Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update
Abstract
:1. Introduction
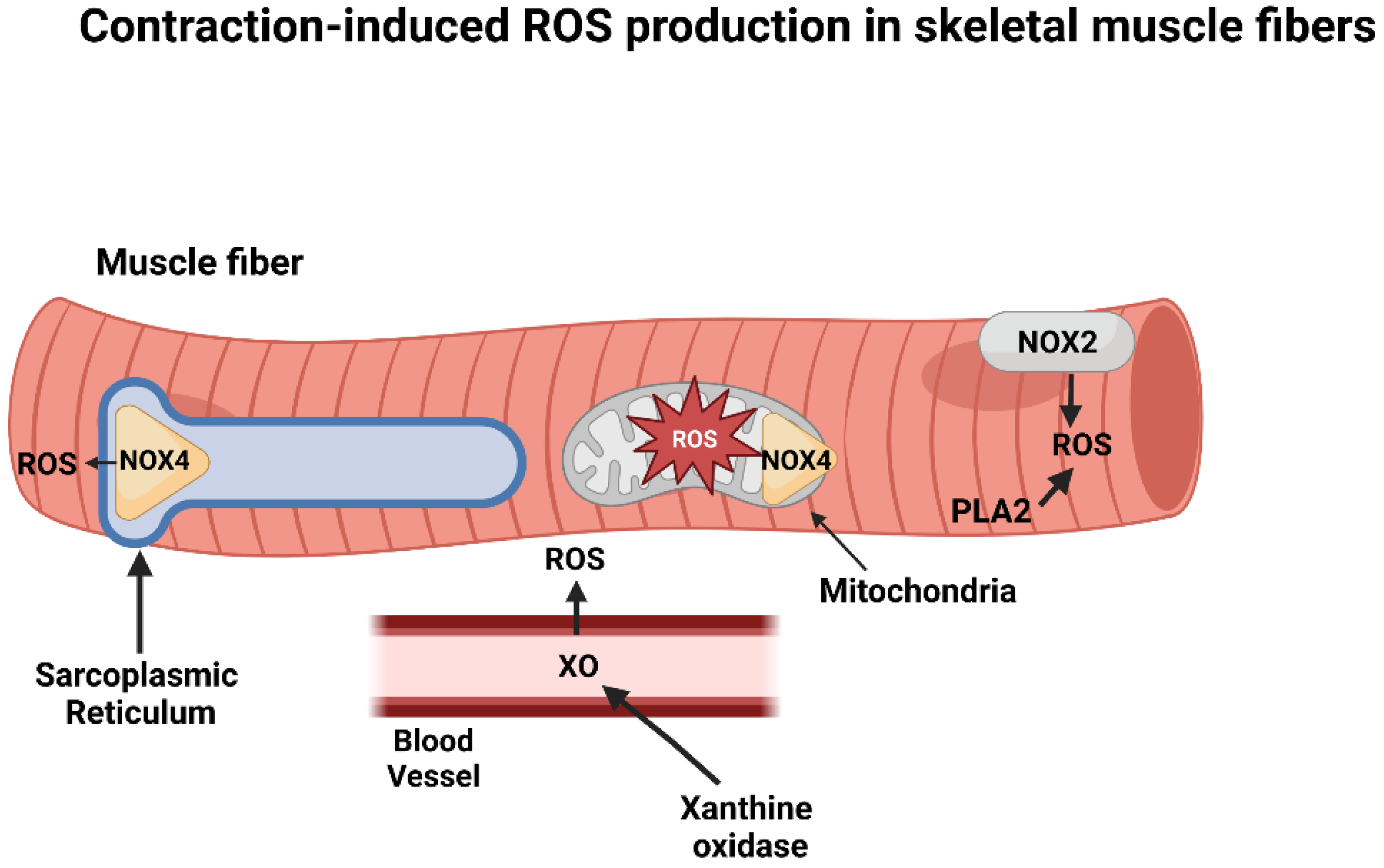
2. Intracellular Sources of ROS in Contracting Skeletal Muscles
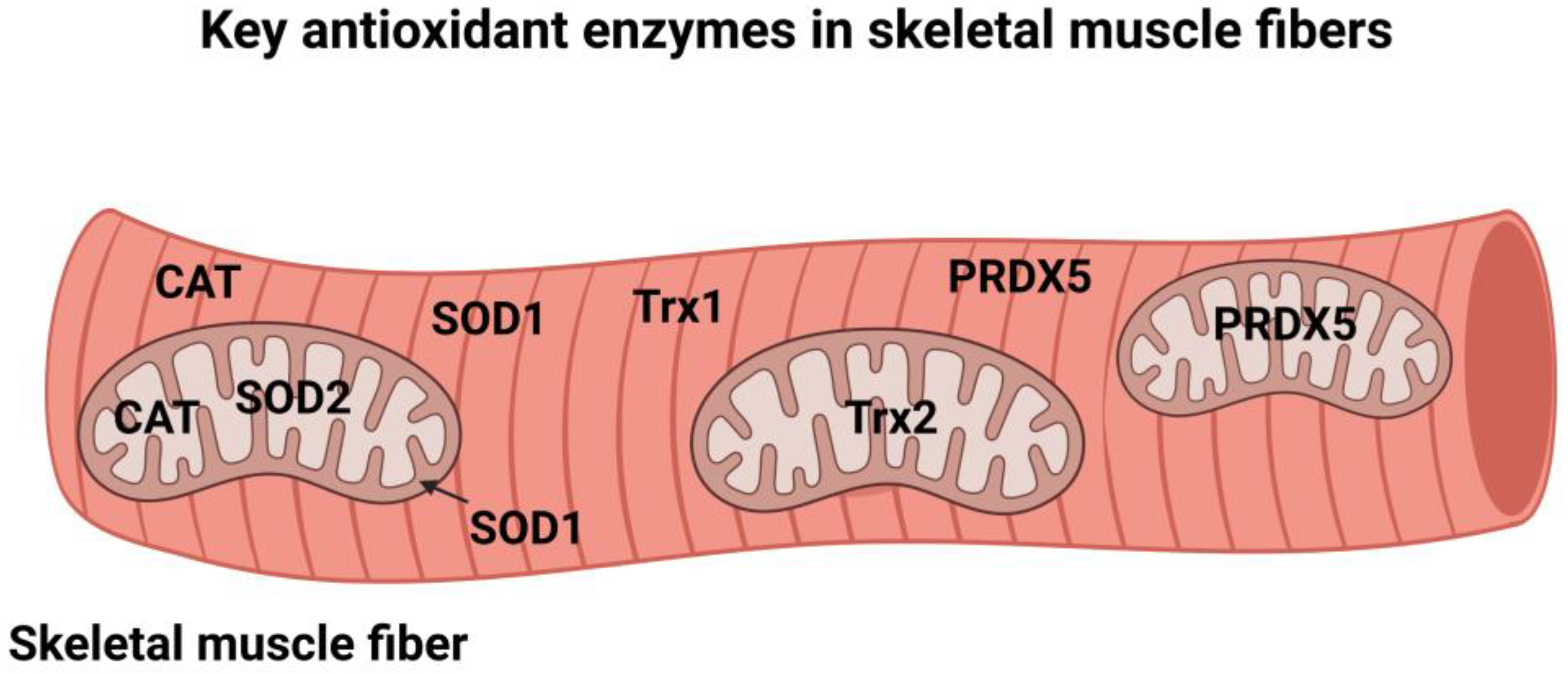
3. Cellular Antioxidant Enzymes
3.1. Superoxide Dismutase (SOD)
3.2. Glutathione Peroxidase (GPX)
3.3. Catalase (CAT)
3.4. Peroxiredoxins (PRDXs)
3.5. Thioredoxins (Trxs)
4. Endurance Exercise Training-Induced Changes in Muscle Antioxidant Enzymes
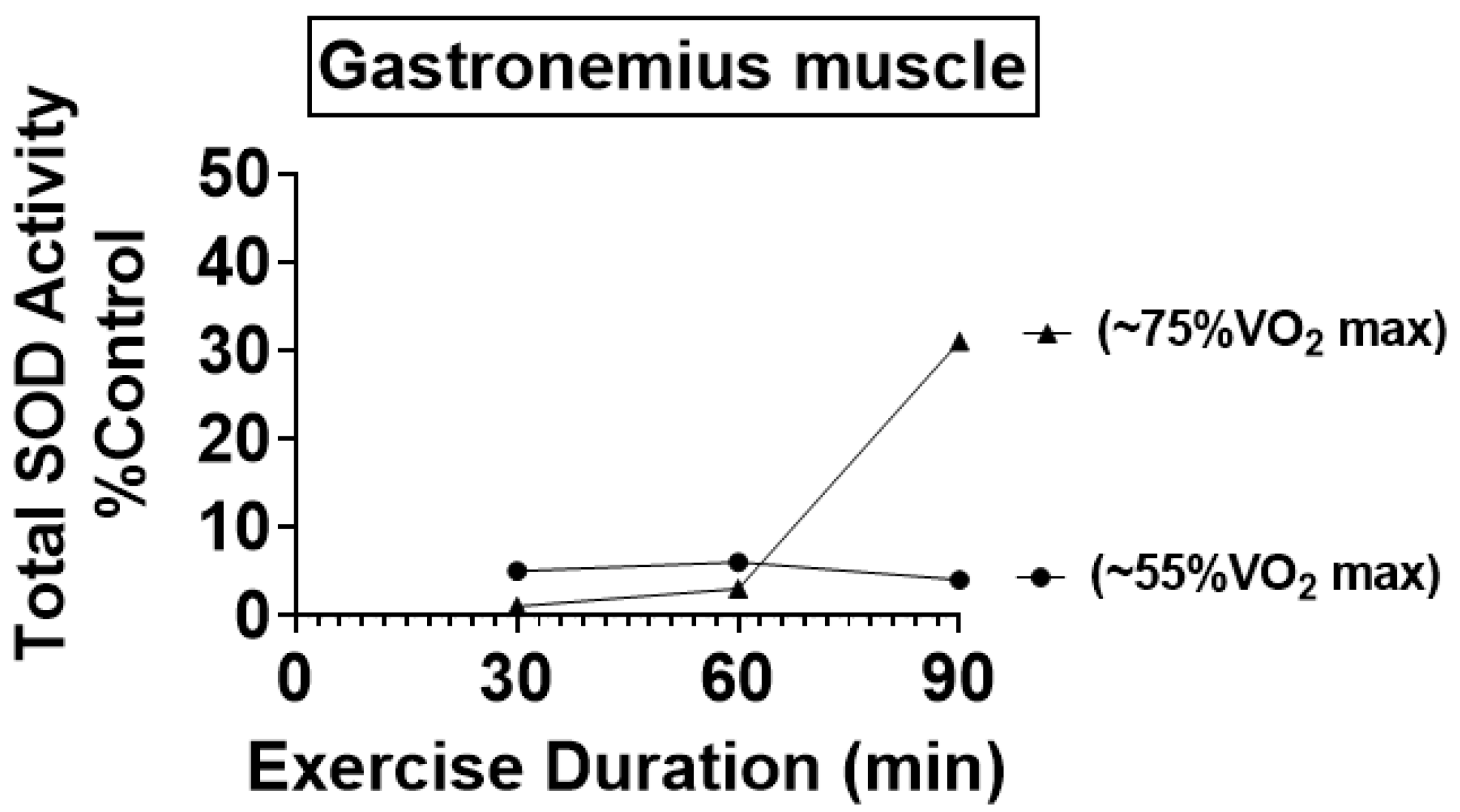
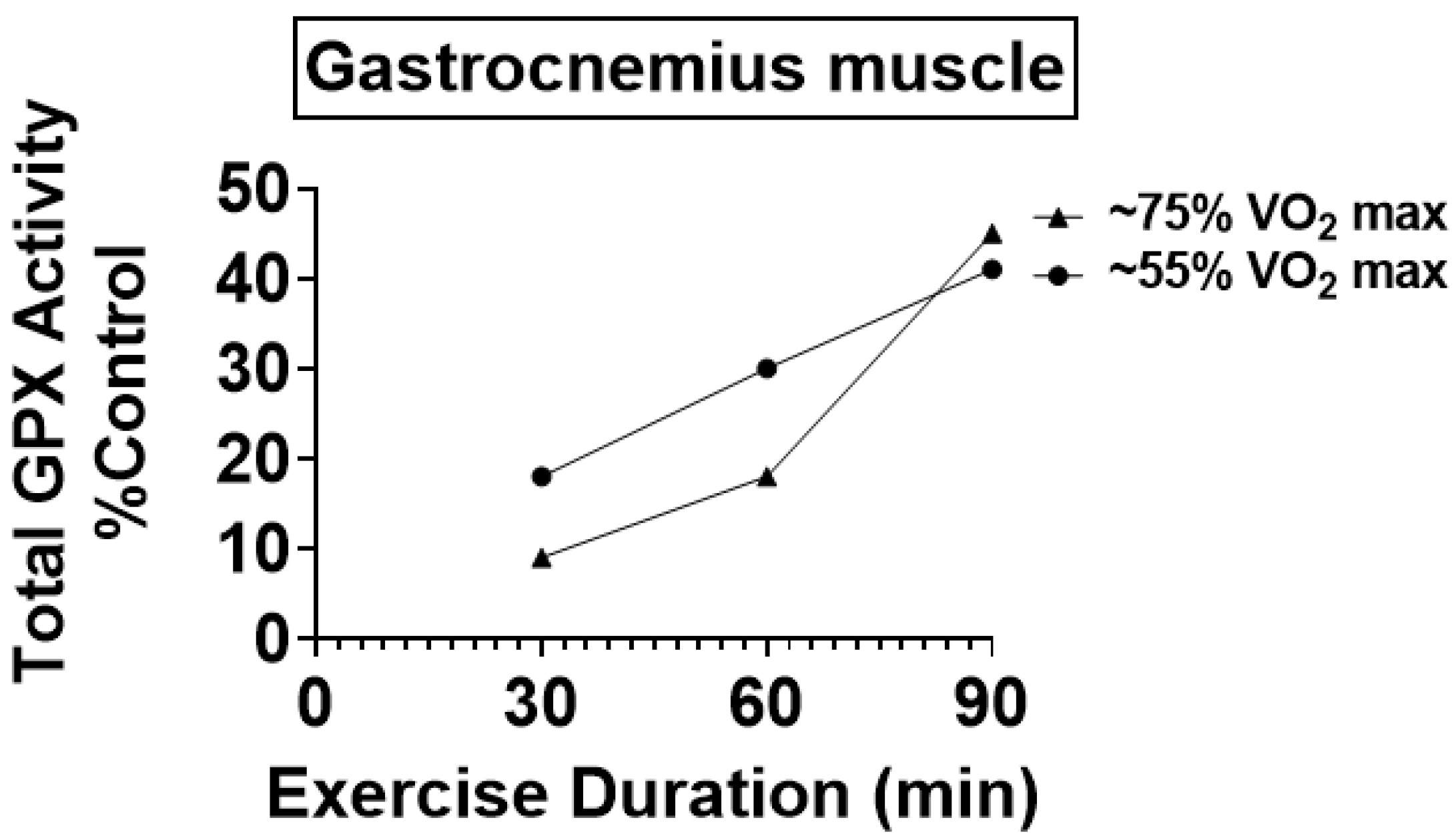
4.1. Preclinical Studies Reveal That Endurance Training Increases the Activity of Key Antioxidant Enzymes in Skeletal Muscles
4.2. Endurance Exercise Training Increases Key Antioxidant Enzymes in Human Skeletal Muscles
5. High-Intensity Interval Training-Induced Changes in Skeletal Muscle Antioxidant Enzymes
5.1. Preclinical Studies
5.2. High-Intensity Interval Training and Antioxidant Enzymes in Human Skeletal Muscles
6. Resistance Training-Induced Increases in Muscle Antioxidant Enzymes
6.1. Preclinical Studies Suggest That Resistance Training Increases Skeletal Muscle Antioxidants
6.2. Resistance Exercise Training Increases Antioxidant Enzyme Activity in Humans
7. Exercise-Induced Improvements in Muscle Antioxidant Enzyme Capacity Protects against ROS-Mediated Oxidative Damage
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Packer, L.; Brooks, G.A. Exercise bioenergetics following sprint training. Arch. Biochem. Biophys. 1982, 215, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, A.T.; Packer, L. Vitamin E, physical exercise and tissue oxidative damage. Ciba Found Symp. 1983, 101, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 2011, 15, 2477–2486. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [Green Version]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.C.; Arbogast, S.; Guo, Z.; Mathenia, J.; Su, W.; Reid, M.B. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J. Appl. Physiol. 2006, 100, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, R.; Basu Thakur, P.; Li, S.; Minard, C.; Rodney, G.G. Real-time imaging of NADPH oxidase activity in living cells using a novel fluorescent protein reporter. PLoS ONE 2013, 8, e63989. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Martinez, A.; Santangelo, G.; Pallardo, F.V.; Sastre, J.; Vina, J. Oxidative stress in marathon runners: Interest of antioxidant supplementation. Br. J. Nutr. 2006, 96 (Suppl. S1), S31–S33. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardo, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef]
- Vogel, J.; Kruse, C.; Zhang, M.; Schroder, K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. 2015, 593, 2145–2154. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Leiva, A.; Pena, M.; Muller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J. Cell Physiol. 2006, 209, 379–388. [Google Scholar] [CrossRef]
- Michaelson, L.P.; Shi, G.; Ward, C.W.; Rodney, G.G. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve 2010, 42, 522–529. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arner, E.S. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Ohno, H.; Oh-ishi, S.; Kizaki, T.; Ookawara, T.; Fujii, J.; Radák, Z.; Taniguchi, N. Superoxide dismutases in exercise and diseases. In Handbood of Oxidants and Antioxidants and Exercise; Sen, C., Packer, L., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 243–295. [Google Scholar]
- Lewandowski, L.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef] [Green Version]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Flohe, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.A.J.G. Free Radicals in Biology and Medicine; Oxford: London, UK, 2015. [Google Scholar]
- Karplus, P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015, 80, 183–190. [Google Scholar] [CrossRef]
- Chae, H.Z.; Robison, K.; Poole, L.B.; Church, G.; Storz, G.; Rhee, S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: Alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 1994, 91, 7017–7021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox. Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, D.; Osama, A.; Fang, J. Natural Molecules Targeting Thioredoxin System and Their Therapeutic Potential. Antioxid. Redox Signal. 2021, 34, 1083–1107. [Google Scholar] [CrossRef]
- Alessio, H.M.; Goldfarb, A.H. Lipid peroxidation and scavenger enzymes during exercise: Adaptive response to training. J. Appl. Physiol. 1988, 64, 1333–1336. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990, 68, 2337–2343. [Google Scholar] [CrossRef]
- Pereira, B.; Costa Rosa, L.F.; Safi, D.A.; Medeiros, M.H.; Curi, R.; Bechara, E.J. Superoxide dismutase, catalase, and glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol. Behav. 1994, 56, 1095–1099. [Google Scholar] [CrossRef]
- Gore, M.; Fiebig, R.; Hollander, J.; Leeuwenburgh, C.; Ohno, H.; Ji, L.L. Endurance training alters antioxidant enzyme gene expression in rat skeletal muscle. Can. J. Physiol. Pharmacol. 1998, 76, 1139–1145. [Google Scholar] [CrossRef]
- Higuchi, M.; Cartier, L.J.; Chen, M.; Holloszy, J.O. Superoxide dismutase and catalase in skeletal muscle: Adaptive response to exercise. J. Gerontol. 1985, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Fiebig, R.; Gore, M.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression in skeletal muscle: Fiber-specific adaptation to endurance training. Am. J. Physiol. 1999, 277, R856–R862. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Smuder, A.J.; Sollanek, K.J.; Morton, A.B.; Roberts, M.D.; Kavazis, A.N. Comparative changes in antioxidant enzymes and oxidative stress in cardiac, fast twitch and slow twitch skeletal muscles following endurance exercise training. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 160–168. [Google Scholar] [PubMed]
- Lawler, J.M.; Kwak, H.B.; Song, W.; Parker, J.L. Exercise training reverses downregulation of HSP70 and antioxidant enzymes in porcine skeletal muscle after chronic coronary artery occlusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1756–R1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeuwenburgh, C.; Fiebig, R.; Chandwaney, R.; Ji, L.L. Aging and exercise training in skeletal muscle: Responses of glutathione and antioxidant enzyme systems. Am. J. Physiol. 1994, 267, R439–R445. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am. J. Physiol. 1997, 272, R363–R369. [Google Scholar] [CrossRef] [PubMed]
- Oh-ishi, S.; Kizaki, T.; Nagasawa, J.; Izawa, T.; Komabayashi, T.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of endurance training on superoxide dismutase activity, content and mRNA expression in rat muscle. Clin. Exp. Pharmacol. Physiol. 1997, 24, 326–332. [Google Scholar] [CrossRef]
- Osorio Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rego Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef]
- Quintanilha, A.T. Effects of physical exercise and/or vitamin E on tissue oxidative metabolism. Biochem. Soc. Trans. 1984, 12, 403–404. [Google Scholar] [CrossRef]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Fiebig, R.; Gore, M.; Ookawara, T.; Ohno, H.; Ji, L.L. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflug. Arch. 2001, 442, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Pushkarenko, J.; Houston, M.E. Lack of antioxidant adaptation to short-term aerobic training in human muscle. Am. J. Physiol. 1996, 271, R832–R836. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Adams, V.; Schulze, P.C.; Erbs, S.; Gielen, S.; Fiehn, E.; Mobius-Winkler, S.; Schubert, A.; Schuler, G.; Hambrecht, R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation 2005, 111, 1763–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, C.; Chung, N.; Schmidt, U.; Kreutz, T.; Lenzen, E.; Schiffer, T.; Geisler, S.; Graf, C.; Montiel-Garcia, G.; Renner, R.; et al. Training alters the skeletal muscle antioxidative capacity in non-insulin-dependent type 2 diabetic men. Scand. J. Med. Sci. Sports 2012, 22, 462–470. [Google Scholar] [CrossRef]
- Samjoo, I.A.; Safdar, A.; Hamadeh, M.J.; Raha, S.; Tarnopolsky, M.A. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr. Diabetes 2013, 3, e88. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, K.L.; Ballard, K.D.; Deal, M.A.; Tagariello, L.C.; Karrow, J.M.; Volk, G.A.; Meisler, A.; Connors, I.D.; Mott, R.E. Associations Among Physical Activity Level and Skeletal Muscle Antioxidants in Older Adults. J. Phys. Act. Health 2020, 17, 895–901. [Google Scholar] [CrossRef]
- Cobley, J.N.; Sakellariou, G.K.; Owens, D.J.; Murray, S.; Waldron, S.; Gregson, W.; Fraser, W.D.; Burniston, J.G.; Iwanejko, L.A.; McArdle, A.; et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014, 70, 23–32. [Google Scholar] [CrossRef]
- Atalay, M.; Seene, T.; Hanninen, O.; Sen, C.K. Skeletal muscle and heart antioxidant defences in response to sprint training. Acta Physiol. Scand. 1996, 158, 129–134. [Google Scholar] [CrossRef]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef]
- Hellsten, Y.; Apple, F.S.; Sjodin, B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J. Appl. Physiol. 1996, 81, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Frandsen, U.; Orthenblad, N.; Sjodin, B.; Richter, E.A. Xanthine oxidase in human skeletal muscle following eccentric exercise: A role in inflammation. J. Physiol. 1997, 498 (Pt 1) Pt 1, 239–248. [Google Scholar] [CrossRef]
- Gomes, M.J.; Pagan, L.U.; Lima, A.R.R.; Reyes, D.R.A.; Martinez, P.F.; Damatto, F.C.; Pontes, T.H.D.; Rodrigues, E.A.; Souza, L.M.; Tosta, I.F.; et al. Effects of aerobic and resistance exercise on cardiac remodelling and skeletal muscle oxidative stress of infarcted rats. J. Cell Mol. Med. 2020, 24, 5352–5362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murlasits, Z.; Lee, Y.; Powers, S.K. Short-term exercise does not increase ER stress protein expression in cardiac muscle. Med. Sci. Sports Exerc. 2007, 39, 1522–1528. [Google Scholar] [CrossRef]
- Scheffer, D.L.; Silva, L.A.; Tromm, C.B.; da Rosa, G.L.; Silveira, P.C.; de Souza, C.T.; Latini, A.; Pinho, R.A. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl. Physiol. Nutr. Metab. 2012, 37, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Beltran Valls, M.R.; Dimauro, I.; Brunelli, A.; Tranchita, E.; Ciminelli, E.; Caserotti, P.; Duranti, G.; Sabatini, S.; Parisi, P.; Parisi, A.; et al. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age 2014, 36, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, D.; Hakkinen, K.; Cuevas, M.J.; Lima, E.; Kauhanen, A.; Mattila, M.; Sillanpaa, E.; Ahtiainen, J.P.; Karavirta, L.; Almar, M.; et al. Effects of strength and endurance training on antioxidant enzyme gene expression and activity in middle-aged men. Scand. J. Med. Sci. Sports 2007, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.H.C.; Lamb, D.A.; Godwin, J.S.; Osburn, S.C.; Ruple, B.A.; Moore, J.H.; Vann, C.G.; Huggins, K.W.; Fruge, A.D.; Young, K.C.; et al. Effects of Resistance Training on the Redox Status of Skeletal Muscle in Older Adults. Antioxidants 2021, 10, 350. [Google Scholar] [CrossRef]
- Parise, G.; Phillips, S.M.; Kaczor, J.J.; Tarnopolsky, M.A. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic. Biol. Med. 2005, 39, 289–295. [Google Scholar] [CrossRef]
- Powers, S.K.; Criswell, D.; Lawler, J.; Martin, D.; Ji, L.L.; Herb, R.A.; Dudley, G. Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir. Physiol. 1994, 95, 227–237. [Google Scholar] [CrossRef]
- Sollanek, K.J.; Burniston, J.G.; Kavazis, A.N.; Morton, A.B.; Wiggs, M.P.; Ahn, B.; Smuder, A.J.; Powers, S.K. Global Proteome Changes in the Rat Diaphragm Induced by Endurance Exercise Training. PLoS ONE 2017, 12, e0171007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh-ishi, S.; Kizaki, T.; Ookawara, T.; Sakurai, T.; Izawa, T.; Nagata, N.; Ohno, H. Endurance training improves the resistance of rat diaphragm to exercise-induced oxidative stress. Am. J. Respir. Crit. Care Med. 1997, 156, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Powers, S.K.; Demirel, H.A.; Coombes, J.S.; Naito, H. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Powers, S.K.; Stewart, D.J.; Demirel, H.A.; Shanely, R.A.; Naito, H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000, 81, 67–74. [Google Scholar] [CrossRef]
- Barreiro, E.; Galdiz, J.B.; Marinan, M.; Alvarez, F.J.; Hussain, S.N.; Gea, J. Respiratory loading intensity and diaphragm oxidative stress: N-acetyl-cysteine effects. J. Appl. Physiol. 2006, 100, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Khawli, F.A.; Reid, M.B. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J. Appl. Physiol. 1994, 77, 317–324. [Google Scholar] [CrossRef]
- Reid, M.B.; Stokic, D.S.; Koch, S.M.; Khawli, F.A.; Leis, A.A. N-acetylcysteine inhibits muscle fatigue in humans. J. Clin. Investig. 1994, 94, 2468–2474. [Google Scholar] [CrossRef]
- Shindoh, C.; DiMarco, A.; Thomas, A.; Manubay, P.; Supinski, G. Effect of N-acetylcysteine on diaphragm fatigue. J. Appl. Physiol. 1990, 68, 2107–2113. [Google Scholar] [CrossRef]
- Vieira Ramos, G.; Choqueta de Toledo-Arruda, A.; Maria Pinheiro-Dardis, C.; Liyoko Suehiro, C.; Luiz de Russo, T.; Vieira, R.P.; Arruda Martins, M.; Salvini, T.F.; Durigan, J.L.Q. Exercise Prevents Diaphragm Wasting Induced by Cigarette Smoke through Modulation of Antioxidant Genes and Metalloproteinases. Biomed. Res. Int. 2018, 2018, 5909053. [Google Scholar] [CrossRef] [Green Version]
- Morton, A.B.; Smuder, A.J.; Wiggs, M.P.; Hall, S.E.; Ahn, B.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Huertas, A.M.; Ozdemir, M.; Yoshihara, T.; et al. Increased SOD2 in the diaphragm contributes to exercise-induced protection against ventilator-induced diaphragm dysfunction. Redox. Biol. 2019, 20, 402–413. [Google Scholar] [CrossRef]
- Smuder, A.J.; Min, K.; Hudson, M.B.; Kavazis, A.N.; Kwon, O.S.; Nelson, W.B.; Powers, S.K. Endurance exercise attenuates ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2012, 112, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smuder, A.J.; Morton, A.B.; Hall, S.E.; Wiggs, M.P.; Ahn, B.; Wawrzyniak, N.R.; Sollanek, K.J.; Min, K.; Kwon, O.S.; Nelson, W.B.; et al. Effects of exercise preconditioning and HSP72 on diaphragm muscle function during mechanical ventilation. J. Cachexia Sarcopenia Muscle 2019, 10, 767–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smuder, A.J.; Kavazis, A.N.; Min, K.; Powers, S.K. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J. Appl. Physiol. 2011, 111, 1190–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, K.R.; Vincent, H.K.; Braith, R.W.; Lennon, S.L.; Lowenthal, D.T. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur. J. Appl. Physiol. 2002, 87, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.; Vincent, K.R. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 2006, 14, 1921–1930. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Jamurtas, A.Z.; Villiotou, V.; Pouliopoulou, S.; Fotinakis, P.; Taxildaris, K.; Deliconstantinos, G. Oxidative stress responses in older men during endurance training and detraining. Med. Sci. Sports Exerc. 2004, 36, 2065–2072. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powers, S.K.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants 2023, 12, 39. https://doi.org/10.3390/antiox12010039
Powers SK, Goldstein E, Schrager M, Ji LL. Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants. 2023; 12(1):39. https://doi.org/10.3390/antiox12010039
Chicago/Turabian StylePowers, Scott K., Erica Goldstein, Matthew Schrager, and Li Li Ji. 2023. "Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update" Antioxidants 12, no. 1: 39. https://doi.org/10.3390/antiox12010039
APA StylePowers, S. K., Goldstein, E., Schrager, M., & Ji, L. L. (2023). Exercise Training and Skeletal Muscle Antioxidant Enzymes: An Update. Antioxidants, 12(1), 39. https://doi.org/10.3390/antiox12010039





