell Dev. Biol. 2021, 9, 634900. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Peng, Q.; Zhang, Y.; Wang, J. Dihydromyricetin improves cardiac insufficiency by inhibiting HMGB1 in diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 641–648. [Google Scholar] [CrossRef]
- Ni, T.; Lin, N.; Lu, W.; Sun, Z.; Lin, H.; Chi, J.; Guo, H. Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc. Drugs Ther. 2020, 34, 291–301. [Google Scholar] [CrossRef]
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. Biomed. Res. Int. 2017, 2017, 3764370. [Google Scholar] [CrossRef]
- Fang, T.; Cao, R.; Wang, W.; Ye, H.; Shen, L.; Li, Z.; Hu, J.; Gao, Q. Alterations in necroptosis during ALDH2 mediated protection against high glucose induced H9c2 cardiac cell injury. Mol. Med. Rep. 2018, 18, 2807–2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, X.; Hua, Y.; Ding, Y.; Meng, G.; Zhang, W. RIPK3-mediated mecroptosis in diabetic cardiomyopathy requires CaMKII activation. Oxid. Med. Cell. Longev. 2021, 2021, 6617816. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Huang, X.; Yue, J.; Xiang, H.; Shaheen, S.; Jiang, Z.; Tao, Y.; Tu, J.; Liu, Z.; Yao, Y.; et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics 2021, 11, 3981–3995. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, X.; Cui, Y.; Guo, Y.; Jia, X.; Cui, Y.; Song, Y.; Wang, S.; Geng, Y.; Li, R.; et al. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 2021, 41, 101915. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T.T.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gong, W.; Xu, M.; Zhang, S.; Shen, J.; Zhu, M.; Wang, Y.; Chen, Y.; Shi, J.; Meng, G. Necroptosis inhibition by hydrogen sulfide alleviated hypoxia-induced cardiac fibroblasts proliferation via Sirtuin 3. Int. J. Mol. Sci. 2021, 22, 11893. [Google Scholar] [CrossRef]
- Wei, L.; Sun, X.; Qi, X.; Zhang, Y.; Li, Y.; Xu, Y. Dihydromyricetin ameliorates cardiac ischemia/reperfusion injury through Sirt3 activation. Biomed. Res. Int. 2019, 2019, 6803943. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Feng, H.; Li, S.; Meng, G.; Liu, S.; Tang, X.; Ma, Y.; Han, Y.; Xiao, Y.; Gu, Y.; et al. SIRT3 mediates the antioxidant effect of hydrogen sulfide in endothelial cells. Antioxid. Redox Signal. 2016, 24, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Li, Y.; Jin, Y.; Chen, Y.; Tian, L.; He, W. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-kappaB/NLRP3 and PGC1a/SIRT3 pathways. Int. Immunopharmacol. 2021, 96, 107728. [Google Scholar] [CrossRef]
- Liu, S.G.; Wang, Y.M.; Zhang, Y.J.; He, X.J.; Ma, T.; Song, W.; Zhang, Y.M. ZL006 protects spinal cord neurons against ischemia-induced oxidative stress through AMPK-PGC-1alpha-Sirt3 pathway. Neurochem. Int. 2017, 108, 230–237. [Google Scholar] [CrossRef]
- Nasoni, M.G.; Carloni, S.; Canonico, B.; Burattini, S.; Cesarini, E.; Papa, S.; Pagliarini, M.; Ambrogini, P.; Balduini, W.; Luchetti, F. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal. Res. 2021, 71, e12747. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zheng, Y.; Chen, R.; Shen, J.; Zhang, S.; Gu, Y.; Shi, J.; Meng, G. Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants 2023, 12, 200. https://doi.org/10.3390/antiox12010200
Chen Y, Zheng Y, Chen R, Shen J, Zhang S, Gu Y, Shi J, Meng G. Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants. 2023; 12(1):200. https://doi.org/10.3390/antiox12010200
Chicago/Turabian StyleChen, Yun, Yangyang Zheng, Ruixiang Chen, Jieru Shen, Shuping Zhang, Yunhui Gu, Jiahai Shi, and Guoliang Meng. 2023. "Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation" Antioxidants 12, no. 1: 200. https://doi.org/10.3390/antiox12010200
APA StyleChen, Y., Zheng, Y., Chen, R., Shen, J., Zhang, S., Gu, Y., Shi, J., & Meng, G. (2023). Dihydromyricetin Attenuates Diabetic Cardiomyopathy by Inhibiting Oxidative Stress, Inflammation and Necroptosis via Sirtuin 3 Activation. Antioxidants, 12(1), 200. https://doi.org/10.3390/antiox12010200







