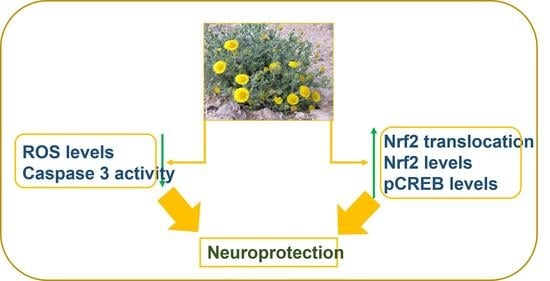
Neuroprotective Effects of Pulicaria incisa Infusion on Human Neuroblastoma Cells and Hippocampal Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Pi Infusion
2.2. Chemical Characterization of Pi Infusion
2.2.1. Sample Preparation
2.2.2. Silylation Derivatization
2.2.3. Gas Chromatography–Mass Spectrometry Analysis
2.2.4. Data Processing and Compound Annotation
2.3. Cell Growth
2.4. Measurement of Cell Viability
2.5. Quantitation of Nrf2 Levels in Nuclear Extracts
2.6. Immunocytochemistry
2.7. Determination of Caspase-3 Activity
2.8. Determination of Phospho-CREB Levels
2.9. Evaluation of Intracellular ROS Levels
2.10. In Vivo Establishment of the Aging Mouse Model
2.11. Hematoxylin and Eosin Staining Assay
2.12. Digital Morphometry and Histopathological Evaluation of Pyknosis in the Hippocampus
2.13. Statistical Analyses
3. Results
3.1. Chemical Characterization of Pi Infusions
3.2. Pi Infusion Protects Neuronal Cells against Oxidative-Stress-Induced Cell Death
3.3. Pi Infusion Inhibits H2O2-Induced Caspase-3 Activity
3.4. The Effect of Pi Infusion on H2O2-Induced ROS Levels in Neuronal Cells
3.5. Pi Infusion Upregulates Cellular Levels of Nrf2 and Induces Its Translocation to the Nucleus
3.6. Pi Infusion Upregulates the Phosphorylation of the Transcription Factor Cyclic AMP Response Element-Binding Protein (CREB)
3.7. Consumption of Pi Infusion Prevents Neuronal Cell Death in the Hippocampus of Aging Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzoni, F.; Scarfò, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System from Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [Green Version]
- Elmann, A.; Beit-Yannai, E.; Telerman, A.; Ofir, R.; Mordechay, S.; Erlank, H.; Borochov-Neori, H. Pulicaria incisa infusion attenuates inflammatory responses of brain microglial cells. J. Funct. Foods 2016, 25, 110–122. [Google Scholar] [CrossRef]
- Elmann, A.; Telerman, A.; Mordechay, S.; Erlank, H.; Ofir, R. Antioxidant and astroprotective effects of a Pulicaria incisa infusion. Oxid. Med. Cell. Longev. 2012, 2012, 157598. [Google Scholar] [CrossRef] [Green Version]
- Mansour, R.M.A.; Ahmed, A.A.; Melek, F.R.; Saleh, N.A.M. The flavonoids of Pulicaria incisa. J. Fitoterapia 1990, 61, 186–187. [Google Scholar]
- Saleh, N.A.M. Global phytochemistry: The Egyptian experience. Phytochemistry 2003, 63, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gleel, W.; Hassanien, M.F.R. Antioxidant properties and lipid profile of Diplotaxis harra, Pulicaria incisa and Avicennia marina. Acta Aliment. 2012, 41, 143–151. [Google Scholar] [CrossRef]
- Bakr, R.O.; Shahat, E.A.; Elissawy, A.E.; Fayez, A.M.; Eldahshan, O.A. Evaluation of the hepatoprotective activity of Pulicaria incisa subspecies candolleana and in silico screening of its isolated phenolics. J. Ethnopharmacol. 2021, 271, 113767. [Google Scholar] [CrossRef] [PubMed]
- El-Sabagh, O.A.; El-Toumy, S.A.; Mounir, R.; Farag, M.A.; Mahrous, E.A. Metabolite profiles of Pulicaria crispa and P. incisa in relation to their in-vitro/in-vivo antioxidant activity and hepatoprotective effect: A comparative mass spectrometry-based metabolomics. J. Pharm. Biomed. Anal. 2020, 194, 113804. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.M.A.; Ramadan, M.F.; Abd El-Gleel, W. Impact of Pulicaria incisa, Diplotaxis harra and Avicennia marina as Hypocholesterolemic Agent. Dtsch. Lebensmitt. Rundsch. 2007, 103, 320–327. [Google Scholar]
- Shabana, M.M.; Mirhom, Y.W.; Genenah, A.A.; Aboutabl, E.A.; Amer, H.A. Study into wild Egyptian plants of potential medicinal activity. Ninth communication: Hypoglycaemic activity of some selected plants in normal fasting and alloxanised rats. Arch. Exp. Veterinärmed. 1990, 44, 389–394. [Google Scholar]
- Woldemichael, G.M.; Gutierrez-Lugo, M.T.; Franzblau, S.G.; Wang, Y.; Suarez, E.; Timmermann, B.N. Mycobacterium tuberculosis growth inhibition by constituents of Sapium haematospermum. J. Nat. Prod. 2004, 67, 598–603. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Amer, M.M.A.; Mansour, H.T.; Wahdan, K.M.; El-Sayed, R.M.; El-Sanhoty, S.; El-Gleel, W.A. Bioactive lipids and antioxidant properties of wild Egyptian Pulicaria incisa, Diplotaxis harra, and Avicennia marina. J. Verbr. Lebensm. 2009, 4, 239–245. [Google Scholar] [CrossRef]
- Mahajna, S.; Kadan, S.; Tietel, Z.; Saad, B.; Khasib, S.; Tumeh, A.; Ginsberg, D.; Zaid, H. In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line. Molecules 2019, 24, 4139. [Google Scholar] [CrossRef] [Green Version]
- Min, A.Y.; Doo, C.N.; Son, E.J.; Sung, N.Y.; Lee, K.J.; Sok, D.E.; Kim, M.R. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem. Biol. Interact. 2015, 242, 153–162. [Google Scholar] [CrossRef]
- Reedy, C.L.; Anderson, J.; Reedy, T.J. Quantitative Porosity Studies of Archaeological Ceramics by Petrographic Image Analysis. Mater. Res. Soc. Symp. Proc. 2017, 1656, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Abu-Reidah, I.M.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MSn. Food Res. Int. 2017, 100, 494–500. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, L. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef] [Green Version]
- Hyslop, P.A.; Zhang, Z.; Pearson, D.V.; Phebus, L.A. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: Correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995, 671, 181–186. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lason, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef]
- Chwastek, J.; Jantas, D.; Lason, W. The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a gammaH2AX/p-p53/caspase-3-independent mechanism: Inhibition of calpain and cathepsin D. Int. J. Biochem. Cell. Biol. 2017, 87, 38–53. [Google Scholar] [CrossRef]
- Cores, A.; Piquero, M.; Villacampa, M.; Leon, R.; Menendez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Cardinaux, J.R. Emerging Roles of CREB-Regulated Transcription Coactivators in Brain Physiology and Pathology. Trends Neurosci. 2017, 40, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G. CREB: A Multifaceted Target for Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. 2007, 274, 3210–3217. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Stebbings, K.A.; Choi, H.W.; Ravindra, A.; Llano, D.A. The impact of aging, hearing loss, and body weight on mouse hippocampal redox state, measured in brain slices using fluorescence imaging. Neurobiol. Aging. 2016, 42, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Zenkov, N.K.; Kozhin, P.M.; Chechushkov, A.V.; Martinovich, G.G.; Kandalintseva, N.V.; Menshchikova, E.B. Mazes of Nrf2 Regulation. Biochemistry 2017, 82, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Mattart, L.; Calay, D.; Simon, D.; Roebroek, L.; Caesens-Koenig, L.; Van Steenbrugge, M.; Tevel, V.; Michiels, C.; Arnould, T.; Boudjeltia, K.Z.; et al. The peroxynitrite donor 3-morpholinosydnonimine activates Nrf2 and the UPR leading to a cytoprotective response in endothelial cells. Cell. Signal. 2012, 24, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, C.; Jiang, H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing. Res. Rev. 2021, 65, 101207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [Green Version]
- Corenblum, M.J.; Ray, S.; Remley, Q.W.; Long, M.; Harder, B.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell 2016, 15, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell. Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Manners, M.T.; Brynildsen, J.K.; Schechter, M.; Liu, X.; Eacret, D.; Blendy, J.A. CREB deletion increases resilience to stress and downregulates inflammatory gene expression in the hippocampus. Brain Behav. Immun. 2019, 81, 388–398. [Google Scholar] [CrossRef]
- Paramanik, V.; Thakur, M.K. Role of CREB signaling in aging brain. Arch. Ital. Biol. 2013, 151, 33–42. [Google Scholar] [CrossRef]
- Yu, X.W.; Oh, M.M.; Disterhoft, J.F. CREB, cellular excitability, and cognition: Implications for aging. Behav. Brain Res. 2017, 322, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Kang, H.; Yun, S.P.; Lee, Y.S.; Lee, Y.I.; Lee, Y. Activation of the Akt1-CREB pathway promotes RNF146 expression to inhibit PARP1-mediated neuronal death. Sci. Signal. 2020, 13, eaax7119. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Xi, Y.; Zhou, Y.; Wang, G. Protective effects of chlorogenic acid on isoflurane-induced cognitive impairment of aged mice. Food Sci. Nutr. 2022, 10, 3492–3500. [Google Scholar] [CrossRef]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug. Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Singh, D. Dietary Flavonoids Interaction with CREB-BDNF Pathway: An Unconventional Approach for Comprehensive Management of Epilepsy. Curr. Neuropharmacol. 2019, 17, 1158–1175. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.M.; Wu, L.Y.; Liu, G.J.; Xu, W.D.; Zhang, X.S.; Gao, Y.Y.; Tao, T.; Zhou, Y.; Lu, Y.; et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J. Neuroinflamm. 2020, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Cheng, X.N.; Bai, F.; Fang, L.Y.; Hu, H.Z.; Sun, D.Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018, 106, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef] [PubMed]










| Compound | Rt | Total Peak Area (%) in the Methanolic Phase | Total Peak Area (%) in the Aqueous Phase |
|---|---|---|---|
| Malic acid | 21.884 | 1.925 | |
| 3-Aminophenol | 25.702 | 1.414 | |
| Citric acid | 27.333 | 1.034 | 3.806 |
| Quininic acid | 28.051 | 1.754 | |
| Fructose | 28.259 | 11.647 | 25.913 |
| Talose | 28.634 | 4.517 | 10.309 |
| Glucose | 28.646 | 9.038 | |
| Myo-Inositol | 29.433 | 6.418 | 25.897 |
| Quinoline, 5-chloro-8-ethoxy-7-iodo- | 29.442 | 1.825 | |
| Galactaric acid | 30.296 | 3.575 | |
| Caffeic acid | 31.520 | 1.327 | |
| Sucrose | 37.814 | 15.348 | 8.261 |
| Turanose | 38.323 | 1.240 | |
| Aucubin | 39.131 | 2.792 | |
| Catechine (2R-E) | 40.487 | 2.115 | |
| Chlorogenic acid 1 | 41.573 | 16.192 | |
| Quercetin | 41.974 | 8.082 | |
| Chlorogenic acid 2 | 42.115 | 1.424 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barak, T.; Miller, O.; Melamed, S.; Tietel, Z.; Harari, M.; Belausov, E.; Elmann, A. Neuroprotective Effects of Pulicaria incisa Infusion on Human Neuroblastoma Cells and Hippocampal Neurons. Antioxidants 2023, 12, 32. https://doi.org/10.3390/antiox12010032
Barak T, Miller O, Melamed S, Tietel Z, Harari M, Belausov E, Elmann A. Neuroprotective Effects of Pulicaria incisa Infusion on Human Neuroblastoma Cells and Hippocampal Neurons. Antioxidants. 2023; 12(1):32. https://doi.org/10.3390/antiox12010032
Chicago/Turabian StyleBarak, Talya, Oshrat Miller, Sarit Melamed, Zipora Tietel, Moti Harari, Eduard Belausov, and Anat Elmann. 2023. "Neuroprotective Effects of Pulicaria incisa Infusion on Human Neuroblastoma Cells and Hippocampal Neurons" Antioxidants 12, no. 1: 32. https://doi.org/10.3390/antiox12010032
APA StyleBarak, T., Miller, O., Melamed, S., Tietel, Z., Harari, M., Belausov, E., & Elmann, A. (2023). Neuroprotective Effects of Pulicaria incisa Infusion on Human Neuroblastoma Cells and Hippocampal Neurons. Antioxidants, 12(1), 32. https://doi.org/10.3390/antiox12010032