Dietary Methionine Level Impacts the Growth, Nutrient Metabolism, Antioxidant Capacity and Immunity of the Chinese Mitten Crab (Eriocheir sinensis) under Chronic Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Design, Sampling and Growth Measurement
2.3. Chemical Composition Analysis
2.4. Enzyme Activity Assay
2.5. Analysis of Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Whole-Body Proximate Composition
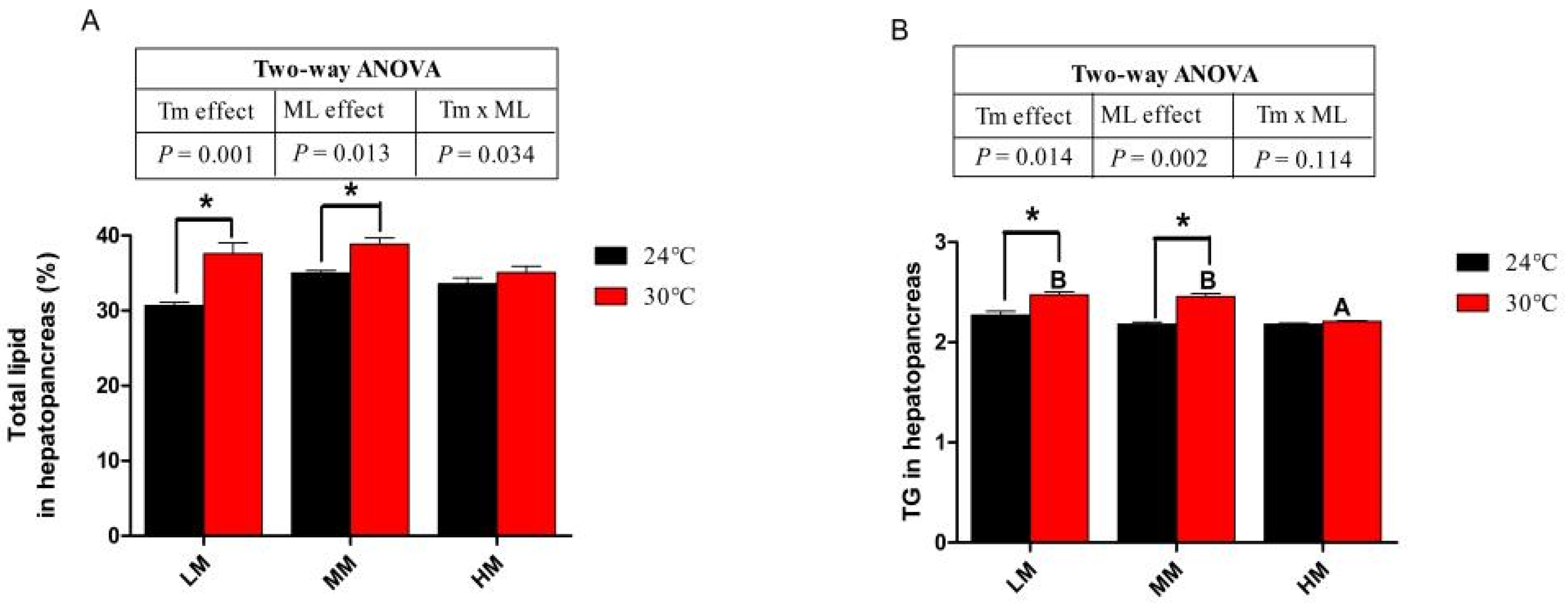
3.3. Hepatopancreas Lipid, Triglyceride (TG) and Gene Expressions of Lipid Metabolism
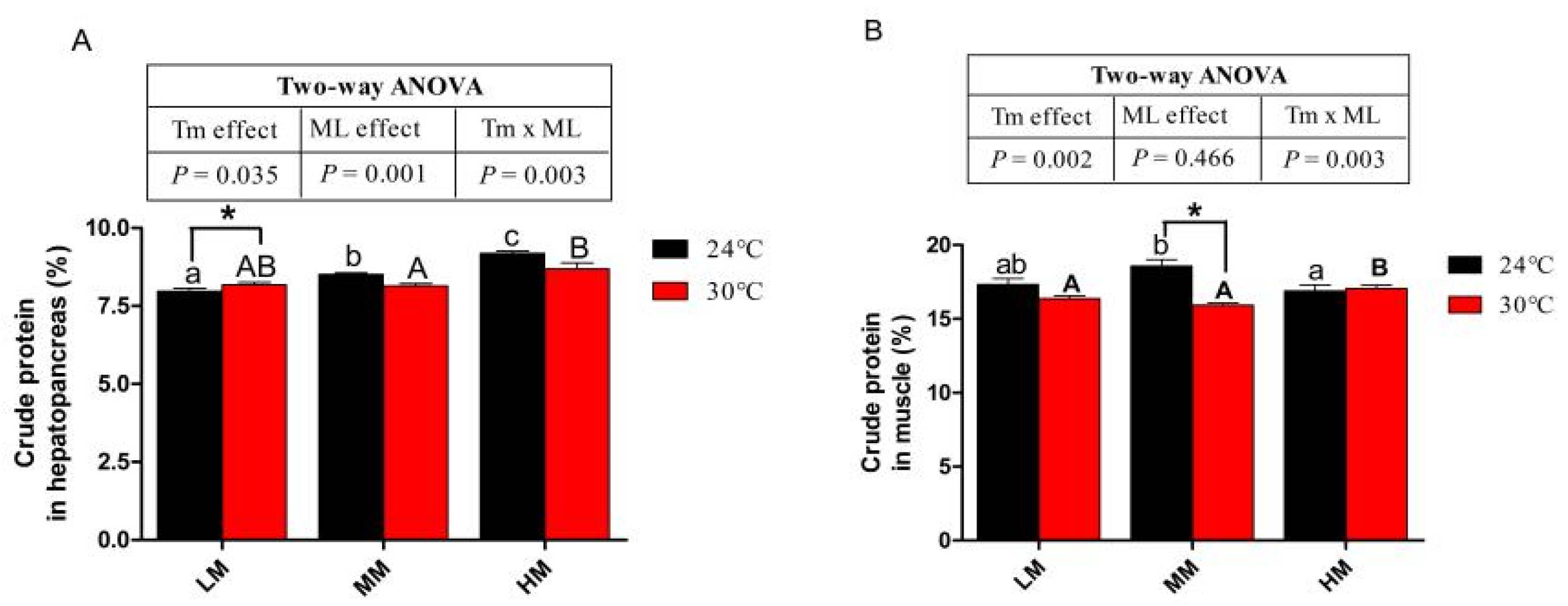
3.4. Hepatopancreas and Muscle Crude Protein Content
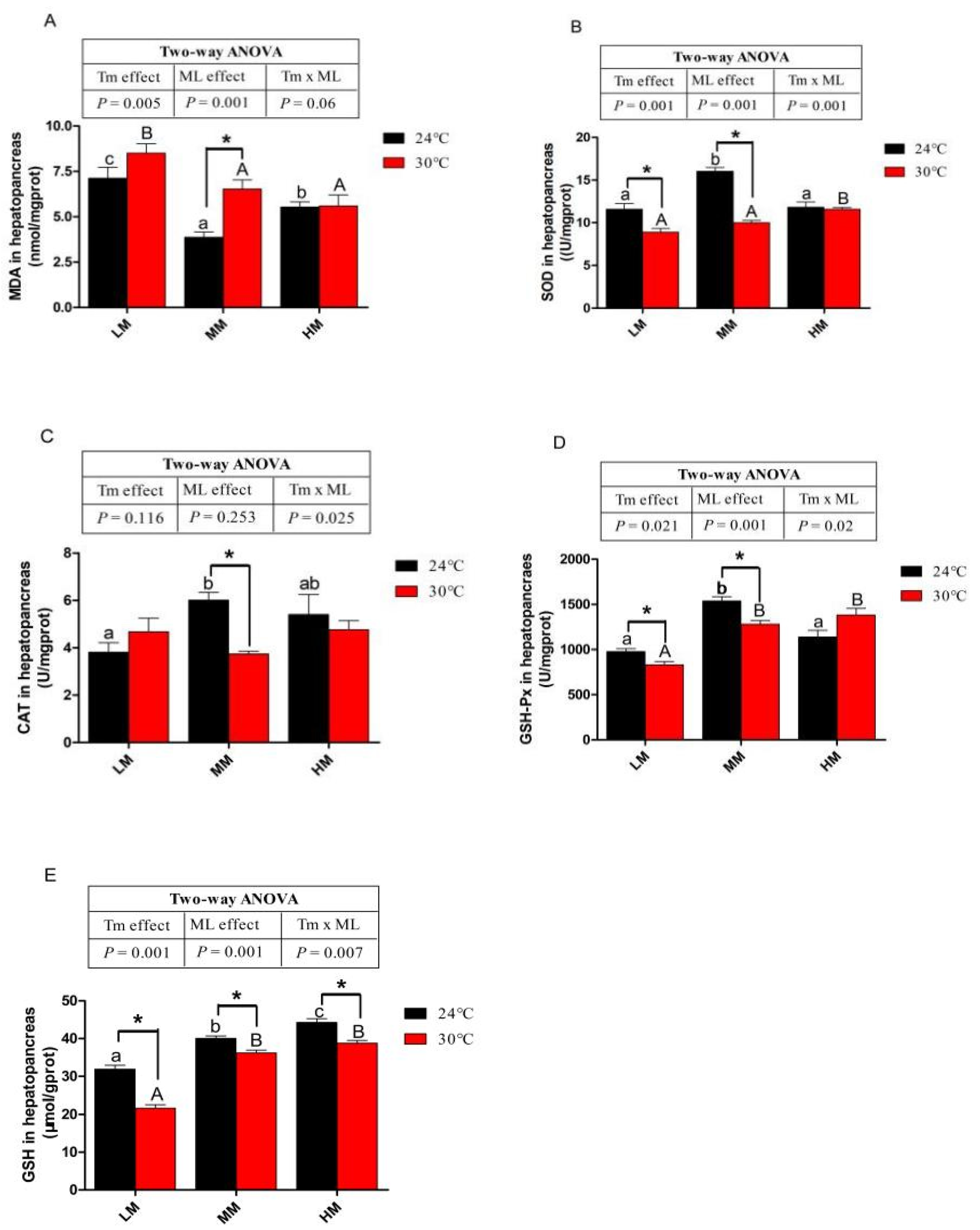
3.5. Antioxidative Capacity
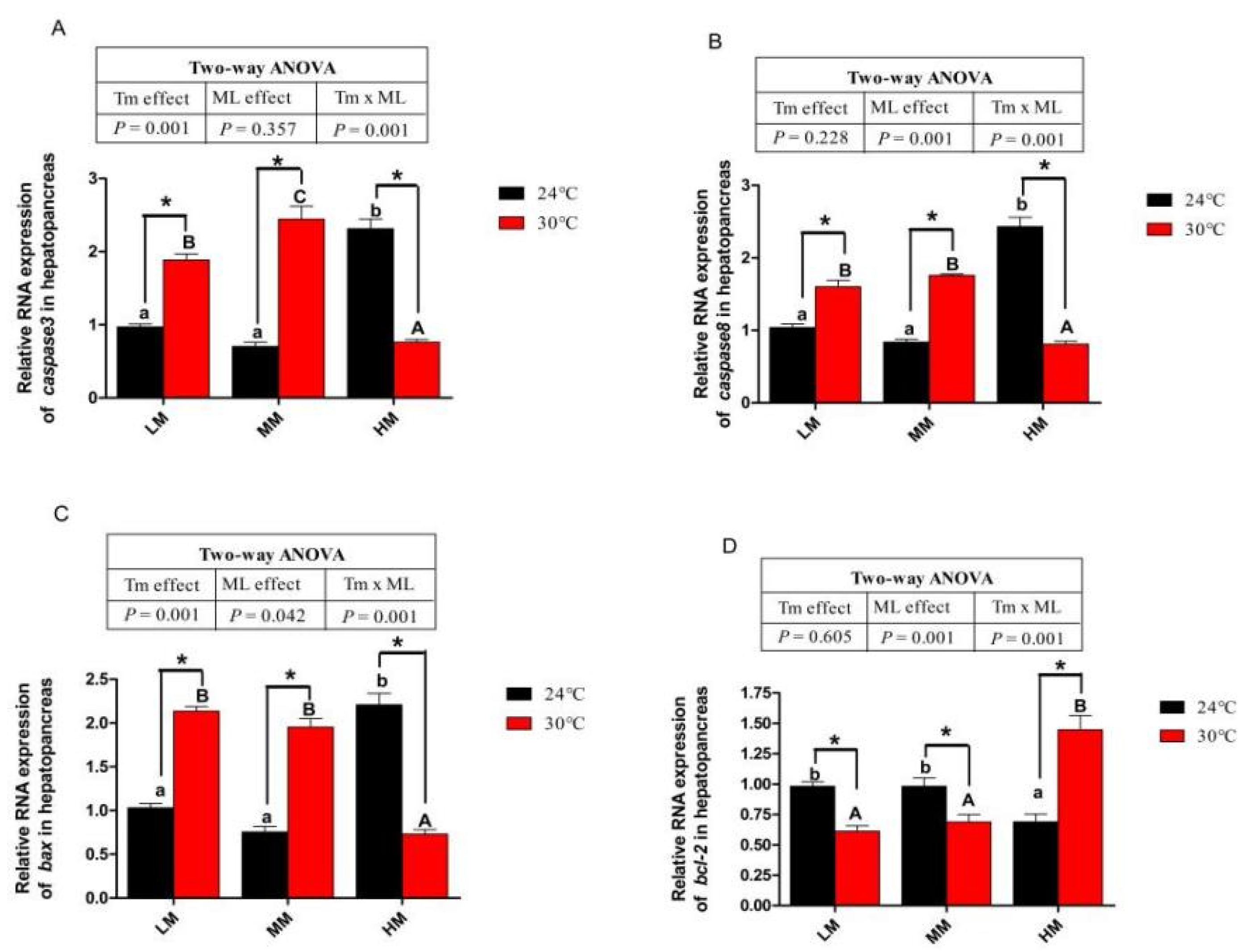
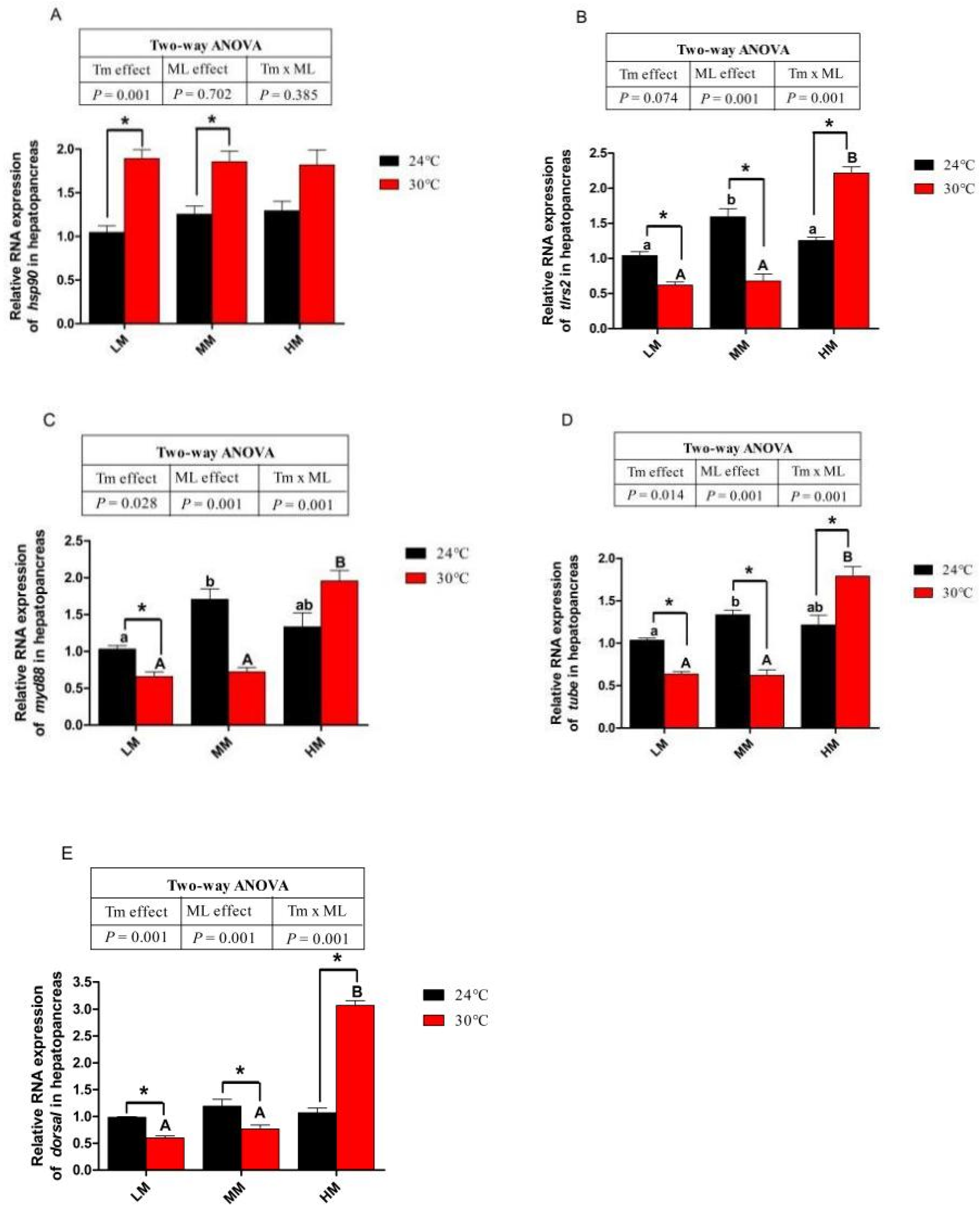
3.6. Expression of Genes about Apoptotic Factors and Tlrs Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Wang, Y.; Zhao, X.; Lu, M.; Tang, P. Construction and application of high temperature heat damage index for river crab culture in Hongze Lake Beach. Agric. Biotechnol. 2019, 8, 128–132. [Google Scholar]
- Liu, G.S.; Cai, X.Y.; Tong, F.; Wang, L.; Zhang, X.M. Investigation of massive death of sea cucumber in artificial reef zone of Shuangdao Bay, Weiha. Fish. Inf. Strategy 2014, 29, 122–129. (In Chinese) [Google Scholar]
- Sephton, D.H.; Driedzic, W.R. Effect of acute and chronic temperature transition on enzymes of cardiac metabolism in white perch (Morone americana), yellow perch (Perca flavescens), and smallmouth bass (Micropterus dolomieui). Can. J. Zool. 1991, 69, 258–262. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens1. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Starkie, R.L.; Hargreaves, M.; Rolland, J.; Febbraio, M.A. Heat stress, cytokines, and the immune response to exercise. Brain, Behav. Immun. 2005, 19, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Kullgren, A.; Jutfelt, F.; Fontanillas, R.; Sundell, K.; Samuelsson, L.; Wiklander, K.; Kling, P.; Koppe, W.; Larsson, D.G.J.; Bjornsson, B.T. The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic Salmon (Salmo salar). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Foss, A.; Sparboe, L.O.; Sigurdsson, S. The effect of temperature and fish size on growth and feed efficiency ratio of juvenile spotted wolffish Anarhichas minor. J. Fish Biol. 2006, 68, 1107–1122. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The Effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic Salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, S.J. Effect of brooding temperature on larval quality of shrimp and crab. Chin. J. Appl. Ecol. 1998, 1, 71–74. (In Chinese) [Google Scholar]
- Yuan, Q.; Qian, J.; Ren, Y.; Zhang, T.; Li, Z.; Liu, J. Effects of stocking density and water temperature on survival and growth of the juvenile Chinese mitten crab, Eriocheir sinensis, reared under laboratory conditions. Aquaculture 2018, 495, 631–636. [Google Scholar] [CrossRef]
- Leung, K.M.Y.; Chu, J.C.W.; Wu, R.S.S. Effects of body weight, water temperature and ration size on ammonia excretion by the areolated grouper (Epinephelus areolatus) and Mangrove Snapper (Lutjanus argentimaculatus). Aquaculture 1999, 170, 215–227. [Google Scholar] [CrossRef]
- Aksit, M.; Yalcin, S.; Ozkan, S.; Metin, K.; Ozdemir, D. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Altan, O.; Pabuccuoglu, A.; Altan, A.; Konyalioglu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.H.; Xu, H.J.; Yong, Y.H.; An, L.L.; Jiao, P.R.; Liao, M. Heat stress upregulation of toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of bama miniature pigs: An in vivo and in vitro study. Animal 2014, 8, 1462–1468. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Feng, G.L.; Xu, Y.L.; Jiang, X.Y.; Li, J.M.; Li, G.Y.; Yang, Y.L. Comparsion of photosynthetic characteristics of two spring wheat seedling to salt stress. J. Gansu Agric. Univ. 2020, 55, 45–55. (In Chinese) [Google Scholar]
- Kalbande, V.H.; Ravikanth, K.; Maini, S.; Rekhe, D.S. Methionine supplementation options in poultry. Int. J. Poult. Sci. 2009, 8, 588–591. [Google Scholar] [CrossRef]
- Zhan, X.A.; Li, J.X.; Xu, Z.R.; Zhao, R.Q. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006, 47, 576–580. [Google Scholar] [CrossRef]
- Pan, F.Y.; Feng, L.; Jiang, W.D.; Jiang, J.; Wu, P.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. Methionine hydroxy analogue enhanced fish immunity via modulation of NF-ΚB, TOR, MLCK, MAPKs and Nrf2 Signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 56, 208–228. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Gasparin, F.R.S.; Constantin, J.; Neto, A.R.O. Effects of methionine supplementation on the redox state of acute heat stress–exposed quails1. J. Anim. Sci. 2014, 92, 806–815. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; de Souza Khatlab, A.; Santana, T.P.; Pozza, P.C.; Menck Soares, M.A.; Brito, C.O.; Barbosa, L.T.; Gasparino, E. Heat stress effect on the intestinal epithelial function of broilers fed methionine supplementation. Livest. Sci. 2020, 240, 10415. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Q.; Zhang, T.; Li, Z.; Liu, J. Effects of water temperature on growth, feeding and molting of juvenile Chinese mitten crab Eriocheir sinensis. Aquaculture 2017, 468, 169–174. [Google Scholar] [CrossRef]
- Jakubowska, M.; Normant, M. Effect of temperature on the physiology and bioenergetics of adults of the Chinese mitten crab Eriocheir sinensis: Considerations for a species invading cooler waters. Mar. Freshw. Behav. Physiol. 2011, 44, 171–183. [Google Scholar] [CrossRef]
- Hamasaki, K. Effects of temperature on the egg incubation period, survival and developmental period of larvae of the mud crab Scylla serrata (Forskal) (Brachyura: Portunidae) Reared in the Laboratory. Aquaculture 2003, 219, 561–572. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Neuparth, T.; Costa, F.O.; Costa, M.H. Effects of temperature and salinity on life history of the marine amphipod gammarus locusta. implications for ecotoxicological testing. Ecotoxicology 2002, 11, 61–73. [Google Scholar] [CrossRef]
- Orbea, A.; Ortiz, Z.M.; Sole, M.; Porte, C.; Cajaraville, M.P. Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia Estuaries (Bay of Biscay). Aquat. Toxicol. 2002, 58, 75–98. [Google Scholar] [CrossRef]
- Han, F.; Wang, X.; Guo, J.; Qi, C.; Xu, C.; Luo, Y.; Li, E.; Qin, J.G.; Chen, L. Effects of glycinin and β-conglycinin on growth performance and intestinal health in juvenile Chinese mitten crabs (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 84, 269–279. [Google Scholar] [CrossRef]
- Wucheng, Y.; Chen, X.; Lu, G.; Chen, J.; Lu, W.; Wang, C.; Huang, S.; Wang, J. Selection of appropriate reference genes for qPCR in the Chinese mitten crab, Eriocheir sinensis (Decapoda, Varunidae). Crustaceana 2017, 90, 275–296. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, X.; Cao, M. Effects of salinity and temperature on survival, growth, and energy budget of juvenile Litopenaeus vannamei. J. Shellfish Res. 2009, 26, 141–146. [Google Scholar] [CrossRef]
- Jones, C.M.; Ruscoe, I.M. Assessment of stocking size and density in the production of redclaw crayfish, Cherax Quadricarinatus (von Martens) (Decapoda: Parastacidae), cultured under earthen pond conditions. Aquaculture 2000, 189, 63–71. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, S.L.; Wang, F. Studies on the bioenergetics of Penaeus chinensis, II. Effects of temperature and body weight on energy budget. J. Ocean. Univ. Qingdao 1998, 28, 228–232. (In Chinese) [Google Scholar]
- Wu, Z.X.; Chen, X.X.; Li, M. Experiment on temperature tolerance of Cherax quadricarinatus. Reserv. Fish. 1997, 3, 2–8. (In Chinese) [Google Scholar]
- An, Z.-H.; Dong, Y.W.; Dong, S.L. A high-performance temperature-control Scheme: Growth of sea cucumber apostichopus japonicus with different modes of diel temperature fluctuation. Aquac. Int. 2009, 17, 459–467. [Google Scholar] [CrossRef]
- Wang, X.; Lei, X.-Y.; Guo, Z.-X.; Wang, S.; Wan, J.-W.; Liu, H.-J. The immuneoreaction and antioxidant status of Chinese mitten crab (Eriocheir sinensis) involve protein metabolism and the response of mTOR signaling pathway to dietary methionine levels. Fish Shellfish Immunol. 2022, 127, 703–714. [Google Scholar] [CrossRef]
- Le, D.T.; Liang, X.; Fomenko, D.E.; Raza, A.S.; Chong, C.K.; Carlson, B.A.; Hatfield, D.L.; Gladyshev, V.N. Analysis of methionine/selenomethionine oxidation and methionine sulfoxide reductase function using methionine-rich proteins and antibodies against their oxidized forms. Biochemistry 2008, 47, 6685–6694. [Google Scholar] [CrossRef]
- Xu, H.; Ren, M.; Liang, H.; Ge, X.; Ji, K.; Huang, D.; Yu, H.; Wu, L. Interactive effects of water salinity and dietary methionine levels on growth performance, whole-body composition, plasma parameters, and expression of major nutrient metabolism genes in juvenile genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737381. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Z.; Shi, H.; Wang, J.; Huang, J.; Li, Y.; Li, J.; Wang, Y. Label-free quantification of protein expression in the rainbow trout (Oncorhynchus mykiss) in response to short-term exposure to heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 158–168. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Feng, S.; Xiang, X.; Mai, K.; Ai, Q. Effects of dietary phospholipid on lipase activity, antioxidant capacity and lipid metabolism-related gene expression in large yellow croaker larvae (Larimichthys crocea). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 201, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Mai, K.; Ai, Q. Regulation of hepatic lipid deposition by phospholipid in large yellow croaker. Br. J. Nutr. 2017, 118, 999–1009. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Huang, D.; Guo, Y.; Wu, Y.; Wu, C.; Zhang, W.; Mai, K. Interactions of dietary carbohydrate and vitamin D3 on growth performance, insulin signaling pathway and glucose metabolism in juvenile Abalone haliotis Discus Hannai. Aquaculture 2021, 542, 736908. [Google Scholar] [CrossRef]
- Aissa, A.F.; Tryndyak, V.; Conti, A.; Melnyk, S.; Gomes, T.D.U.H.; Bianchi, M.L.P.; James, S.J.; Beland, F.A.; Antunes, L.M.G.; Pogribny, I.P. Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol. Nutr. Food Res. 2014, 58, 1502–1512. [Google Scholar] [CrossRef]
- Nuez-Ortin, W.G.; Carter, C.G.; Nichols, P.D.; Cooke, I.R.; Wilson, R. Liver proteome response of pre-harvest Atlantic Salmon following exposure to elevated temperature. BMC Genom. 2018, 19, 133–137. [Google Scholar] [CrossRef]
- Roy, P.K.; Lall, S.P. Dietary phosphorus requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture 2003, 221, 451–468. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, L.; Li, J.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef]
- Yun, Y.; Song, D.; He, Z.; Mi, J.; Wang, L.; Nie, G. Effects of methionine supplementation in plant protein based diet on growth performance and fillet quality of iuveniles yellow river carp (Cyprinus carpio haematopterus). Aquaculture 2022, 549, 737810. [Google Scholar] [CrossRef]
- Aledo, J.C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 2019, 28, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Bagnyukova, T.V. Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, P.; Li, J.; Gao, B.Q.; Chen, P. Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: Antioxidant defense and heat shock proteins. Aquaculture 2014, 434, 11–17. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Zhou, H.; Zhang, Y.; Gao, W.; Zhang, W.; Mai, K. Reduced glutathione supplementation in practical diet improves the growth, anti-oxidative capacity, disease resistance and gut morphology of shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 73, 152–157. [Google Scholar] [CrossRef]
- Perez, J.A.; Peres, H.; Rubio, V.C.; Oliva, T.A. The effect of hypoxia on intermediary metabolism and oxidative status in gilthead sea bream (Sparus aurata) fed on diets supplemented with methionine and white tea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 506–516. [Google Scholar] [CrossRef]
- Rubin, L.L.; Canal, C.W.; Ribeiro, A.L.M.; Kessler, A.; Silva, I.; Trevizan, L.; Viola, T.; Raber, M.; Gonçalves, T.A.; Kras, R. Effects of methionine and arginine dietary levels on the immunity of broiler chickens submitted to immunological stimuli. Braz. J. Poult. Sci. 2007, 9, 241–247. [Google Scholar] [CrossRef]
- Machado, M.; Moura, J.; Peixoto, D.; Castro, M.; Conceicao, L.E.C.; Dias, J.; Costas, B. Dietary methionine as a strategy to improve innate immunity in rainbow trout (Oncorhynchus mykiss) juveniles. Gen. Comp. Endocrinol. 2021, 302, 113690. [Google Scholar] [CrossRef]
- Grobner, S.; Adkins, I.; Schulz, S.; Richter, K.; Borgmann, S.; Wesselborg, S.; Ruckdeschel, K.; Micheau, O.; Autenrieth, I.B. Catalytically active yersinia outer protein P induces cleavage of RIP and caspase-8 at the level of the DISC independently of death receptors in dendritic cells. Apoptosis 2007, 12, 1813–1825. [Google Scholar] [CrossRef]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Velez, D.A.; Wang, N.P.; Hewan, K.O.; Nakamura, M.; Guyton, R.A.; Vinten, J.J. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis 2001, 6, 279–290. [Google Scholar] [CrossRef]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Mi, H.; Pan, L.; Yu, H. The immunoreaction and antioxidant capacity of iuvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-ΚB signal pathways in response to dietary methionine levels. Fish Shellfish Immunol. 2020, 105, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Flohe, S.B.; Bruggemann, J.; Lendemans, S.; Nikulina, M.; Meierhoff, G.; Flohe, S.; Kolb, H. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype1. J. Immunol. 2003, 170, 2340–2348. [Google Scholar] [CrossRef]
- Bose, S.; Weikl, T.; Bügl, H.; Buchner, J. Chaperone function of Hsp90-associated proteins. Science 1996, 274, 1715–1717. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156–160. [Google Scholar] [CrossRef]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. CMLS Cell. Mol. Life Sci. 2002, 59, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Queitsch, C.; Sangster, T.A.; Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 2002, 417, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef]
- Welch, W.J.; Feramisco, J.R. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982, 257, 14949–14959. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile Tilapia (Oreochromis niloticus) exposed to heat stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.-F.; Gao, B. Heat shock proteins and immune system. J. Leukoc. Biol. 2009, 85, 905–910. [Google Scholar] [CrossRef]
- Fu, J.; Shi, H.; Cao, N.; Wu, S.; Zhan, T.; Xie, L.; Wang, Z.; Ye, L.; Li, C.; Shen, Y.; et al. Toll-like receptor 9 signaling promotes autophagy and apoptosis via divergent functions of the P38/JNK pathway in human salivary gland cells. Exp. Cell Res. 2019, 375, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.P.; Liu, Y.L.; Ding, K.N.; Hou, X.J.; Qin, J.J.; Zhang, Y.A.; Liu, H.X.; Shen, X.L.; He, Y.M. Chai hu oral liquid enhances the immune functions of both spleen and bursa of fabricius in heat-stressed broilers through Strengthening TLR4-TBK1 signaling pathway. Poult. Sci. 2021, 100, 101302. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Azeredo, R.; Diaz, R.P.; Afonso, A.; Peres, H.; Oliva, T.A.; Costas, B. Dietary tryptophan and methionine as modulators of European Seabass (Dicentrarchus Labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.C.H.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef]

| Ingredients | Experiment Diets | ||
|---|---|---|---|
| LM | MM | HM | |
| Fermented soybean meal | 30 | 30 | 30 |
| Cottonseed meal | 15 | 15 | 15 |
| Corn gluten meal | 12 | 12 | 12 |
| Chicken meal | 12 | 12 | 12 |
| α-starch | 12 | 12 | 12 |
| Fish oil | 4.5 | 4.5 | 4.5 |
| Lecithin | 1 | 1 | 1 |
| Cholesterol | 0.5 | 0.5 | 0.5 |
| Choline chloride | 0.5 | 0.5 | 0.5 |
| Vitamin premix a | 3 | 3 | 3 |
| Mineral premix b | 2 | 2 | 2 |
| Coated lysine | 1.37 | 1.37 | 1.37 |
| Butylated hydroxytoluene | 0.05 | 0.05 | 0.05 |
| Cellulose | 2.48 | 1.68 | 0.88 |
| Carboxymethyl cellulose | 2 | 2 | 2 |
| Glycine | 1.6 | 0.8 | 0 |
| Coated methionine | 0 | 1.6 | 3.2 |
| Proximate composition | |||
| Moisture | 13.20 | 12.42 | 13.10 |
| Crude protein | 38.24 | 38.13 | 38.19 |
| Crude lipid | 9.13 | 9.11 | 9.20 |
| Amino Acid | Experimental Diets | ||
|---|---|---|---|
| LM | MM | HM | |
| Asparagine | 3.42 | 3.60 | 3.55 |
| Threonine | 1.40 | 1.43 | 1.44 |
| Serine | 1.65 | 1.71 | 1.73 |
| Glutamic acid | 6.86 | 6.99 | 7.05 |
| Glycine | 3.09 | 2.48 | 1.77 |
| Alanine | 1.99 | 2.04 | 2.03 |
| Cystine | 0.46 | 0.45 | 0.47 |
| Valine | 1.63 | 1.67 | 1.67 |
| Methionine | 0.48 | 1.05 | 1.72 |
| Isoleucine | 1.45 | 1.48 | 1.49 |
| Leucine | 3.17 | 3.23 | 3.20 |
| Tyrosine | 1.15 | 1.23 | 1.18 |
| Phenylalanine | 1.86 | 1.93 | 1.86 |
| Lysine | 2.35 | 2.35 | 2.35 |
| Histidine | 0.92 | 0.96 | 0.95 |
| Arginine | 2.60 | 2.60 | 2.60 |
| Proline | 2.11 | 2.22 | 2.16 |
| Gene | Position | Primer Sequence | Product Size (bp) |
|---|---|---|---|
| srebp-1 | Forward | TCTTCACACCCTCTGGACGC | 162 |
| Reverse | CCAAGGTTGTAATGGCACGC | ||
| elovl6 | Forward | TGAGAAGCGGCAATGGATGAAG | 164 |
| Reverse | TGGAGAAGAGGGCCAGGAAGAC | ||
| fas | Forward | GTCCCTTCTTCTACGCCATCC | 127 |
| Reverse | CGCTCTCCAGGTCAATCTTCAC | ||
| cpt-1α | Forward | CATCTGGACACCCACCTCCA | 183 |
| Reverse | ATCTCCTCACCCGGCACTCT | ||
| caat | Forward | CATCAAGAGCCAGGAGCCCA | 172 |
| Reverse | CTTCAACAGCAGCCCGCAAA | ||
| mttp | Forward | TAGGACAAGCAGGACTTTCCTCA | 138 |
| Reverse | CCACATCCACAAACACATCAACA | ||
| hsp90 | Forward | TCACCAACGACTGGGAGGAT | 83 |
| Reverse | CAGGAAGAGGAGTGCCCTGA | ||
| tlr2 | Forward | CATACCAGGACGACGAAC | 135 |
| Reverse | AGACATTGAGCGAGGAGA | ||
| myd88 | Forward | GCCATCGCAGTCGCCAAGTT | 148 |
| Reverse | GGCATCCTGTTCATCCAGTTCTGAC | ||
| dorsal | Forward | CGTCAGCAGCACAGCAGAGAAT | 272 |
| Reverse | CCCGTATTTCCTCCCTCAACTTCAG | ||
| tube | Forward | ATTGTGCTGCTGGAGTTGCTGAC | 207 |
| Reverse | CATCGTCGGTCGCTTCTTCTTGG | ||
| caspase8 | Forward | CATGGTGATGAGAATGAC | 120 |
| Reverse | TTGGATGAAGTAGAGACG | ||
| caspase3 | Forward | AGGAAAAGTTCACGCCGCTA | 103 |
| Reverse | GGCTGCCTTCTGTCAGGATT | ||
| bax | Forward | AGAGATGAAGCAGACCACGC | 106 |
| Reverse | TTCTACGGTGGGTGAGTCCA | ||
| bcl-2 | Forward | CATCATCTCCCTCTTCGCGG | 100 |
| Reverse | CAGTCCCATCACGTCGATCA | ||
| β-actin | Forward | TCGTGCGAGACATCAAGGAAA | 178 |
| Reverse | AGGAAGGAAGGCTGGAAGAGTG |
| Parameters | Experiment Diets | Two-Way ANOVA (p Value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NT-LM | NT-MM | NT-HM | HT-LM | HT-MM | HT-HM | TL | ML | TL × ML | |
| WG (%) | 147.56 ± 6.63 a,* | 184.43 ± 12.3 b | 158.31 ± 6.95 ab | 215.87 ± 11.28 AB,* | 247.65 ± 22.47 B | 181.08 ± 5.69 A | 0.001 | 0.007 | 0.170 |
| SGR (%/day) | 2.52 ± 0.09 a,* | 3.18 ± 0.17 b | 2.70 ± 0.1 a | 3.64 ± 0.18 AB,* | 4.22 ± 0.38 B | 3.04 ± 0.09 A | 0.001 | 0.003 | 0.139 |
| Survival (%) | 79.05 ± 0.9 a,* | 91.43 ± 4.36 b,* | 80.95 ± 3.43 ab | 54.17 ± 6.34 A,* | 52.08 ± 4.54 A,* | 75.71 ± 0.71 B | 0.001 | 0.036 | 0.003 |
| MR (%) | 106.67 ± 3.43 a | 120.95 ± 2.52 b | 105.71 ± 4.95 a | 120.95 ± 5.79 AB | 127.62 ± 3.43 B | 107.62 ± 2.52 A | 0.036 | 0.003 | 0.324 |
| FI (g) | 1.29 ± 0.03 a,* | 1.30 ± 0.02 a,* | 1.29 ± 0.02 a,* | 1.58 ± 0.01 B,* | 1.62 ± 0.01 B,* | 1.52 ± 0.02 A,* | 0.001 | 0.028 | 0.087 |
| FCR (%) | 1.3 ± 0.03 b,* | 1.16 ± 0.02 a | 1.21 ± 0.06 ab | 1.11 ± 0.04 A,* | 1.15 ± 0.02 AB | 1.24 ± 0.02 B | 0.069 | 0.061 | 0.003 |
| PER (%) | 2.01 ± 0.03 a,* | 2.44 ± 0.01 c | 2.29 ± 0.01 b | 2.58 ± 0.01 C,* | 2.48 ± 0.01 B | 2.23 ± 0.02 A | 0.002 | 0.001 | 0.001 |
| Parameters | Experiment Diets | Two-Way ANOVA(p Value) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NT-LM | NT-MM | NT-HM | HT-LM | HT-MM | HT-HM | TL | ML | TL × ML | |
| Moisture (%) | 67.83 ± 0.77 | 68.16 ± 0.68 | 64.8 ± 1.89 | 69.33 ± 3.11 | 68.85 ± 1.71 | 65.66 ± 1.79 | 0.512 | 0.165 | 0.973 |
| Ash (%) | 12.21 ± 0.07 | 12.09 ± 0.04 | 12.19 ± 0.08 | 12.14 ± 0.07 | 12.27 ± 0.05 | 12.21 ± 0.11 | 0.469 | 0.911 | 0.267 |
| Crude protein (%) | 11.95 ± 0.36 a | 12.94 ± 0.2 ab,* | 14.01 ± 0.48 b | 10.74 ± 0.37 A | 10.8 ± 0.41 A,* | 13.06 ± 0.59 B | 0.001 | 0.001 | 0.357 |
| Crude lipid (%) | 3.66 ± 0.23 a,* | 4.17 ± 0.31 ab,* | 4.98 ± 0.32 b | 4.78 ± 0.18 * | 5.11 ± 0.08 * | 5.01 ± 0.39 | 0.008 | 0.045 | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, C.; Wang, X.; Li, X.; Huang, Q.; Wang, H.; Miao, Y.; Li, E.; Qin, J.; Chen, L. Dietary Methionine Level Impacts the Growth, Nutrient Metabolism, Antioxidant Capacity and Immunity of the Chinese Mitten Crab (Eriocheir sinensis) under Chronic Heat Stress. Antioxidants 2023, 12, 209. https://doi.org/10.3390/antiox12010209
Liu J, Zhang C, Wang X, Li X, Huang Q, Wang H, Miao Y, Li E, Qin J, Chen L. Dietary Methionine Level Impacts the Growth, Nutrient Metabolism, Antioxidant Capacity and Immunity of the Chinese Mitten Crab (Eriocheir sinensis) under Chronic Heat Stress. Antioxidants. 2023; 12(1):209. https://doi.org/10.3390/antiox12010209
Chicago/Turabian StyleLiu, Jiadai, Cong Zhang, Xiaodan Wang, Xinyu Li, Qincheng Huang, Han Wang, Yixin Miao, Erchao Li, Jianguang Qin, and Liqiao Chen. 2023. "Dietary Methionine Level Impacts the Growth, Nutrient Metabolism, Antioxidant Capacity and Immunity of the Chinese Mitten Crab (Eriocheir sinensis) under Chronic Heat Stress" Antioxidants 12, no. 1: 209. https://doi.org/10.3390/antiox12010209
APA StyleLiu, J., Zhang, C., Wang, X., Li, X., Huang, Q., Wang, H., Miao, Y., Li, E., Qin, J., & Chen, L. (2023). Dietary Methionine Level Impacts the Growth, Nutrient Metabolism, Antioxidant Capacity and Immunity of the Chinese Mitten Crab (Eriocheir sinensis) under Chronic Heat Stress. Antioxidants, 12(1), 209. https://doi.org/10.3390/antiox12010209











