Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings
Abstract
:1. Background
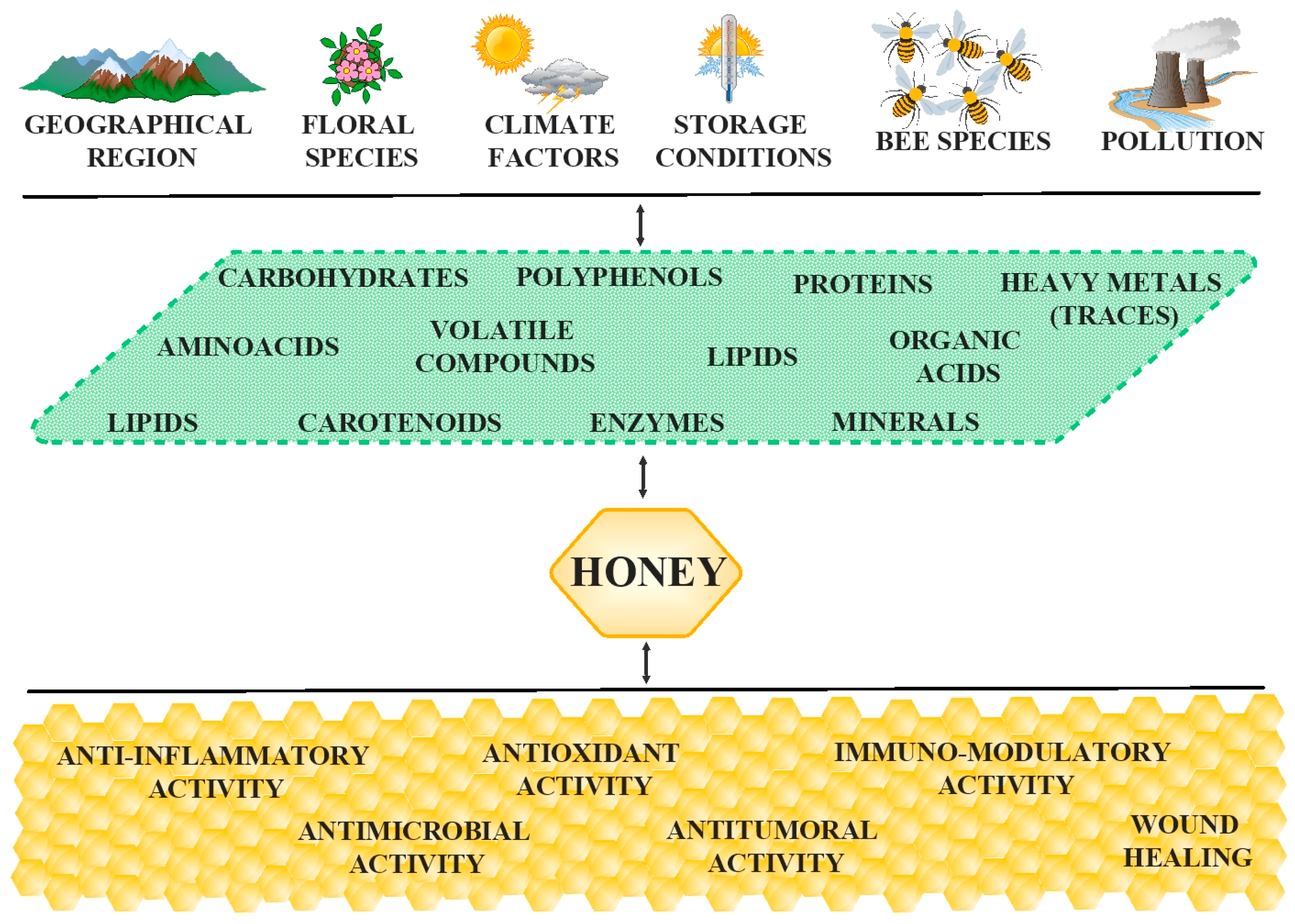
2. The Composition-Related Activity of Honey
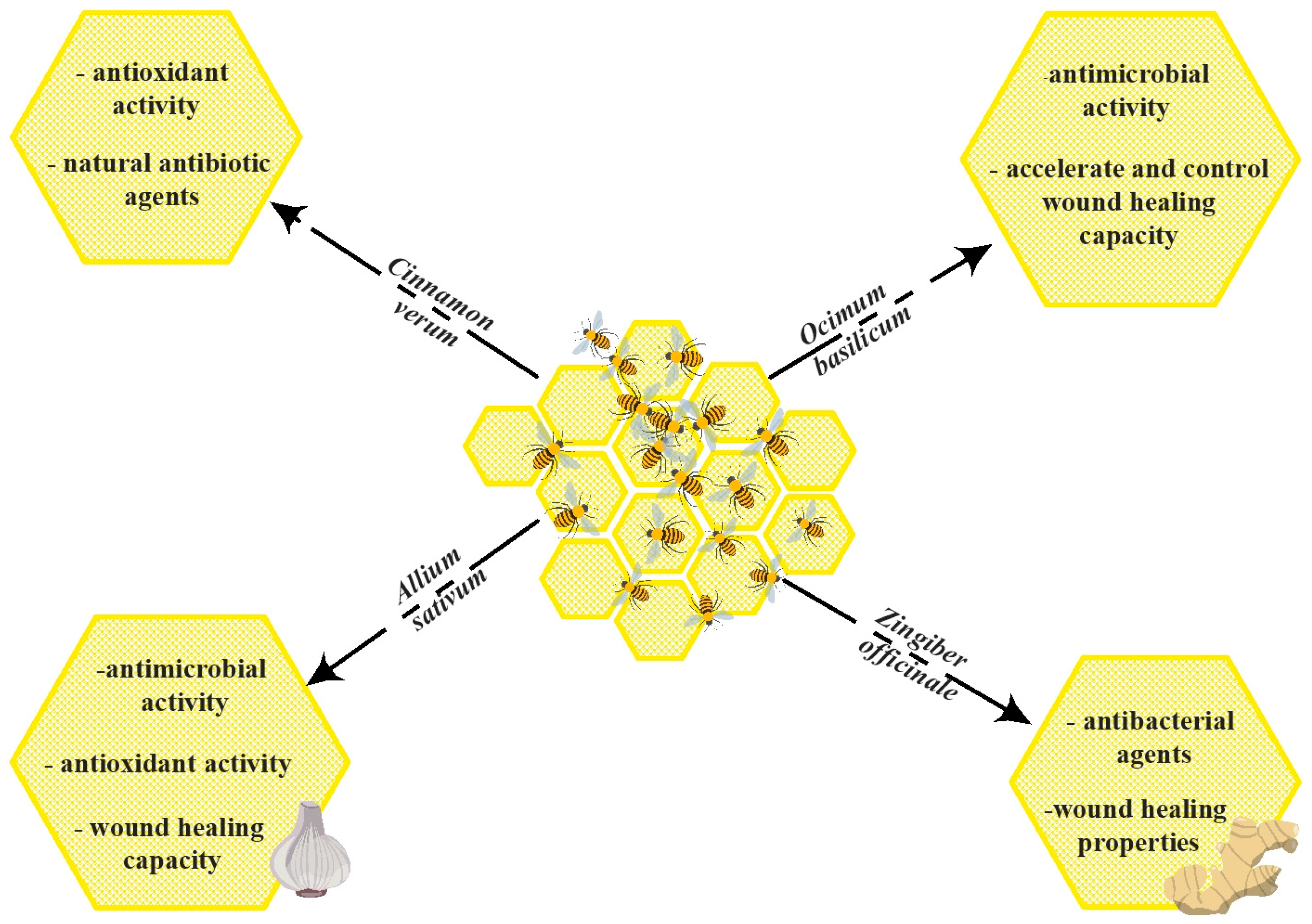
3. Synergic Effect of Honey with Other Natural Products
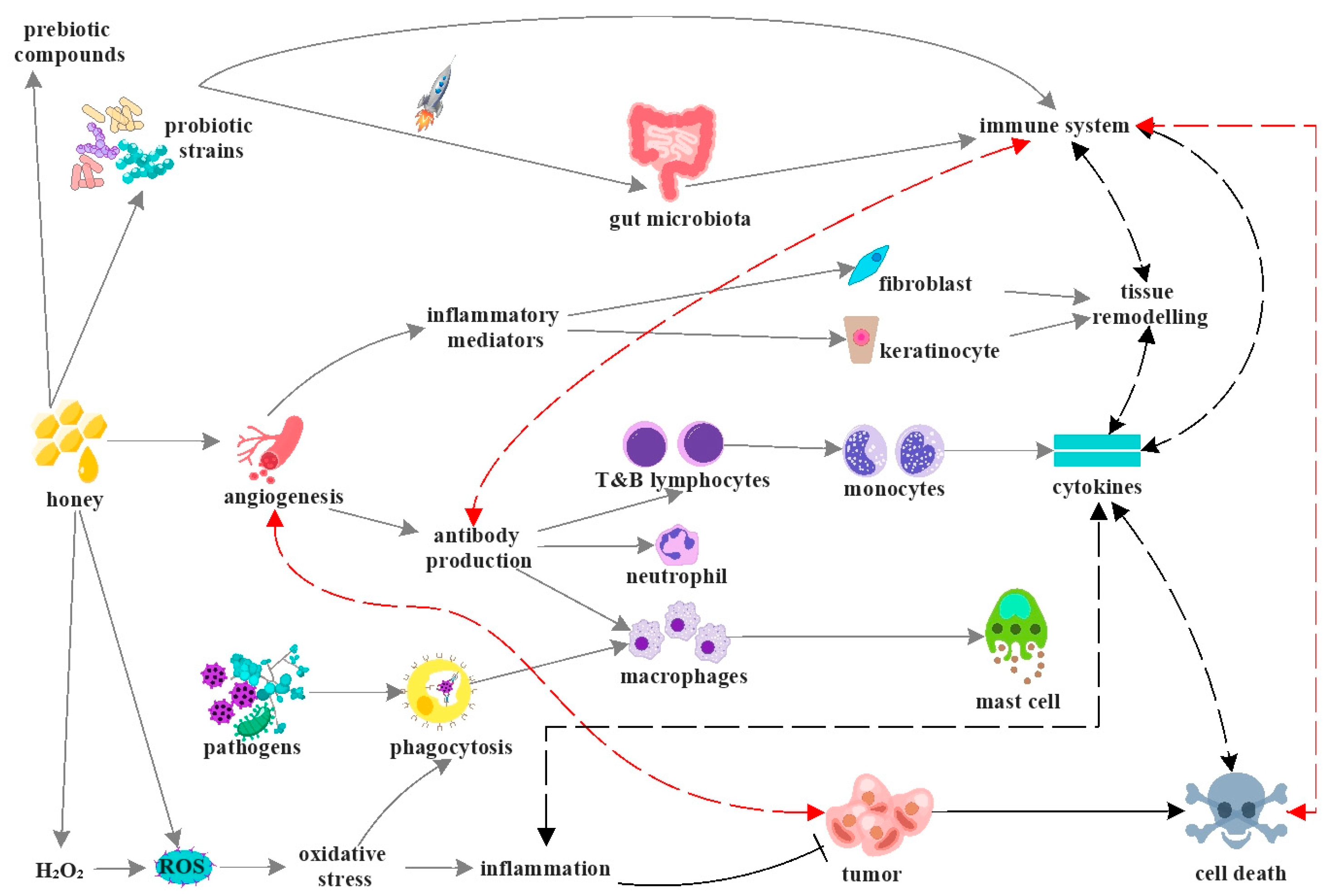
4. Mechanisms of Action and Biological Activity of Honey
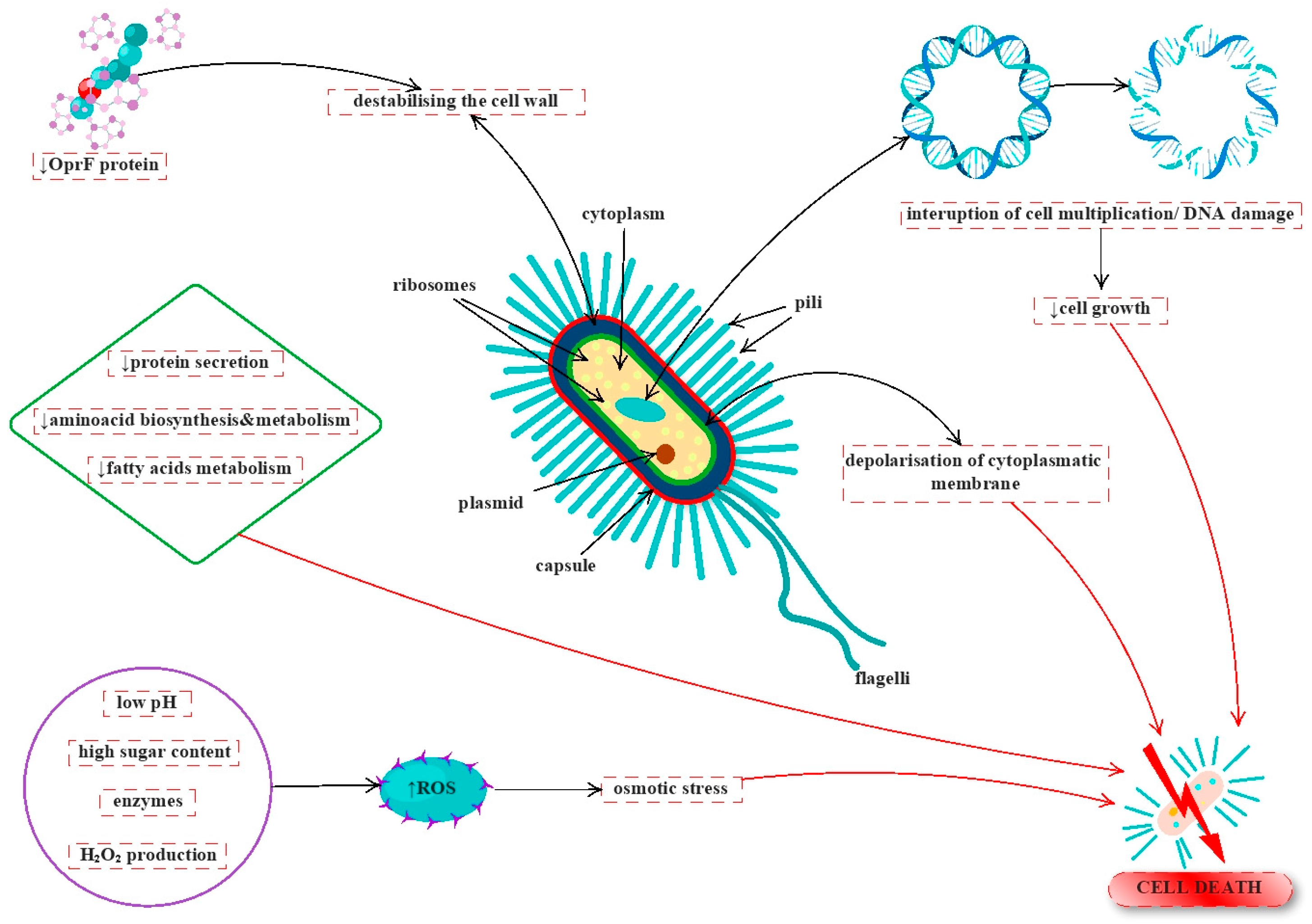
5. Antimicrobial Activity
6. Antioxidant Activity
7. Anti-Inflammatory Activity
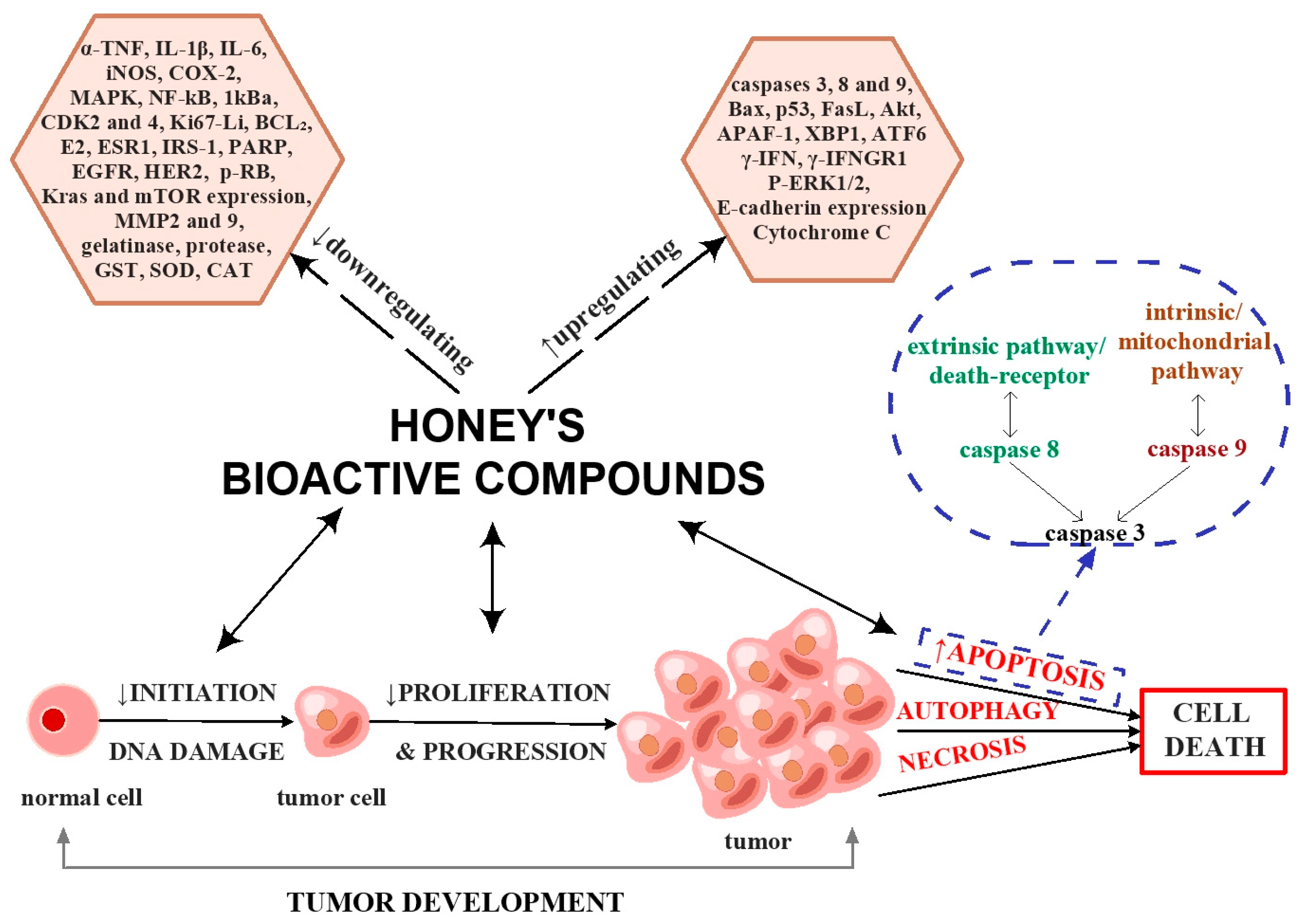
8. Antitumor Activity
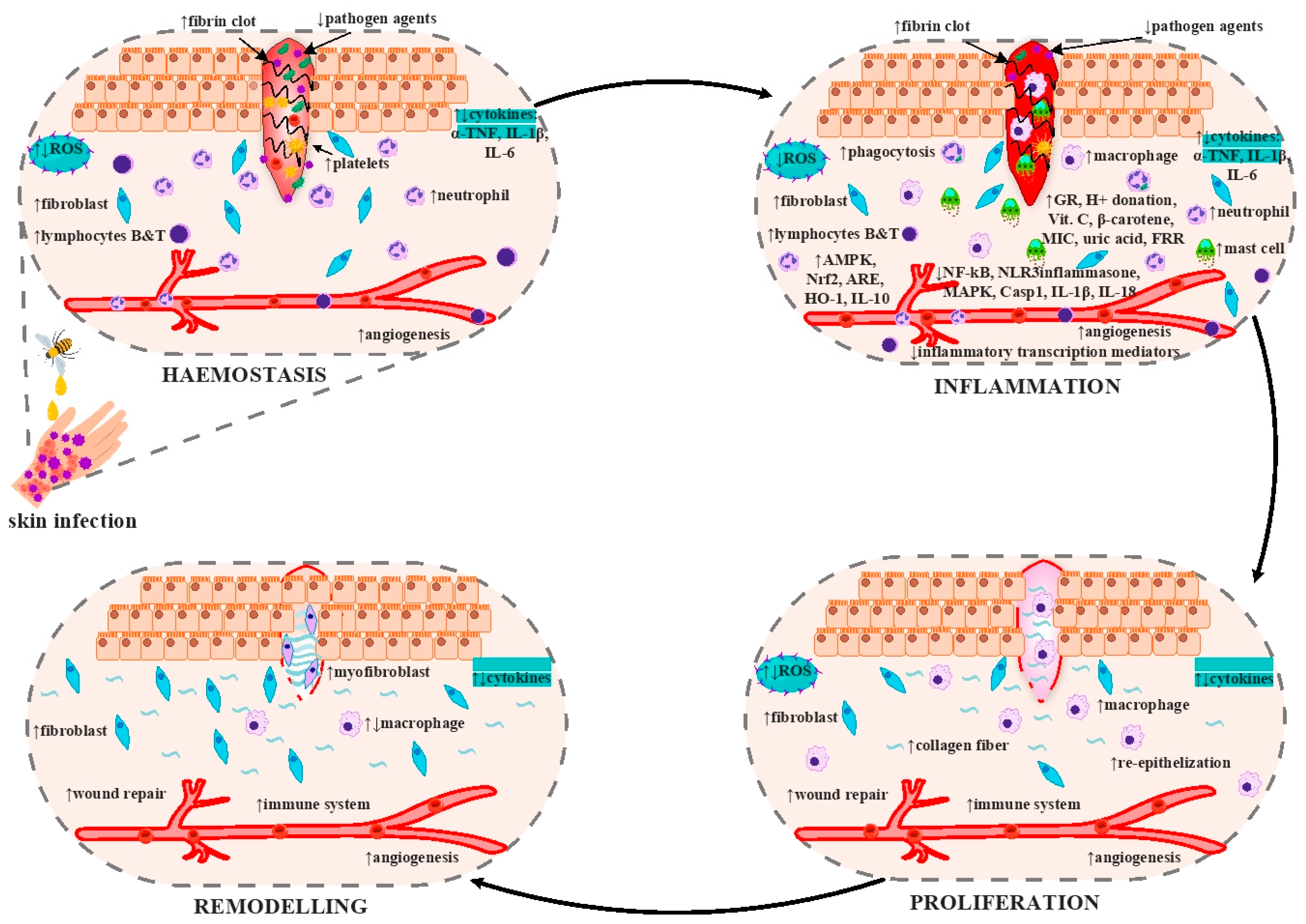
9. Wound Healing Activity
10. Preclinical and Clinical Trials Using Honey and Honey-Based Products
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crane, E. The Archaeology of Beekeeping; Cornell University Press: Ithaca, NY, USA, 1983. [Google Scholar]
- Kvavadze, E.; Gambashidze, I.; Mindiashvili, G.; Gogochuri, G. The first find in southern Georgia of fossil honey from the Bronze Age, based on palynological data. Veg. Hist. Archaeobotany 2007, 16, 399–404. [Google Scholar] [CrossRef]
- Lomsadze, G. Georgia, Unearths the World’s Oldest Honey. Available online: https://eurasianet.org/report-georgia-unearths-the-worlds-oldest-honey (accessed on 6 June 2022).
- The World’s First Winemakers Were the World’s First Beekeepers. Available online: https://guildofscientifictroubadours.com/ (accessed on 6 June 2022).
- Pećanac, M.; Janjić, Z.; Komarcević, A.; Pajić, M.; Dobanovacki, D.; Misković, S.S. Burns treatment in ancient times. Med. Pregl. 2013, 66, 263–270. [Google Scholar] [PubMed]
- Bresson, A. The Making of the Ancient Greek Economy; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. Gen. Med. Open Access 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, F.A.; Mehmood, M.H.; Malik, A.; Siddiqui, R.; Khan, N.A. Antiacanthamoebic properties of natural and marketed honey in Pakistan. Asian Pac. J. Trop. Biomed. 2016, 6, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Mediterr. J. Nutr. Metab. 2009, 3, 15–23. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernández, T.Y.; et al. Apis mellifera vs. Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT 2018, 87, 272–279. [Google Scholar] [CrossRef]
- Khan, F.R.; Ul Abadin, Z.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Vit, P.; Medina, M.; Eunice Enríquez, M. Quality standards for medicinal uses of Meliponinae honey in Guatemala, Mexico and Venezuela. Bee World 2004, 85, 2–5. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, W.M.; Hassan, H.A.; Nour, S.E.; Elmageed, A.Z.E.; Matrougui, K. Hepatoprotective effects of Nigella sativa and bees’ honey on hepatotoxicity induced by administration of sodium nitrite and sunset yellow. FASEB J. 2009, 23, 733.2. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.S.; Gurtu, S. Hepatoprotective effect of tualang honey supplementation in streptozotocin-induced diabetic rats. Int. J. Appl. Res. Nat. Prod. 2012, 4, 37–41. [Google Scholar]
- Biluca, F.C.; da Silva, B.; Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; et al. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Ezz El-Arab, A.M.; Girgis, S.M.; Hegazy, E.M.; Abd El-Khalek, A.B. Effect of dietary honey on intestinal microflora and toxicity of mycotoxins in mice. BMC Complement. Altern. Med. 2006, 6, 6. [Google Scholar] [CrossRef]
- McGovern, D.P.B.; Abbas, S.Z.; Vivian, G.; Dalton, H.R. Manuka Honey against Helicobacter Pylori; Gastrointestinal Department, Royal Cornwall Hospital: Cornwall, UK, 1999. [Google Scholar]
- Somall, N.A.I.; Coley, K.E.; Molan, P.C.; Hancock, B.M. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 1994, 87, 9–12. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mustafa, M.R.; Mohd, M.A.; Yusoff, K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010, 30, 650–659. [Google Scholar] [CrossRef]
- Nasuti, C.; Gabbianelli, R.; Falcioni, G.; Cantalamessa, F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr. Res. 2006, 26, 130–137. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, F.; Sadiq, M.; Jehangir, A. Anti-hyperlipidemic effect of Acacia Honey (Desi Kikar) in cholesterol-diet induced hyperlipidemia in rats. Biomedica 2011, 27, 62–67. [Google Scholar]
- Adewoye, E.O.; Omolekulo, T. Effect of Honey on Altered Thyroid State in Female Wistar Rats. Arch. Basic Appl. Med. 2014, 2, 63–68. [Google Scholar]
- Roubik, D.; Vit, P.; Pedro, S. Pot-Honey: A Legacy of Stingless Bees; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Ávila, S.; Beux, M.R.; Ribani, R.H.; Zambiazi, R.C. Stingless bee honey: Quality parameters, bioactive compounds, health-promotion properties and modification detection strategies. Trends Food Sci. Technol. 2018, 81, 37–50. [Google Scholar] [CrossRef]
- Jalil, A.M.A.; Kasmuri, A.R.; Hadi, H. Stingless Bee Honey, the Natural Wound Healer: A Review. Skin Pharm. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef]
- De Queiroz Pimentel, R.B.; da Costa, C.A.; Albuquerque, P.M.; Junior, S.D. Antimicrobial activity and rutin identification ofhoney produced by the stingless bee Melipona compressipes manaosensis and commercial honey. Complement. Altern. Med. 2013, 13, 151. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, L.; Heyneman, A.; Hoeksema, H.; Verbelen, J.; Monstrey, S. Honey in modern wound care: A systematic review. Burns 2013, 39, 1514–1525. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Estevinho, M.L.; Afonso, S.E.; Feas, X. Antifungal effect of lavender honey against Candida albicans, Candida krusei and Cryptococcus neoformans. J. Food Sci. Technol. 2011, 48, 640–643. [Google Scholar] [CrossRef]
- Moussa, A.; Noureddine, D.; Saad, A.; Abdelmelek, M.; Abdelkader, B. Antifungal activity of four honeys of different types from Algeria against pathogenic yeast: Candida albicans and Rhodotorula sp. Asian Pac. J. Trop. Biomed. 2012, 2, 554–557. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Tulipani, S.; Diaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, A.M.S. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Man, N.N.M.; Hassan, R.; Ang, C.Y.; Abdullah, A.D.; Radzi, M.M.A.; Sulaiman, S.A. Antileukemic Effect of Tualang Honey on Acute and Chronic Leukemia Cell Lines. Biomed Res. Int. 2015, 2015, 307094. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Nascimento, K.S.; Sattler, G.J.A.; Macedo, L.L.F.; González, S.C.V.; Pereira de Melo, I.L.; da Silva Araújo, E.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids-food sources and health benefits. Rocz. Państwowego Zakładu Hig. 2014, 65, 79–85. [Google Scholar]
- Aal, A.M.; El-Hadidy, M.R.; El Mashad, N.; El-Sebaie, A. Antimicrobial Effect of Bee Honey in Comparison to Antibiotics on Organisms Isolated From Infected Burns. Ann. Burn Fire Disasters 2007, 20, 83–88. [Google Scholar]
- Theunissen, F.; Grobler, S.; Gedalia, I. The antifungal action of three South African honeys on Candida albicans. Apidologie 2001, 32, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.S. Natural Honey Lowers Plasma Glucose, C-Reactive Protein, Homocysteine, and Blood Lipids in Healthy, Diabetic, and Hyperlipidemic Subjects: Comparison with Dextrose and Sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Al-Himyari, F.A. The use of honey as a natural preventive therapy of cognitive decline and dementia in the middle east. Alzheimer’s Dement. 2009, 5, P247. [Google Scholar] [CrossRef]
- Othman, Z.; Zakaria, R.; Hussain, N.H.N.; Hassan, A.; Shafin, N.; Al-Rahbi, B.; Ahmad, A.H. Potential Role of Honey in Learning and Memory. Med. Sci. 2015, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chepulis, L.M.; Starkey, N.J.; Waas, J.R.; Molan, P.C. The effects of long-term honey, sucrose or sugar-free diets on memory and anxiety in rats. Physiol. Behav. 2009, 97, 359–368. [Google Scholar] [CrossRef]
- Chew, C.Y.; Chua, L.S.; Soontorngun, N.; Lee, C.T. Discovering potential bioactive compounds from Tualang honey. Agric. Nat. Resour. 2018, 52, 361–365. [Google Scholar] [CrossRef]
- Liu, J.-R.; Ye, Y.-L.; Lin, T.-Y.; Wang, Y.-W.; Peng, C.-C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013, 139, 938–943. [Google Scholar] [CrossRef]
- Mračević, Đ.S.; Krstić, M.; Lolić, A.; Ražić, S. Comparative study of the chemical composition and biological potential of honey from different regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Zaidi, H.; Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Debbache, N.; Pacheco, R.; Serralheiro, M.L.; Araujo, M.E. Biological properties of phenolic compound extracts in selected Algerian honeys—The inhibition of acetylcholinesterase and α-glucosidase activities. Eur. J. Integr. Med. 2019, 25, 77–84. [Google Scholar] [CrossRef]
- Bilandzic, N.; Gajger, T.I.; Kosanovic, M.; Calopek, B.; Sedak, M.; Kolanovic, S.B.; Varenina, I.; Luburic, D.B.; Varga, I.; Dokic, M. Essential and toxic element concentrations in monofloral honeys from southern Croatia. Food Chem. 2017, 234, 245–253. [Google Scholar] [CrossRef]
- Ciulu, M.; Solinas, S.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Spano, N.; Sanna, G. RP-HPLC determination of water-soluble vitamins in honey. Talanta 2011, 83, 924–929. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef]
- Ariefdjohan, M.W.; Martin, B.R.; Lachcik, P.J.; Weaver, C.M. Acute and Chronic Effects of Honey and Its Carbohydrate Constituents on Calcium Absorption in Rats. J. Agric. Food Chem. 2008, 56, 2649–2654. [Google Scholar] [CrossRef]
- Gul, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef]
- Da Costa, A.C.V.; Sousa, J.M.B.; da Silva, M.A.A.P.; Garruti, D.d.S.; Madruga, M.S. Sensory and volatile profiles of monofloral honeys produced by native stingless bees of the brazilian semiarid region. Food Res. Int. 2018, 105, 110–120. [Google Scholar] [CrossRef]
- Ávila, S.; Hornung, P.S.; Teixeira, G.L.; Malunga, L.N.; Apea-Bah, F.B.; Beux, M.R.; Beta, T.; Ribani, R.H. Bioactive compounds and biological properties of Brazilian stingless bee honey have a strong relationship with the pollen floral origin. Food Res. Int. 2019, 123, 1–10. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Review of the Medicinal Effects of Tualang Honey and a Comparison with Manuka Honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar]
- Yarsan, E.; Karacal, F.; Ibrahim, I.G.; Dikmen, B.; Koksal, A.; Das, Y.K. Contents of Some Metals in Honeys from Different Regions in Turkey. Bull. Environ. Contam. Toxicol. 2007, 79, 255–258. [Google Scholar] [CrossRef]
- Bilandžić, N.; Gačić, M.; Đokić, M.; Sedak, M.; Šipušić, Đ.I.; Končurat, A.; Gajger, I.T. Major and trace elements levels in multifloral and unifloral honeys in Croatia. J. Food Compos. Anal. 2014, 33, 132–138. [Google Scholar] [CrossRef]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Pellerano, R.G.; Uñates, M.A.; Cantarelli, M.A.; Camiña, J.M.; Marchevsky, E.J. Analysis of trace elements in multifloral Argentine honeys and their classification according to provenance. Food Chem. 2012, 134, 578–582. [Google Scholar] [CrossRef]
- Leyva-Jimenez, F.J.; Lozano-Sanchez, J.; Borras-Linares, I.; Cadiz-Gurrea, M.d.l.L.; Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram negative bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Lianda, R.L.P.; D’Oliveira Sant’Ana, L.; Echevarriaa, A.; Castro, R.N. Antioxidant Activity and Phenolic Composition of Brazilian Honeys and their Extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef]
- Cigic, I.K.; Prosen, H. An overview of conventional and emerging analytical methods for the determination of mycotoxins. Int. J. Mol. Sci. 2009, 10, 62–115. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef]
- Massaro, C.F.; Shelley, D.; Heard, T.A.; Brooks, P. In Vitro Antibacterial Phenolic Extracts from “Sugarbag” Pot-Honeys of Australian Stingless Bees (Tetragonula carbonaria). J. Agric. Food Chem. 2014, 62, 12209–12217. [Google Scholar] [CrossRef]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F. Cerumen of Australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften 2011, 98, 329–337. [Google Scholar] [CrossRef]
- Da Silva, I.A.; da Silva, T.M.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Soledade, L.E.; Lima Ede, O.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.; da Silva, G.S.; de Novais, J.S.; dos Santos, F.; de Assis, R.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, A.; Bruni, R.; Maietti, S.; Poli, F.; Damiano, R.; Paganetto, G.; Muzzoli, M.; Scalvenzi, L.; Sacchetti, G. Ecuadorian stingless bee (Meliponinae) honey: A chemical and functional profile of an ancient health product. Food Chem. 2009, 114, 1413–1420. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Dezmirean, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Fortuna, T. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009, 113, 568–574. [Google Scholar] [CrossRef]
- Yancheva, S.; Mavromatis, P.; Georgieva, L. Polyphenol profile and antioxidant activity of extracts from olive leaves. J. Cent. Eur. Agric. 2016, 17, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Balík, J.; Kyseláková, M.; Vrchotová, N.; Tříska, J.; Kumšta, M.; Veverka, J.; Híc, P.; Totušek, J.; Lefnerová, D. Relations between polyphenols content and antioxidant activity in vine grapes and leaves. Czech J. Food Sci. 2008, 26, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, A.; Bhardwaj, R.; Thukral, A.K. Polyphenol profiling in the leaves of plant from the carchment area of river Beas, India. Int. J. Pharma Bio Sci. 2015, 6, 1005–1012. [Google Scholar]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Epibrassinolide-imidacloprid interaction enhances non-enzymatic antioxidants in Brassica juncea L. Indian J. Plant Physiol. 2016, 21, 70–75. [Google Scholar] [CrossRef]
- Mahfoudhi, A.; Prencipe, F.P.; Mighri, Z.; Pellati, F. Metabolite profiling of polyphenols in the Tunisian plant Tamarix aphylla (L.) Karst. J. Pharm. Biomed. Anal. 2014, 99, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Jaafar, H.Z. HPLC and GC-MS determination of bioactive compounds in microwave obtained extracts of three varieties of Labisia pumila Benth. Molecules 2011, 16, 6791–6805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Thiruvengadam, M.; Chung, I.-M.; Nagella, P. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop Sci. 2013, 12, 1921–1926. [Google Scholar]
- Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trac. Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Kamaruddin, M.Y.; Aljadi, A. Isolation and Identification of Phenolic Acids in Malaysian Honey with Antibacterial Activities. Turk. J. Med. Sci. 2003, 33, 229–236. [Google Scholar]
- Ramanauskiene, K.; Stelmakiene, A.; Briedis, V.; Ivanauskas, L.; Jakstas, V. The quantitative analysis of biologically active compounds in Lithuanian honey. Food Chem. 2012, 132, 1544–1548. [Google Scholar] [CrossRef]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Barku, V.Y.A.; Boye, A.; Quansah, N. Antioxidant and wound healing studies on the extracts of Corchorus olitorius leaf. Sci. Res. Rev. J. 2013, 1, 67–73. [Google Scholar]
- Abderrahim, L.A.; Taibi, K.; Ait Abderrahim, N.; Boussaid, M.; Rios-Navarro, C.; Ruiz-Sauri, A. Euphorbia honey and garlic: Biological activity and burn wound recovery. Burns 2019, 45, 1695–1706. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar]
- Khashan, A.A. Antibacterial activity of garlic extract (Allium sativum) against Staphylococcus aureus in vitro. J. Bio-Sci. Biotechnol. 2014, 3, 346–348. [Google Scholar]
- Khan, M.S.; Quershi, N.A.; Jabeen, F.; Asghar, M.S.; Shakeel, S. Analysis of minerals profile, phenolic compounds and potential of Garlic (Allium sativum) as antioxidant scavenging the free radicals. Int. J. Biosci. (IJB) 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Andualem, B. Synergistic Antimicrobial Effect of Tenegn Honey (Trigona iridipennis) and Garlic Against Standard and Clinical Pathogenic Bacterial Isolates. Int. J. Microbiol. Res. 2013, 4, 16–22. [Google Scholar] [CrossRef]
- Bettar, I.; González-Miret, M.L.; Hernanz, D.; Marconi, A.; Heredia, F.J.; Terrab, A. Characterisation of Moroccan Spurge (Euphorbia) honeys by their physicochemical characteristics, mineral contents and colour. Arab. J. Chem. 2019, 12, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Escuredo, O.; Miguez, M.; Fernandez-Gonzalez, M.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Muller, P.; Alber, D.G.; Turnbull, L.; Schlothauer, R.C.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS ONE 2013, 8, e57679. [Google Scholar] [CrossRef]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S.E. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar] [CrossRef] [Green Version]
- Wasihun, A.G.; Kasa, B.G. Evaluation of antibacterial activity of honey against multidrug resistant bacteria in Ayder Referral and Teaching Hospital, Northern Ethiopia. Springerplus 2016, 5, 842. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, C. Investigation of in vitro antifungal activity of honey. J. Med. Plants Res. 2012, 6, 3512–3516. [Google Scholar] [CrossRef]
- Alves, A.; Ramos, A.; Gonçalves, M.M.; Bernardo, M.; Mendes, B. Antioxidant activity, quality parameters and mineral content of Portuguese monofloral honeys. J. Food Compos. Anal. 2013, 30, 130–138. [Google Scholar] [CrossRef]
- Fernández-Torres, R.; Pérez-Bernal, J.L.; Bello-López, M.Á.; Callejón-Mochón, M.; Jiménez-Sánchez, J.C.; Guiraúm-Pérez, A. Mineral content and botanical origin of Spanish honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Bai, L.; Ho, C.-T.; Bai, N. Characteristic Components, Biological Activities and Future Prospective of Fructus Mori: A Review. Curr. Pharmacol. Rep. 2018, 4, 210–219. [Google Scholar] [CrossRef]
- Čeksteryte, V. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Angioi, R.; Morrin, A.; White, B. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Appl. Sci. 2021, 11, 15192. [Google Scholar] [CrossRef]
- Tuhin, R.H.; Begum, M.M.; Rahman, M.S.; Karim, R.; Begum, T.; Ahmed, S.U.; Mostofa, R.; Hossain, A.; Abdel-Daim, M.; Begum, R. Wound healing effect of Euphorbia hirta linn. (Euphorbiaceae) in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 423. [Google Scholar] [CrossRef]
- Khalil, A.T.; Khan, I.; Ahmad, K.; Khan, Y.A.; Khan, M.; Khan, M.J. Synergistic antibacterial effect of honey and Herba Ocimi Basilici against some bacterial pathogens. J. Tradit Chin. Med. 2013, 33, 810–814. [Google Scholar] [CrossRef]
- Svecova, E.; Neugebauerova, J. A study of 34 cultivars of basil (Ocimum L.) and their morphological, economic and biochemical characteristics, using standardized descriptors. Acta Univ. Sapientiae Aliment. 2010, 3, 118–135. [Google Scholar]
- Namias, N. Honey in the Management of Infections. Surg. Infect. 2003, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Özcan, M. Mineral contents of some plants used as condiments in Turkey. Food Chem. 2004, 84, 437–440. [Google Scholar] [CrossRef]
- Sajjadi, S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. DARU J. Pharm. Sci. 2006, 14, 128–130. [Google Scholar]
- Salmah, I.; Mahmood, A.A.; Sidik, K. Synergistic effects of alcoholic extract of sweet basil (Ocimum basilicum L) leaves and honey on cutaneous wound healing in rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 220–224. [Google Scholar]
- Rezvani, M.B.; Niakan, M.; Kamalinejad, M.; Ahmadi, F.S.; Hamze, F. The synergistic effect of honey and cinnamon against Streptococcus mutans bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 314–320. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef] [Green Version]
- Fani, M.; Kohanteb, J. Inhibitory activity of aloe vera gel on some clinically isolated cariogenic and periodontopathic bacteria. J. Oral Sci. 2012, 54, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Ferrazzano, G.F.; Roberto, L.; Amato, I.; Cantile, T.; Sangianantoni, G.; Ingenito, A. Antimicrobial properties of green tea extract against cariogenic microflora: An in vivo study. J. Med. Food 2011, 14, 907–911. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Probst, I.S.; Sforcin, J.M.; Rall, V.L.M.; Fernandes, A.A.H.; Fernandes Júnior, A. Antimicrobial activity of propolis and essential oils and synergism between these natural products. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.V.; Thaker, V.T.; Patel, V.K. Antimicrobial activity of ginger and honey on isolates of extracted carious teeth during orthodontic treatment. Asian Pac. J. Trop. Biomed. 2011, 1, S58–S61. [Google Scholar] [CrossRef]
- Ahmed, M.; Aissat, S.; Djebli, N.; Boulkaboul, A.; Abdelmalek, M.; Khiati, B. The Influence of Starch of Ginger on the Antibacterial Activity of Honey of Different Types from Algeria against Escherichia coli and Staphylococcus aureus. Int. J. Microbiol. Res. 2011, 2, 258–262. [Google Scholar]
- Maeda, Y.; Loughrey, A.; Earle, J.A.; Millar, B.C.; Rao, J.R.; Kearns, A.; McConville, O.; Goldsmith, C.E.; Rooney, P.J.; Dooley, J.S.; et al. Antibacterial activity of honey against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Complement Ther. Clin. Pract. 2008, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Masad, R.J.; Haneefa, S.M.; Mohamed, Y.A.; Al-Sbiei, A.; Bashir, G.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. The Immunomodulatory Effects of Honey and Associated Flavonoids in Cancer. Nutrients 2021, 13, 1269. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Tonks, A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003, 21, 242–247. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef]
- Chepulis, L.M. The effect of honey compared to sucrose, mixed sugars, and a sugar-free diet on weight gain in young rats. J. Food Sci. 2007, 72, S224–S229. [Google Scholar] [CrossRef]
- Ahmed, S.; Othman, N.H. Honey as a potential natural anticancer agent: A review of its mechanisms. Evid. Based Complement. Altern. Med. 2013, 2013, 829070. [Google Scholar] [CrossRef] [Green Version]
- Schell, K.R.; Fernandes, K.E.; Shanahan, E.; Wilson, I.; Blair, S.E.; Carter, D.A.; Cokcetin, N.N. The Potential of Honey as a Prebiotic Food to Re-engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef]
- De Melo, F.H.C.; Menezes, F.; de Sousa, J.M.B.; Dos Santos Lima, M.; da Silva Campelo Borges, G.; de Souza, E.L.; Magnani, M. Prebiotic activity of monofloral honeys produced by stingless bees in the semi-arid region of Brazilian Northeastern toward Lactobacillus acidophilus LA-05 and Bifidobacterium lactis BB-12. Food Res. Int. 2020, 128, 108809. [Google Scholar] [CrossRef]
- Mustar, S.; Ibrahim, N. A Sweeter Pill to Swallow: A Review of Honey Bees and Honey as a Source of Probiotic and Prebiotic Products. Foods 2022, 11, 2102. [Google Scholar] [CrossRef]
- Kowalska, G.; Rosicka-Kaczmarek, J.; Miskiewicz, K.; Zaklos-Szyda, M.; Rohn, S.; Kanzler, C.; Wiktorska, M.; Niewiarowska, J. Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response-In Vitro Study. Nutrients 2022, 14, 2529. [Google Scholar] [CrossRef]
- Kwakman, P.H.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Kwakman, P.H.; Te Velde, A.A.; de Boer, L.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 2011, 6, e17709. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The intracellular effects of manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar] [CrossRef] [Green Version]
- Henriques, A.F.; Jenkins, R.E.; Burton, N.F.; Cooper, R.A. The effect of manuka honey on the structure of Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, R.; Roberts, A.; Brown, H.L. On the antibacterial effects of manuka honey: Mechanistic insights. Res. Rep. Biol. 2015, 6, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the Mechanism of Action of an Ancient Antimicrobial, Manuka Honey, against Pseudomonas aeruginosa Using Modern Transcriptomics. mSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; G. Rodrigues, A.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaheen, Z.; Yatoo, A.; Ali, S.; Niamat, A.; Majid, S.; Rasool, S.; Mudasir Rashid, S.; Ahmad, S.B.; Mir, M.; Zehra, U. Honey: Types, Composition and Antimicrobial Mechanisms. In Therapeutic Applications of Honey and Its Phytochemicals; Muneeb, U., Rehman, S.M., Eds.; Springer Nature Sibgapore Pte Ltd.: Singapore, 2020; pp. 193–214. [Google Scholar] [CrossRef]
- Elston, D.M. Community-acquired methicillin-resistant Staphylococcus aureus. J. Am. Acad Derm. 2007, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Khan, K.A.; Alshehri, A.M.A. Antibacterial potential of some Saudi honeys from Asir region against selected pathogenic bacteria. Saudi J. Biol. Sci. 2019, 26, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical properties, antibacterial and cellular antioxidant activities of buckwheat honey in comparison to manuka honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Guthami, F.M.; Gethami, A.F.; Allah, F.M.; Saleh, A.A.; Fouad, E.A. Potential antibacterial activity of some Saudi Arabia honey. Vet. World 2017, 10, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Basma, A.A.; Zuraini, Z.; Sasidharan, S. A transmission electron microscopy study of the diversity of Candida albicans cells induced by Euphorbia hirta L. leaf extract in vitro. Asian Pac. J. Trop. Biomed. 2011, 1, 20–22. [Google Scholar] [CrossRef] [Green Version]
- Molero, G.; Díez-Orejas, R.; Navarro-García, F.; Monteoliva, L.; Pla, J.; Gil, C.; Sánchez-Pérez, M.; Nombela, C. Candida albicans: Genetics, dimorphism and pathogenicity. Int. Microbiol. 1998, 2, 95–106. [Google Scholar]
- Fesharaki, S.H.; Aghili, S.R.; Shokohi, T.; Boroumand, M.A. Catheter-related candidemia and identification of causative Candida species in patients with cardiovascular disorder. Curr. Med. Mycol. 2018, 4, 7–13. [Google Scholar] [CrossRef]
- Tuon, F.F.; de Almeida, G.M.F.; Costa, S.F. Central venous catheter-associated fungemia due to Rhodotorula spp.—A systematic review. Med. Mycol. 2007, 45, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari-Moghaddam, M.; Hadi-Dabanlou, R.; Khajeh, M.; Rakhshanipour, M.; Shameli, K. Green synthesis of silver nanoparticles using plant extracts. Korean J. Chem. Eng. 2014, 31, 548–557. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Aromal, S.A.; Philip, D. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2011, 83, 392–397. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K.F. Antibacterial and Cytotoxic Potential of Biosynthesized Silver Nanoparticles by Some Plant Extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Haiza, H.; Azizan, A.; Mohidin, A.H.; Halin, D.S.C. Green Synthesis of Silver Nanoparticles Using Local Honey. Nano Hybrids 2013, 4, 87–98. [Google Scholar] [CrossRef]
- Al-Brahim, J.S.; Mohammed, A.E. Antioxidant, cytotoxic and antibacterial potential of biosynthesized nanoparticles using bee honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 363–373. [Google Scholar] [CrossRef]
- White, J.W.; Subers, J.M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Allen, K.L.; Molan, P.C.; Reid, G.M. A survey of the antibacterial activity of some New Zealand honeys. J. Pharm. Pharmacol. 1991, 43, 817–822. [Google Scholar] [CrossRef]
- Russell, K.M.; Molan, P.C.; Wilkins, A.L.; Holland, P.T. Identification of some antibacterial constituents of New Zealand manuka honey. J. Agric. Food Chem. 1990, 38, 10–13. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Lu, Y.; Molan, P.C. Extractable organic substances from New Zealand unifloral manuka (Leptospermum scoparium) honeys. J. Apic. Res. 2015, 32, 3–9. [Google Scholar] [CrossRef]
- Blair, S.E.; Carte, D.A. The potential for honey in the management of wounds and infection. Aust. Infect. Control 2005, 10, 24–31. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants 2019, 10, 1–28. [Google Scholar]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacityin vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Mushtaq, S.; Imtiyaz, Z.; Wali, A.F.; Khan, A.; Rashid, S.M.; Amin, I.; Ali, A.; Rehman, M.U.; Arafah, A. Honey: A Powerful Natural Antioxidant and Its Possible Mechanism of Action. In Therapeutic Applications of Honey and Its Phoitochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; pp. 11–31. [Google Scholar] [CrossRef]
- Nweze, J.A.; Olovo, C.V.; Nweze, E.I.; John, O.O.; Paul, C. Therapeutic Properties of Honey. In Honey Analysis; de Toledo, V.A.A., Chambó, E.D., Eds.; IntechOpen: London, UK, 2019; p. 21. [Google Scholar]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant Properties of Honey and Its Role in Preventing Health Disorder. Open Nutraceuticals J. 2010, 3, 6–16. [Google Scholar] [CrossRef]
- Chaikham, P.; Prangthip, P. Alteration of antioxidative properties of longan flower-honey after high pressure, ultra-sonic and thermal processing. Food Biosci. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of daily consumption of honey solution on hematological indices and blood levels of minerals and enzymes in normal individuals. J. Med. Food 2003, 6, 135–140. [Google Scholar] [CrossRef]
- Kupeli Akkol, E.; Orhan, D.D.; Gurbuz, I.; Yesilada, E. In vivo activity assessment of a “honey-bee pollen mix” formulation. Pharm. Biol. 2010, 48, 253–259. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Salam, S.K.; Salleh, M.S.; Gurtu, S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.S. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Abdul Aziz, A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose-Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, I. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2017, 20, 864–876. [Google Scholar] [CrossRef] [Green Version]
- Konuskan, B.D.; Mungan, B. Effects of Variety, Maturation and Growing Region on Chemical Properties, Fatty Acid and Sterol Compositions of Virgin Olive Oils. J. Am. Oil Chem. Soc. 2016, 93, 1499–1508. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2009, 27, 677–689. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, A.E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S. Antidotal Effects of Curcumin Against Agents-Induced Cardiovascular Toxicity. Cardiovasc. Haematol. Disord.-Drug Targets 2016, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular mechanism-based therapeutic properties of honey. Biomed. Pharm. 2020, 130, 110590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J.; et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Javed, A.; Mushtaq, Z.; Hassan, S.; Shariati, M.A.; Khan, M.U.; Majeed, M.; Nigam, M.; Mishra, A.P.; et al. Honey and cancer: A mechanistic review. Clin. Nutr. 2019, 38, 2499–2503. [Google Scholar] [CrossRef]
- Susin, S.A.; Zamzami, N.; Kroemer, G. Mitochondria as regulators of apoptosis: Doubt no more. Biochim. Biophys. Acta 1998, 1366, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Maiuri, M.C.; Vitale, I.; Zischka, H.; Castedo, M.; Zitvogel, L.; Kroemer, G. Cell death modalities: Classification and pathophysiological implications. Cell Death Diffe.r 2007, 14, 1237–1243. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Andersen, M.H.; Becker, J.C.; Straten, P. Regulators of apoptosis: Suitable targets for immune therapy of cancer. Nat. Rev. Drug Discov. 2005, 4, 399–409. [Google Scholar] [CrossRef]
- Jaganathan, S.K. Honey Constituents and their apoptotic effect in colon cancer cells. J. Apiproduct Apimedical Sci. 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Investig. New Drugs 2010, 28, 624–633. [Google Scholar] [CrossRef]
- Tomasin, R.; Gomes-Marcondes, M.C. Oral administration of Aloe vera and honey reduces Walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. Phytother. Res. 2011, 25, 619–623. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef]
- Wee, L.H.; Morad, N.A.; Aan, G.J.; Makpol, S.; Wan Ngah, W.Z.; Mohd Yusof, Y.A. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/beta-catenin Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 6549–6556. [Google Scholar] [CrossRef] [Green Version]
- Tahir, A.A.; Sani, N.F.; Murad, N.A.; Makpol, S.; Ngah, W.Z.; Yusof, Y.A. Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr. J. 2015, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen 2014, 22, 187–192. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Cognasse, F.; Hamzeh, H.; Chavarin, P.; Acquart, S.; Genin, C.; Garraud, O. Evidence of Toll-like receptor molecules on human platelets. Immunol. Cell Biol. 2005, 83, 196–198. [Google Scholar] [CrossRef]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, C.L.; Arpey, C.J. Normal Cutaneous Wound Healing: Clinical Correlation with Cellular and Molecular Events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Y.; Chen, L.; Xie, J.; Tang, J.; Zhao, J.; Shu, B.; Qi, S.; Chen, J.; Liang, G.; et al. Dendritic epidermal T cells facilitate wound healing in diabetic mice. Am. J. Transl. Res. 2016, 8, 2375–2384. [Google Scholar]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonte, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Sandseter, E.B.H.; Kennair, L.E.O. Children’s Risky Play from an Evolutionary Perspective: The Anti-Phobic Effects of Thrilling Experiences. Evol. Psychol. J. 2011, 9, 257–284. [Google Scholar] [CrossRef]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef]
- Maddocks, S.; Jenkins, R. Honey: A sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Molan, P.; Rhodes, T. Honey: A Biologic Wound Dressing. Wounds 2015, 27, 141–151. [Google Scholar]
- Kateel, R.; Adhikari, P.; Augustine, A.J.; Ullal, S. Topical honey for the treatment of diabetic foot ulcer: A systematic review. Complement. Ther. Clin. Pract. 2016, 24, 130–133. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, Y.; Li, J.; Li, L.; Li, Z. Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats. Eur. J. Pharmacol. 2018, 836, 83–88. [Google Scholar] [CrossRef]
- Holland, L.C.; Norris, J.M. Medical grade honey in the management of chronic venous leg ulcers. Int. J. Surg. 2015, 20, 17–20. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukhopadhyay, A.; Chaudhary, A.; Rajput, M.; Pawar, H.S.; Mukherjee, R.; Das, A.K.; Banerjee, P.; Chatterjee, J. Therapeutic interfaces of honey in diabetic wound pathology. Wound Med. 2017, 18, 21–32. [Google Scholar] [CrossRef]
- Khiati, B.; Bacha, S.; Aissat, S.; Ahmed, M. The use of Algerian honey on cutaneous wound healing: A case report and review of the literature. Asian Pac. J. Trop. Dis. 2014, 4, S867–S869. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Doner, L.W. The sugars of honey—A review. J. Sci. Food Agric. 1977, 28, 443–456. [Google Scholar] [CrossRef]
- Markowicz, D.B.; Monaro, E.; Siguemoto, E.; Séfora, M. Maillard reaction products in processed foods: Pros and cons. In Food industrial Processes-Methods And Equipment, 1st ed.; Valdez, B., Ed.; InTech: Rijeka, Croatia, 2012; pp. 281–300. [Google Scholar]
- Alimentarius, C. Revised codex standard for honey. Codex Stan 2001, 12, 1982. [Google Scholar]
- Official Journal of the European Union. Regulation (EU) No 1151/2012 of the European Parliament and of the Council on quality schemes for agricultural products and foodstuffs; 2012; Volume L 343, pp. 1–29. [Google Scholar]
- Glatt, H.; Schneider, H.; Liu, Y. V79-hCYP2E1-hSULT1A1, a cell line for the sensitive detection of genotoxic effects induced by carbohydrate pyrolysis products and other food-borne chemicals. Mutat. Res. 2005, 580, 41–52. [Google Scholar] [CrossRef]
- Monien, B.H.; Engst, W.; Barknowitz, G.; Seidel, A.; Glatt, H. Mutagenicity of 5-hydroxymethylfurfural in V79 cells expressing human SULT1A1: Identification and mass spectrometric quantification of DNA adducts formed. Chem Res Toxicol 2012, 25, 1484–1492. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.-M.; Jing, H. Demonstration of Antioxidant and Anti-inflammatory Bioactivities from Sugar–Amino Acid Maillard Reaction Products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Yamada, P.; Nemoto, M.; Shigemori, H.; Yokota, S.; Isoda, H. Isolation of 5-(hydroxymethyl)furfural from Lycium chinense and its inhibitory effect on the chemical mediator release by basophilic cells. Planta Med. 2011, 77, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. The Effect of Acid-Hydrolysed Sucrose on Honeybees. J. Apic. Res. 1966, 5, 127–136. [Google Scholar] [CrossRef]
- Moghazy, A.M.; Shams, M.E.; Adly, O.A.; Abbas, A.H.; El-Badawy, M.A.; Elsakka, D.M.; Hassan, S.A.; Abdelmohsen, W.S.; Ali, O.S.; Mohamed, B.A. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res. Clin. Pract. 2010, 89, 276–281. [Google Scholar] [CrossRef]
- Emsen, I.M. A different and safe method of split thickness skin graft fixation: Medical honey application. Burns 2007, 33, 782–787. [Google Scholar] [CrossRef]
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2008, 343, 651–659. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Yusoff, M.K.; Makpol, S.; Yusof, M.Y.A. Gelam Honey Attenuates Carrageenan-Induced Rat Paw Inflammation via NF-κB Pathway. PLoS ONE 2013, 8, e72365. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.; Soundharrajan, I.; Govindan, N. Nutraceuticals as potential therapeutic agents for colon cancer: A review. Acta Pharm. Sin. B 2014, 4, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Lazaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Cione, E.; Aiello, F.; Caroleo, M.C. From the hive: Honey, a novel weapon against cancer. Eur. J. Med. Chem. 2017, 142, 290–299. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B: Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Detta, N.; Ylikauppila, H.; Nikkola, L.; Ashammakhi, N.; Chiellini, F.; Chiellini, E. Poly(vinyl alcohol)-based electrospun meshes as potential candidate scaffolds in regenerative medicine. J. Bioact. Compat. Polym. 2010, 26, 20–34. [Google Scholar] [CrossRef]
- Neres Santos, A.M.; Duarte Moreira, A.P.; Piler Carvalho, C.W.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Fernandes Mendes, M.; Nunes Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. Journal of ethnopharmacology 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, S.S.; Kim, J.-H.; Bae, C.S.; Choi, S.H. Inhibitory effect of whole bee venom in adjuvant-induced arthritis. In Vivo 2005, 19, 801–805. [Google Scholar]
- Lee, W.R.; Kim, S.J.; Park, J.H.; Kim, K.H.; Chang, Y.C.; Park, Y.Y.; Lee, K.G.; Han, S.M.; Yeo, J.H.; Pak, S.C.; et al. Bee venom reduces atherosclerotic lesion formation via anti-inflammatory mechanism. Am. J. Chin. Med. 2010, 38, 1077–1092. [Google Scholar] [CrossRef]
- Abou, Z.S.S.; Abdellatif, A.; Azzazy, H.M.E. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181. [Google Scholar] [CrossRef]
- Costeloe, A.; Vandjelovic, N.D.; Evans, M.A.; Saraiya, S.S. The use of honey in cochlear implant associated wounds in pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 80–83. [Google Scholar] [CrossRef]
| No. | Polyphenols [µg/g] | Plant-Based Food | Species Name | Country | Reference |
|---|---|---|---|---|---|
| 1 | 226.00 | Rosemary plant | Rosmarinus officinalis | Portugal | [80] |
| 2 | 406.00 | Viper’s Bugloss Plant | Echium vulgare | Portugal | [80] |
| 3 | 728.00 | Heather plant | Calluna vulgaris | Portugal | [80] |
| 4 | 20.00 | Acacia plant | Robinia pseudoacacia | Romania | [81] |
| 5 | 128.00 | Lime plant | Tipia sp. | Romania | [81] |
| 6 | 450.00 | Honeydew | Mountain multi-flora | Romania | [81] |
| 7 | 131.00 | Apiaceae fruit | Lagoecia cuminoides | Greece | [82] |
| 8 | 716.00 | Asteraceae herb | Artemisia arborescens | Greece | [82] |
| 9 | 54.00 | Asteraceae leaves | Taraxacum officinale | Greece | [82] |
| 10 | 802.00 | Caprifoliaceae flower | Sambucus nigra | Greece | [82] |
| 11 | 158.00 | Lamiaceae herb | Hyssopus officinalis | Greece | [82] |
| 12 | 71.00 | Lamiaceae flower | Lavandula vera | Greece | [82] |
| 13 | 1018.00 | Lamiaceae leaves | Mentha pulegium | Greece | [82] |
| 14 | 1349.00 | Araceae rhizome | Acorus calamus | Poland | [83] |
| 15 | 7693.00 | Asteraceae herbal | Achillea millefolium | Poland | [83] |
| 16 | 7985.00 | Asteraceae leaves | Echinacea purpurea | Poland | [83] |
| 17 | 11,020.00 | Lamiaceae herbal | Rosmarinus officinalis | Poland | [83] |
| 18 | 2893.50 | Papaveraceae herbal | Chelidonium majus | Poland | [83] |
| 19 | 50.82 | Raspberry herb | Rubus sp. | Australia | [84] |
| 20 | 16.56 | Thyme herb | Thymus vulgaris | Australia | [84] |
| 21 | 6.76 | Camomile herb | Matricaria chamomilla | Australia | [84] |
| 22 | 2560.60 | Oleaceae leaves | Olea europaea | Bulgaria | [85] |
| 23 | 826.10 | Oleaceae leaves | Olea europaea | Bulgaria | [85] |
| 24 | 186.70 | Vitaceae berries | Vitis vinifera | Czech Republic | [86] |
| 25 | 1243.10 | Vitaceae stem | Vitis vinifera | Czech Republic | [86] |
| 26 | 659.40 | Vitaceae leaves | Vitis vinifera | Czech Republic | [86] |
| 27 | 50.12 | Amaranthaceae leaves | Achyranthes aspera | India | [87] |
| 28 | 196.39 | Brassicaceae leaves | Brassica juncea | India | [88] |
| 29 | 179.57 | Brassicaceae seedlings | Brassica juncea | India | [88] |
| 30 | 92.20 | Tamaricaceace stem | Tamarix aphylla | Tunisia | [89] |
| 31 | 668.00 | Tamaricaceace leaves | Tamarix aphylla | Tunisia | [89] |
| 32 | 594.45 | Primulaceae leaves | Labisia pumila | Malaysia | [90] |
| 33 | 1192.12 | Primulaceae leaves | Labisia pumila | Malaysia | [90] |
| 34 | 307.64 | Asteraceae leaves | Artemisia absinthium | South Korea | [91] |
| No. | Natural Products | Key Features | References |
|---|---|---|---|
| 1 | garlic raw extract | It has been shown that there is synergic antimicrobial activity between raw garlic extract and honey, but the antioxidant activity of the mixture is lower than that of A. sativum. Moreover, the mix of honey–A. sativum extract presented increased wound healing capacity compared to Euphorbia honey. | [98] |
| 2 | garlic | The mixture of garlic extracts and Tengen honey has significant sensitivity against Gram-positive and Gram-negative bacteria, which leads to their potential to treat infectious diseases. | [102] |
| 3 | rifampicin | Mixing Medihoney and rifampicin against laboratory and clinical strains of S. aureus, including MRSA strains, demonstrated a good synergism effect. Also, results support the potential of combining Manuka honey and antibiotics in treating S. aureus-related skin infections. | [105] |
| 4 | basil | The combined effect of honey and plants showed synergism. This fact is explained by the antibacterial effect that can be extremely useful in treating infested lesions and can also be used as a potential antimicrobial. | [115] |
| 5 | sweet basil | The synergic effects of honey combined with the alcoholic extract of O. basilicum appeared to have essential properties that made it useful as a wound dressing. | [120] |
| 6 | cinnamon | The study reveals that cinnamon could improve honey’s antibacterial effect against S. mutans. | [121] |
| 7 | ginger | In vitro data from this study shows antimicrobial activity has a synergic effect by combining ginger and honey against bacterial cells isolated from carious teeth. This investigation suggests that a paste made by blending ginger and honey could be used as a mouthwash in treating dental caries, mouth sores, and sore throats and could be incorporated into toothpaste to prevent dental caries. | [127] |
| 8 | ginger | Results confirmed that ginger strongly improves the MIC of honey, thus letting hope for a honey benefit and would constitute an alternative way against the resistance to bacteria. | [128] |
| No. | Type of Bee Product | Mode of Administration | Effects of Honey | References |
|---|---|---|---|---|
| 1 | honey (from the lime tree, chestnut, rapeseed, eucalyptus, and heather) | Oral administration of 1.2 g honey/kg body | ↑ (high) β-carotene ↑ GSH (glutathione) ↑ vitamin C ↑ uric acid | [175] |
| 2 | honey | Oral administration of 1.2 g honey/kg body weight (7 men and 3 women) for 2 weeks | ↑ serum vitamin C, iron, and copper levels ↑ β-carotene ↑ uric acid ↑ GSH ↑lymphocyte and eosinophil % ↓ (low) liver enzymes (AST—aspartate aminotransferase, ALT-alanine aminotransferase) ↓ serum Ig E (imunoglobulin E) ↓ lactic acid dehydrogenase ↓ creatinine kinase ↓ monocytes up to 50% | [176] |
| 3 | honey-pollen mix | in vivo induced liver injury through oral administration of acetaminophen on mice | ↑ TBARS (thiobarbituric acid reactive substance) ↓ GSH in liver | [177] |
| 4 | honey | Oral administration of honey combined with metformin or glibenclamide on rats | ↑ CAT (catalase), GR (glutathione reductase), TAS (total antioxidant status) and GSH for honey combined with metformin or glibenclamide | [178] |
| 5 | honey | 0.2, 1.2 and 2.4 g honey/kg body weight/day -oral administration on rats | ↑SOD (superoxide dismutase) ↓ TAS, CAT, GPx (glutathione peroxidase), GR and GST (glutathione S-transferase) | [179] |
| 6 | honey | Intravenous administration (2 g honey/kg body weight of sheep) | ↑ NO (nitric oxide) levels | [176] |
| 7 | Tualang honey | Oral administration of 1.5 g/kg or 0.75 g honey/kg body weight on two groups of woman athletes | ↑ ROS levels in athletes that consumed 1.5 g honey/ kg body | [180] |
| 8 | honey | Oral consumption: 1, 1.5 and 2 g/kg/day for every 2 weeks | ↓ body weight, total cholesterol, low-density lipoprotein cholesterol, and triglyceride ↑ high-density lipoprotein-cholesterol, haemoglobin A1c | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings. Antioxidants 2023, 12, 34. https://doi.org/10.3390/antiox12010034
Spoială A, Ilie C-I, Ficai D, Ficai A, Andronescu E. Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings. Antioxidants. 2023; 12(1):34. https://doi.org/10.3390/antiox12010034
Chicago/Turabian StyleSpoială, Angela, Cornelia-Ioana Ilie, Denisa Ficai, Anton Ficai, and Ecaterina Andronescu. 2023. "Synergic Effect of Honey with Other Natural Agents in Developing Efficient Wound Dressings" Antioxidants 12, no. 1: 34. https://doi.org/10.3390/antiox12010034






