Dietary Supplement of Grape Wastes Enhances Honeybee Immune System and Reduces Deformed Wing Virus (DWV) Load
Abstract
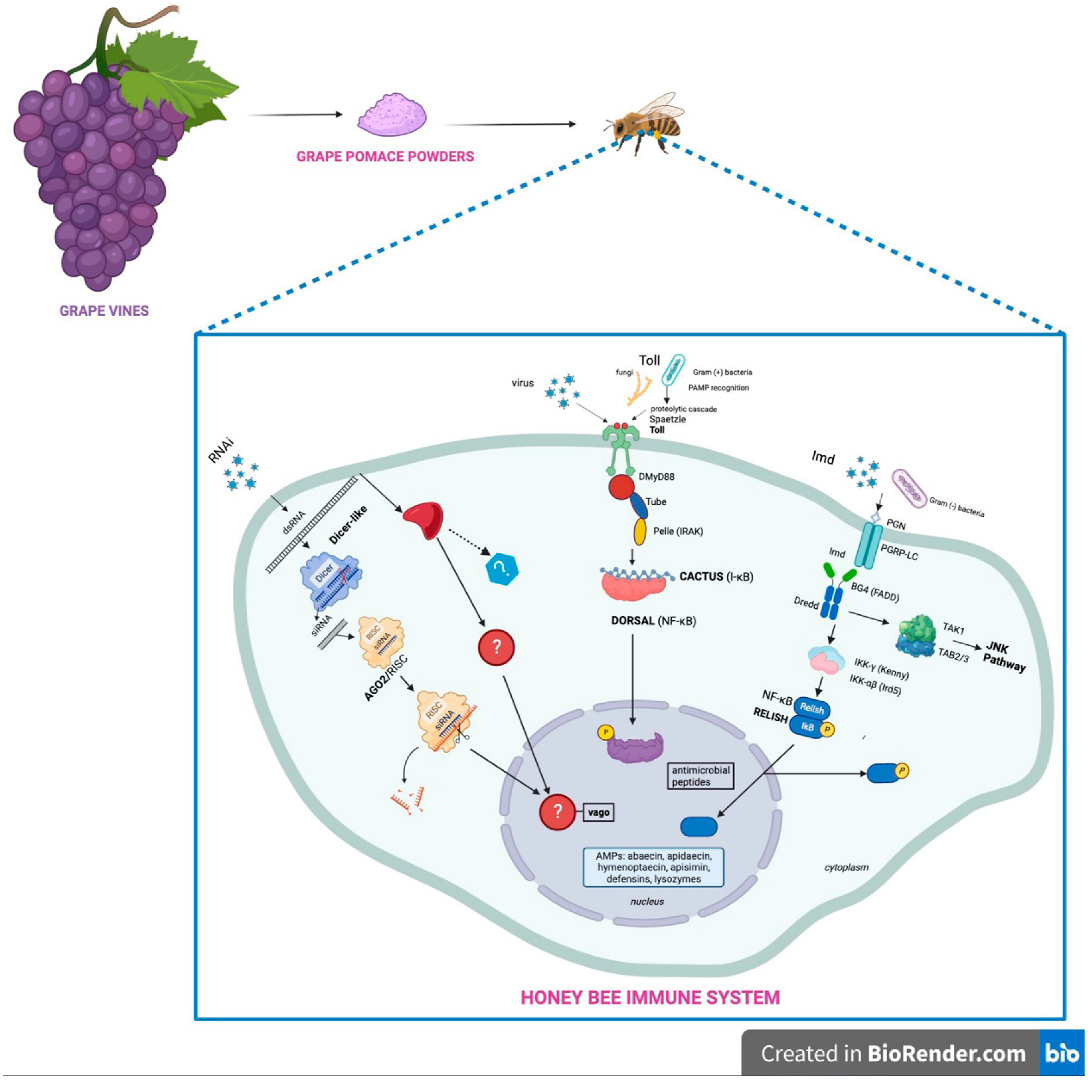
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of the Microencapsulated Supplement by Spray Drying and Characterization of the Grape Pomace Powder (GPP)
2.3. Characterization and Quantification of Phenolic Compounds and Antioxidant Properties from Grape Pomace Powder
2.4. Inoculum DWV-A Preparation
2.5. Bee Inoculation and Experimental Design
2.6. RNA Extraction and cDNA Synthesis
2.7. Real-Time PCR Quantification (qPCR)
2.8. Data Analysis
3. Results and Discussion
3.1. Characterization of the Grape Pomace Dietary Supplement
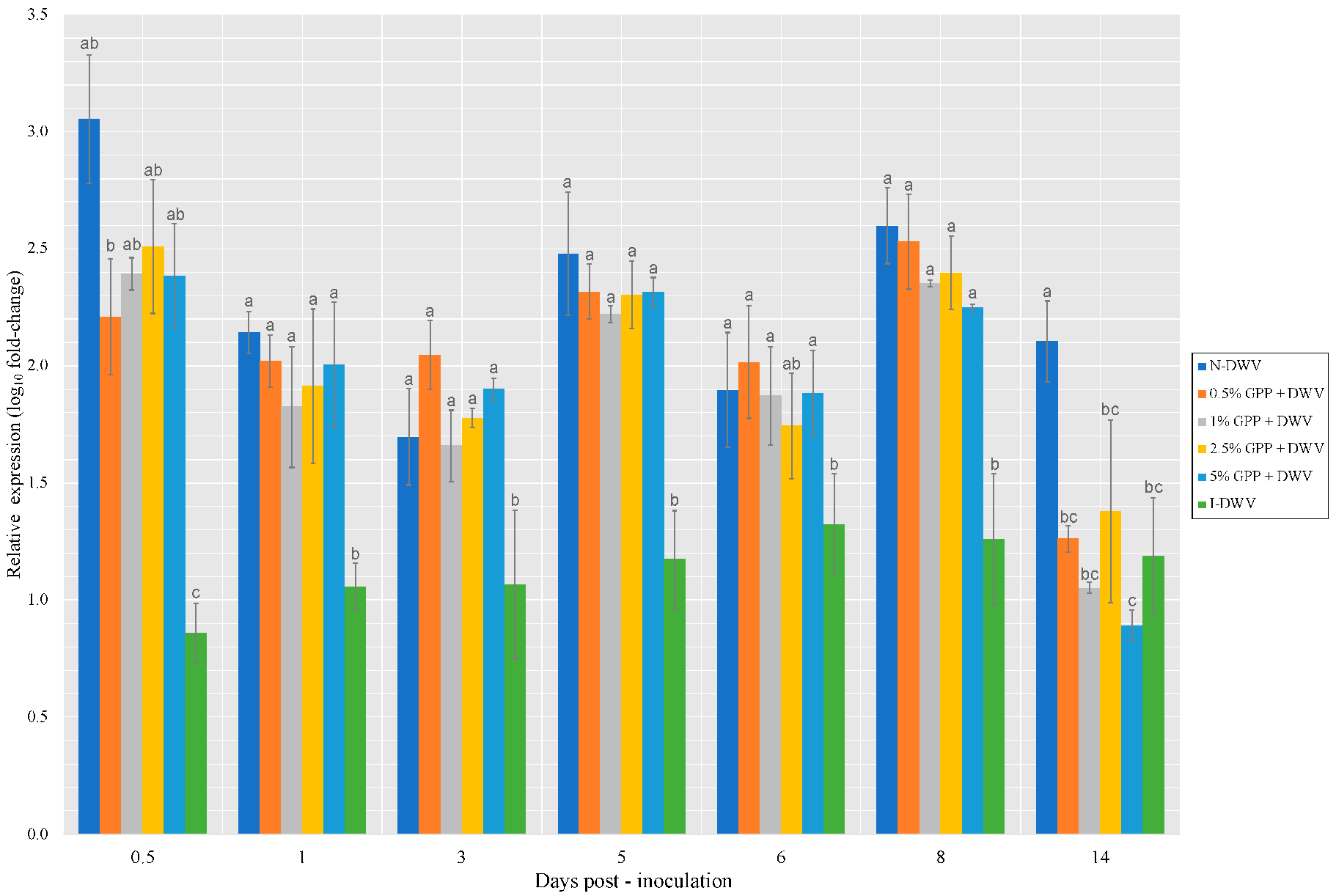
3.2. DWV Load after Feeding with a Grape Pomace Supplement
3.3. Response in Honeybee Gene Expression after Feeding with a Grape Pomace Supplement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasileiadou, M.A.; Altiparmaki, G.; Moustakas, K.; Vakalis, S. Quality of Hydrochar from Wine Sludge under Variable Conditions of Hydrothermal Carbonization: The Case of Lesvos Island. Energies 2022, 15, 3574. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.A.; Cheng, V.J.; Zhang, H.; Mros, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Bekhit, A.A.; McConnell, M. Effect of extraction system and grape variety on anti-influenza compounds from wine production residue. Food Control 2019, 99, 180–189. [Google Scholar] [CrossRef]
- Konowalchuk, J.; Speirs, J.I. Virus inactivation by grapes and wines. Appl. Environ. Microbiol. 1976, 32, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; López, M.D.; Vargas, M.; Aranda, M.; Cañumir, J.A. Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties. Foods 2022, 11, 1604. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2020, 11, 11. [Google Scholar] [CrossRef]
- Hýbl, M.; Mráz, P.; Šipoš, J.; Hoštičková, I.; Bohatá, A.; Čurn, V.; Kopec, T. Polyphenols as Food Supplement Improved Food Consumption and Longevity of Honey Bees (Apis mellifera) Intoxicated by Pesticide Thiacloprid. Insects 2021, 12, 572. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef]
- Loope, K.J.; Baty, J.W.; Lester, P.J.; Wilson Rankin, E.E. Pathogen shifts in a honeybee predator following the arrival of the Varroa mite. Proc. R. Soc. B Boil. Sci. 2019, 286, 20182499. [Google Scholar] [CrossRef]
- Iqbal, J.; Mueller, U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B Boil. Sci. 2007, 274, 1517–1521. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Èntomol. 2019, 64, 205–226. [Google Scholar] [CrossRef]
- Hall, D.M.; Steiner, R. Insect pollinator conservation policy innovations at subnational levels: Lessons for lawmakers. Environ. Sci. Policy 2019, 93, 118–128. [Google Scholar] [CrossRef]
- Van der Steen, J.; Vejsnæs, F. Varroa Control: A Brief Overview of Available Methods. Bee World 2021, 98, 50–56. [Google Scholar] [CrossRef]
- Tritschler, M.; Vollmann, J.J.; Yañez, O.; Chejanovsky, N.; Crailsheim, K.; Neumann, P. Protein nutrition governs within-host race of honey bee pathogens. Sci. Rep. 2017, 7, 14988. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a Functional Food for Apis mellifera. Annu. Rev. Èntomol. 2021, 66, 185–208. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Nally, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts of Polypore Mushroom Mycelia Reduce Viruses in Honey Bees. Sci. Rep. 2018, 8, 13936. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Tozkar, C.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and Pollen Phytochemicals Stimulate Honey Bee (Hymenoptera: Apidae) Immunity to Viral Infection. J. Econ. Èntomol. 2017, 110, 1959–1972. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Flenniken, M.L. RNAi and Antiviral Defense in the Honey Bee. J. Immunol. Res. 2015, 2015, 941897. [Google Scholar] [CrossRef]
- Chejanovsky, N.; Ophir, R.; Schwager, M.S.; Slabezki, Y.; Grossman, S.; Cox-Foster, D. Characterization of viral siRNA populations in honey bee colony collapse disorder. Virology 2014, 454–455, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and dsRNA-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The Iflaviruses Sacbrood virus and Deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. Peerj 2016, 4, e1591. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- López-Belchí, M.D.; Caamaño, E.F.; Pascual, G.; Noriega, F.; Fierro-Morales, P.; Romero-Román, M.E.; Jara, P.; Schoebitz, M.; Serra, I.; Moreno, D.A. Spray-Dried Formulations Rich in Malvidin from Tintorera Grape Wastes: Characterization, Stability, and Storage. Processes 2021, 9, 518. [Google Scholar] [CrossRef]
- Romero-Román, M.E.; Schoebitz, M.; Fuentealba, J.; García-Viguera, C.; Belchí, M.D.L. Phenolic Compounds in Calafate Berries Encapsulated by Spray Drying: Neuroprotection Potential into the Ingredient. Antioxidants 2021, 10, 1830. [Google Scholar] [CrossRef]
- Pantelić, M.; Zagorac, D.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.; Tešić, Z.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Gusachenko, O.N.; Woodford, L.; Balbirnie-Cumming, K.; Ryabov, E.V.; Evans, D.J. Evidence for and against deformed wing virus spillover from honey bees to bumble bees: A reverse genetic analysis. Sci. Rep. 2020, 10, 16847. [Google Scholar] [CrossRef]
- Vargas, M.; Arismendi, N.; Riveros, G.; Zapata, N.; Bruna, A.; Vidal, M.; Rodríguez, M.; Gerding, M. Viral and intestinal diseases detected in Apis mellifera in Central and Southern Chile. Chil. J. Agric. Res. 2017, 77, 243–249. [Google Scholar] [CrossRef]
- Porrini, M.P.; Garrido, P.M.; Eguaras, M.J. Individual feeding of honey bees: Modification of the Rinderer technique. J. Apic. Res. 2013, 52, 194–195. [Google Scholar] [CrossRef]
- Silva, D.; Ceballos, R.; Arismendi, N.; Dalmon, A.; Vargas, M. Variant A of the Deformed Wings Virus Alters the Olfactory Sensitivity and the Expression of Odorant Binding Proteins on Antennas of Apis mellifera. Insects 2021, 12, 895. [Google Scholar] [CrossRef]
- Riveros, G.; Arismendi, N.; Zapata, N.; Evans, D.; Pérez, I.; Aldea, P.; Vargas, M. Occurrence, prevalence and viral load of deformed wing virus variants in Apis mellifera colonies in Chile. J. Apic. Res. 2019, 59, 63–68. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef]
- Evans, J.D. Beepath: An ordered quantitative-PCR array for exploring honey bee immunity and disease. J. Invertebr. Pathol. 2006, 93, 135–139. [Google Scholar] [CrossRef]
- Zhao, Y.; Heerman, M.; Peng, W.; Evans, J.D.; Rose, R.; DeGrandi-Hoffman, G.; Simone-Finstrom, M.; Li, J.; Li, Z.; Cook, S.C.; et al. The Dynamics of Deformed Wing Virus Concentration and Host Defensive Gene Expression after Varroa Mite Parasitism in Honey Bees, Apis mellifera. Insects 2019, 10, 16. [Google Scholar] [CrossRef]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, X.; Kadowaki, T. Characterization of the Copy Number and Variants of Deformed Wing Virus (DWV) in the Pairs of Honey Bee Pupa and Infesting Varroa destructor or Tropilaelaps mercedesae. Front. Microbiol. 2017, 8, 1558. [Google Scholar] [CrossRef]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. R. Soc. B Boil. Sci. 2019, 286, 20190331. [Google Scholar] [CrossRef] [PubMed]
- Annoscia, D.; Zanni, V.; Galbraith, D.; Quirici, A.; Grozinger, C.; Bortolomeazzi, R.; Nazzi, F. Elucidating the mechanisms underlying the beneficial health effects of dietary pollen on honey bees (Apis mellifera) infested by Varroa mite ectoparasites. Sci. Rep. 2017, 7, 6258. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Yeler, H.B.; Nas, S. Optimization of extraction time and temperature for natural antioxidants of öküzgözü grape pomace using various solvent ratios. Food Sci. Technol. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; García-Martínez, T.; Varo, M.; Mérida, J.; Serratosa, M.P. Phenolic compounds, antioxidant activity and color in the fermentation of mixed blueberry and grape juice with different yeasts. LWT 2021, 146, 111661. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Valls-Fonayet, J.; Krisa, S.; Garcia, F.; Saucier, C.; Richard, T.; Hornedo-Ortega, R. Polyphenolic Characterization of Merlot, Tannat and Syrah Skin Extracts at Different Degrees of Maturity and Anti-Inflammatory Potential in RAW 264.7 Cells. Foods 2021, 10, 541. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Moreno-Osorio, L.; Orellana-Palma, P.; Petzold, G. Effects of cryoconcentrate blueberry juice incorporation on gelatin gel: A rheological, textural and bioactive properties study. LWT 2021, 138, 110674. [Google Scholar] [CrossRef]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The Microencapsulation of Maqui (Aristotelia chilensis (Mol.) Stuntz) Juice by Spray-Drying and Freeze-Drying Produces Powders with Similar Anthocyanin Stability and Bioaccessibility. Molecules 2018, 23, 1227. [Google Scholar] [CrossRef]
- Felicioli, A.; Forzan, M.; Sagona, S.; D’Agostino, P.; Baido, D.; Fronte, B.; Mazzei, M. Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.). Vet. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Childers, A.K.; Lopez, D.; Grubbs, K.; Posada-Florez, F.; Weaver, D.; Girten, W.; Vanengelsdorp, D.; Chen, Y.; Evans, J.D. Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biol. 2019, 17, e3000502. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Gammon, D.B.; Mello, C.C. RNA interference-mediated antiviral defense in insects. Curr. Opin. Insect Sci. 2015, 8, 111–120. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Daughenbaugh, K.F.; Parekh, F.; Pizzorno, M.C.; Flenniken, M.L. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395. [Google Scholar] [CrossRef]
- Nazzi, F.; Pennacchio, F. Honey Bee Antiviral Immune Barriers as Affected by Multiple Stress Factors: A Novel Paradigm to Interpret Colony Health Decline and Collapse. Viruses 2018, 10, 159. [Google Scholar] [CrossRef]
- Larsen, A.; Reynaldi, F.J.; Guzmán-Novoa, E. Fundaments of the immune system of honey bee (Apis mellifera). Revision. Rev. Mex. Cienc. Pecu. 2019, 10, 705–728. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, H.; Zhang, Y.; Zhu, F.; Lü, P.; Yao, Q.; Chen, K. Research progress on the immune mechanism of the silkworm Bombyx mori. Physiol. Èntomol. 2018, 43, 159–168. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic Parasite-Pathogen Interactions Mediated by Host Immunity Can Drive the Collapse of Honeybee Colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- Zuo, H.; Yuan, J.; Chen, Y.; Li, S.; Su, Z.; Wei, E.; Li, C.; Weng, S.; Xu, X.; He, J. A MicroRNA-Mediated Positive Feedback Regulatory Loop of the NF-κB Pathway in Litopenaeus vannamei. J. Immunol. 2016, 196, 3842–3853. [Google Scholar] [CrossRef]
- Zanni, V.; Galbraith, D.A.; Annoscia, D.; Grozinger, C.M.; Nazzi, F. Transcriptional signatures of parasitization and markers of colony decline in Varroa-infested honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2017, 87, 1–13. [Google Scholar] [CrossRef]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Wu, C.-P.; Tang, C.-K.; Lin, Y.-H.; Maaroufi, H.O.; Chuang, Y.-C.; Wu, Y.-L. Identification of Immune Regulatory Genes in Apis mellifera through Caffeine Treatment. Insects 2020, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.M.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverría, J. The Signaling Pathways, and Therapeutic Targets of Antiviral Agents: Focusing on the Antiviral Approaches and Clinical Perspectives of Anthocyanins in the Management of Viral Diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]





| Primers | Sequences | Reference | |
|---|---|---|---|
| DWV-A | F | TATCTTCATTAAAGCCACCTGGAA | [36] |
| R | TTTCCTCATTAACTGTGTCGTTGAT | ||
| β-Actin | F | ATGCCAACACTGTCCTTTCTGG | [36] |
| R | GACCCACCAATCCATACGGA | ||
| Relish | F | GCAGTGTTGAAGGAGCTGAA | [37] |
| R | CCAATTCTGAAAAGCGTCCA | ||
| Cactus | F | CACAAGATCTGGAGCAACGA | [37] |
| R | GCATTCTTGAAGGAGGAACG | ||
| Argo-2 | F | ACCTGCTGAGTTATGCACAGT | [38] |
| R | AGCCTTTAGAACTCTTGCTGGT | ||
| Dicer | F | AGCAGTAGCTGATTGTGTGGA | [38] |
| R | TGAAGGATGTGTAAACGCCTGT | ||
| Dorsal | F | CTCATCGGAAGACATGACAGTGA | [38] |
| R | TGAATTCAAAGCCAGTTCGAAAA | ||
| Amel102 | F | CAACTCCAGAATTGGAAATAGCA | [39] |
| R | TTTGCAATAGGAAAAGCAGTTG | ||
| Compounds | GP | GPP | Anthocyanin Content Losses (%) |
|---|---|---|---|
| Anthocyanins | |||
| Cyanidin 3-O-hexoside | 175.79 a | 96.25 b | 45 |
| Peonidin 3-O-hexoside | 40.34 a | 18.87 b | 53 |
| Delphinidin 3-O-hexoside | 180.90 a | 108.03 b | 40 |
| Petunidin 3-O-hexoside | 403.70 a | 227.93 b | 43 |
| Malvidin 3-O-hexoside | 682.34 a | 420.44 b | 38 |
| Malvidin 3-O-(6-acetyl)-glucoside | 42.09 a | 26.35 b | 37 |
| Delphinidin 3,5-O-di-hexoside | 38.26 a | 18.42 b | 51 |
| Malvidin 3,5-O-di-hexoside | 189.39 a | 111.56 b | 41 |
| Petunidin 3,5-O-di-hexoside | 34.21 a | 21.65 b | 36 |
| Malvidin derivatives | 86.26 a | 53.14 b | 38 |
| Total identified | 1873.29 a | 1102.65 b | 41 |
| Hydroxybenzoic acids | |||
| Gallic acid | 6.02 a | 3.09 b | 49 |
| Syringic acid | 153.65 a | 75.33 b | 50 |
| Total identified | 159.67 a | 78.42 b | 49 |
| Hydroxycinnamic acids | |||
| Chlorogenic acid | 0.47 a | 0.20 b | 57 |
| Caftaric acid | 0.79 a | 0.31 b | 60 |
| Caffeic acid | 2.94 a | 0.89 b | 69 |
| p-coumaric acid | 6.17 a | 2.90 b | 52 |
| Ferulic acid | 5.16 a | 2.12 b | 58 |
| Total identified | 15.53 a | 6.42 b | 58 |
| Stilbene | |||
| Trans-Resveratrol | 4.05 a | 1.88 b | 53 |
| Total identified | 4.05 a | 1.88 b | 53 |
| Flavanols | |||
| (+)-Catechin | 67.93 a | 43.79 b | 35 |
| (−)-Epicatechin | 19.57 a | 8.57 b | 56 |
| (−)-Epigallocatechin gallate | 0.82 a | 0.43 b | 47 |
| Total identified | 88.32 a | 52.79 b | 40 |
| Flavonols | |||
| Quercetin 3-glucoside | 6.56 a | 2.93 b | 55 |
| Kaempferol 3-glucoside | 3.73 a | 2.92 a | 21 |
| Myricetin derivatives | 0.44 a | 0.22 b | 50 |
| Quercetin derivatives | 1.54 a | 0.93 a | 39 |
| Total identified | 12.27 a | 7.00 b | 42 |
| Phenylethanoids | |||
| Hydroxytyrosol | 1.83 a | 0.98 b | 46 |
| Tyrosol | 1.68 a | 0.73 b | 56 |
| Total identified | 3.51 a | 1.31 b | 62 |
| Sample | DPPH* | ORAC |
|---|---|---|
| GP | 9340 a | 8153 a |
| GPP | 2988 b | 4921 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, G.; Silva, D.; Vargas, M.; Aranda, M.; Cañumir, J.A.; López, M.D. Dietary Supplement of Grape Wastes Enhances Honeybee Immune System and Reduces Deformed Wing Virus (DWV) Load. Antioxidants 2023, 12, 54. https://doi.org/10.3390/antiox12010054
Pascual G, Silva D, Vargas M, Aranda M, Cañumir JA, López MD. Dietary Supplement of Grape Wastes Enhances Honeybee Immune System and Reduces Deformed Wing Virus (DWV) Load. Antioxidants. 2023; 12(1):54. https://doi.org/10.3390/antiox12010054
Chicago/Turabian StylePascual, Guillermo, Diego Silva, Marisol Vargas, Mario Aranda, Juan Antonio Cañumir, and María Dolores López. 2023. "Dietary Supplement of Grape Wastes Enhances Honeybee Immune System and Reduces Deformed Wing Virus (DWV) Load" Antioxidants 12, no. 1: 54. https://doi.org/10.3390/antiox12010054
APA StylePascual, G., Silva, D., Vargas, M., Aranda, M., Cañumir, J. A., & López, M. D. (2023). Dietary Supplement of Grape Wastes Enhances Honeybee Immune System and Reduces Deformed Wing Virus (DWV) Load. Antioxidants, 12(1), 54. https://doi.org/10.3390/antiox12010054









