MicroRNAs in the Regulation of NADPH Oxidases in Vascular Diabetic and Ischemic Pathologies: A Case for Alternate Inhibitory Strategies?
Abstract
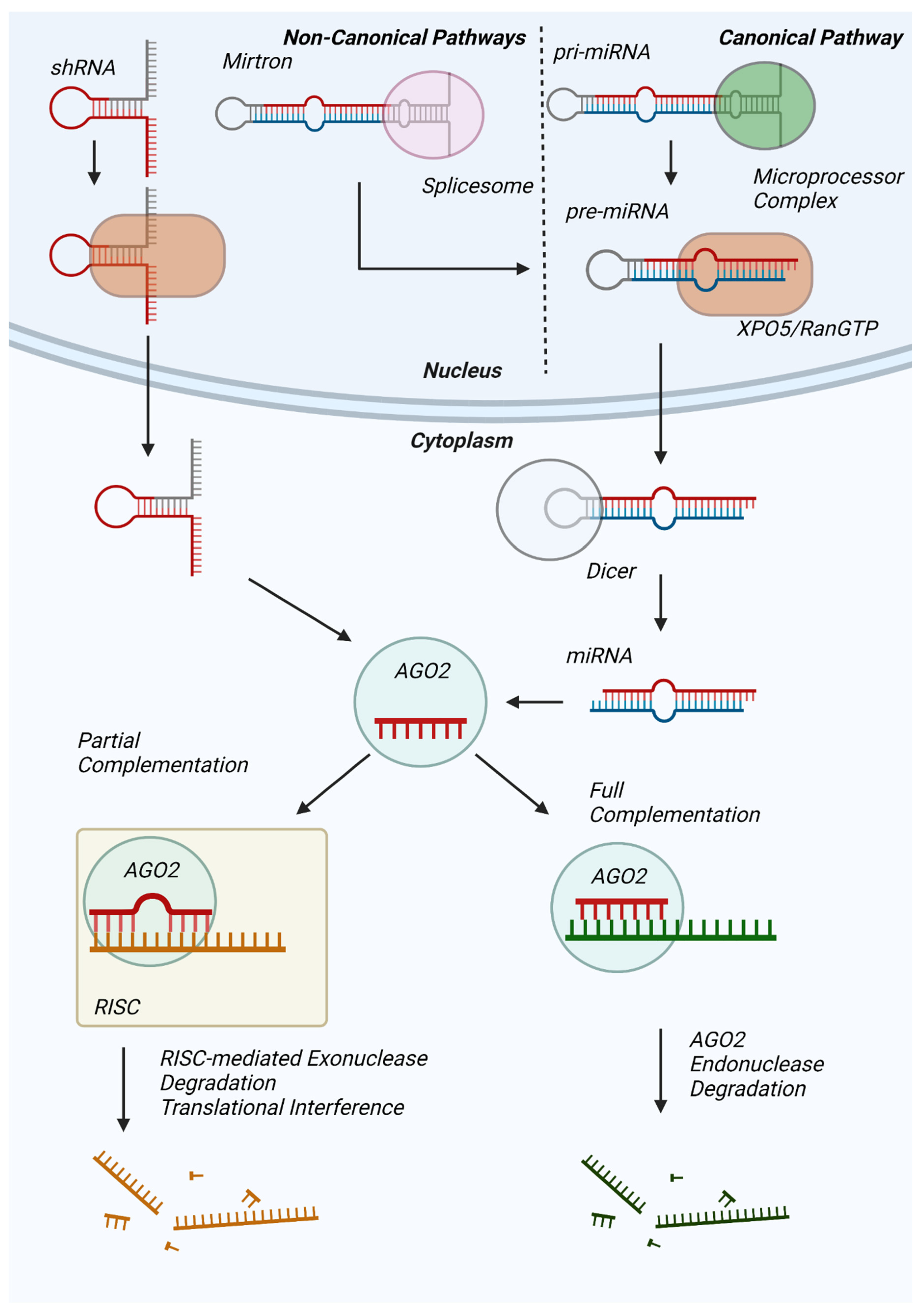
1. Introduction
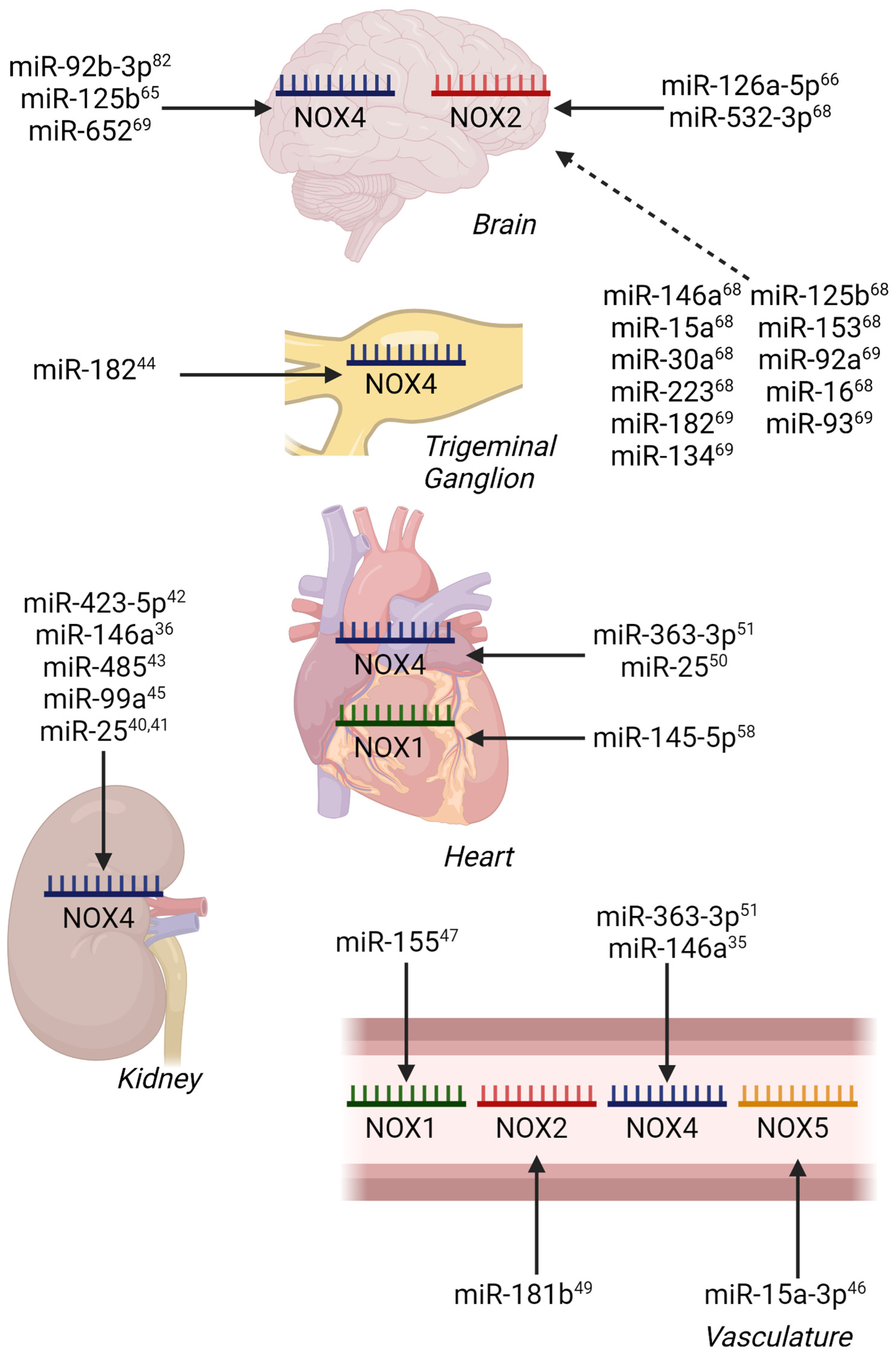
2. miRNA Control in Diabetes and Diabetes-Related Cardiovascular Diseases
3. Atherosclerosis
4. Ischemia/Reperfusion Injury Models
5. Conclusions and Perspectives
| Pathology | Model | miRNA Identified | NOX Regulation | Ref. |
|---|---|---|---|---|
| MI/R | SD rats LAD artery ligation for 30 min/reperfusion for 3 or 24 h | ↓ miR-145-5p | ↑ NOX1 | [58] |
| CI/R | SD rats occlusion of ICA, PCA, MCA, ACA for 2h/reperfusion for 24 h | ↓ miR-126a-5p | ↑ NOX2 | [66] |
| CI/R | SD rats occlusion of ICA, PCA, MCA, ACA for 2 h/reperfusion for 24 h | ↓ miR-454 | ↑ NOX4 | [67] |
| CI/R | SD rats occlusion of ICA, PCA, MCA, ACA for 2 h/reperfusion for 24 h | ↓ miR-652 | ↑ NOX2 | [69] |
| CI/R | SD rats occlusion of MCA for 2 h/reperfusion for 24 h | ↓ miR-92b-3p | ↑ NOX4 | [82] |
| CI/R | SD rats occlusion of ICA for 2 h/reperfusion for 24h | ↓ miR-523-3p | ↑ NOX2 | [68] |
| AD | APPswe/PS1dE9 mice APP/PS1 mice, 6 mos old | ↓ miR-204-3p | ↑ NOX4 | [71] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science 2009, 326, 1275–1279. [Google Scholar] [CrossRef]
- Zhang, H.; Kolb, F.A.; Jaskiewicz, L.; Westhof, E.; Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 2004, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Maurin, T.; Robine, N.; Rasmussen, K.D.; Jeffrey, K.L.; Chandwani, R.; Papapetrou, E.P.; Sadelain, M.; O’Carroll, D.; Lai, E.C. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 15163–15168. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.H.; Shin, S.; Jung, S.R.; Kim, E.; Song, J.J.; Hohng, S. Human Argonaute 2 Has Diverse Reaction Pathways on Target RNAs. Mol. Cell 2015, 59, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef]
- Braun, J.E.; Truffault, V.; Boland, A.; Huntzinger, E.; Chang, C.T.; Haas, G.; Weichenrieder, O.; Coles, M.; Izaurralde, E. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat. Struct. Mol. Biol. 2012, 19, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Steitz, J.A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 2007, 128, 1105–1118. [Google Scholar] [CrossRef]
- Orom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Al Ghouleh, I.; Khoo, N.K.; Knaus, U.G.; Griendling, K.K.; Touyz, R.M.; Thannickal, V.J.; Barchowsky, A.; Nauseef, W.M.; Kelley, E.E.; Bauer, P.M.; et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic. Biol. Med. 2011, 51, 1271–1288. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Mortezaee, K. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: A review. Cell Biochem. Funct. 2018, 36, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Li, Y.; Pagano, P.J. Microvascular NADPH oxidase in health and disease. Free Radic. Biol. Med. 2017, 109, 33–47. [Google Scholar] [CrossRef]
- De Deken, X.; Wang, D.; Many, M.C.; Costagliola, S.; Libert, F.; Vassart, G.; Dumont, J.E.; Miot, F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000, 275, 23227–23233. [Google Scholar] [CrossRef]
- Van der Vliet, A.; Danyal, K.; Heppner, D.E. Dual oxidase: A novel therapeutic target in allergic disease. Br. J. Pharmacol. 2018, 175, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H.; Refetoff, S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J. Biol. Chem. 2006, 281, 18269–18272. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Gorlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.A.; Demir, M. MicroRNA and Cardiovascular Diseases. Balkan Med. J. 2020, 37, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Fernandez-Hernando, C.; Ramirez, C.M.; Goedeke, L.; Suarez, Y. MicroRNAs in metabolic disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185. [Google Scholar] [CrossRef]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. (Oxf.) 2017, 219, 346–361. [Google Scholar] [CrossRef]
- Feng, J.; Xing, W.; Xie, L. Regulatory Roles of MicroRNAs in Diabetes. Int. J. Mol. Sci. 2016, 17, 1729. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Zhang, X. The Profiling and Role of miRNAs in Diabetes Mellitus. J. Diabetes Clin. Res. 2019, 1, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Dimmeler, S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef]
- Neag, M.A.; Mitre, A.O.; Burlacu, C.C.; Inceu, A.I.; Mihu, C.; Melincovici, C.S.; Bichescu, M.; Buzoianu, A.D. miRNA Involvement in Cerebral Ischemia-Reperfusion Injury. Front. Neurosci. 2022, 16, 901360. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Huang, Y.L.; Shih, Y.Y.; Wu, H.Y.; Peng, C.T.; Lo, W.Y. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediat. Inflamm. 2014, 2014, 379537. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.J.; Li, Y.H. MicroRNA146a/NAPDH oxidase4 decreases reactive oxygen species generation and inflammation in a diabetic nephropathy model. Mol. Med. Rep. 2018, 17, 4759–4766. [Google Scholar] [CrossRef]
- Albers, J.W.; Pop-Busui, R. Diabetic neuropathy: Mechanisms, emerging treatments, and subtypes. Curr. Neurol. Neurosci. Rep. 2014, 14, 473. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Kashihara, N.; Haruna, Y.; Kondeti, V.K.; Kanwar, Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhang, B.; Wen, Z.; Fang, H.; Pang, Q.; Yi, F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010, 32, 581–589. [Google Scholar] [CrossRef]
- Oh, H.J.; Kato, M.; Deshpande, S.; Zhang, E.; Das, S.; Lanting, L.; Wang, M.; Natarajan, R. Inhibition of the processing of miR-25 by HIPK2-Phosphorylated-MeCP2 induces NOX4 in early diabetic nephropathy. Sci. Rep. 2016, 6, 38789. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Fan, L.; He, X. miR-423-5p suppresses high-glucose-induced podocyte injury by targeting Nox4. Biochem. Biophys. Res. Commun. 2018, 505, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, K.; Zhu, M.; Xie, X.; Ding, Y.; Shao, X.; Chen, Y.; Liu, J.; Xu, M.; Xu, Y.; et al. miR-485 suppresses inflammation and proliferation of mesangial cells in an in vitro model of diabetic nephropathy by targeting NOX5. Biochem. Biophys. Res. Commun. 2020, 521, 984–990. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Wu, X.; Dai, Y.; Chen, P.; Xie, L. microRNA-182 Mediates Sirt1-Induced Diabetic Corneal Nerve Regeneration. Diabetes 2016, 65, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fan, C.; Cai, Y.; Fang, S.; Zeng, Y.; Zhang, Y.; Lin, X.; Zhang, H.; Xue, Y.; Guan, M. Transplantation of brown adipose tissue up-regulates miR-99a to ameliorate liver metabolic disorders in diabetic mice by targeting NOX4. Adipocyte 2020, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Yu, T.; Yan, C.; Zhou, W.; Cao, F.; You, X.; Zhang, Y.; Sun, Y.; Liu, J.; et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany N. Y.) 2020, 12, 8968–8986. [Google Scholar] [CrossRef]
- Tang, Y.; Song, H.; Shen, Y.; Yao, Y.; Yu, Y.; Wei, G.; Long, B.; Yan, W. MiR-155 acts as an inhibitory factor in atherosclerosis-associated arterial pathogenesis by down-regulating NoxA1 related signaling pathway in ApoE−/− mouse. Cardiovasc. Diagn. Ther. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Rodriguez, A.I.; Csanyi, G.; Ranayhossaini, D.J.; Feck, D.M.; Blose, K.J.; Assatourian, L.; Vorp, D.A.; Pagano, P.J. MEF2B-Nox1 signaling is critical for stretch-induced phenotypic modulation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 430–438. [Google Scholar] [CrossRef]
- Tian, Q.; Leung, F.P.; Chen, F.M.; Tian, X.Y.; Chen, Z.; Tse, G.; Ma, S.; Wong, W.T. Butyrate protects endothelial function through PPARdelta/miR-181b signaling. Pharmacol. Res. 2021, 169, 105681. [Google Scholar] [CrossRef]
- Varga, Z.V.; Kupai, K.; Szucs, G.; Gaspar, R.; Paloczi, J.; Farago, N.; Zvara, A.; Puskas, L.G.; Razga, Z.; Tiszlavicz, L.; et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell Cardiol. 2013, 62, 111–121. [Google Scholar] [CrossRef]
- Zhou, T.; Li, S.; Yang, L.; Xiang, D. microRNA-363-3p reduces endothelial cell inflammatory responses in coronary heart disease via inactivation of the NOX4-dependent p38 MAPK axis. Aging (Albany NY) 2021, 13, 11061–11082. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kracun, D.; Dustin, C.M.; El Massry, M.; Yuan, S.; Goossen, C.J.; DeVallance, E.R.; Sahoo, S.; St Hilaire, C.; Gurkar, A.U.; et al. Forestalling age-impaired angiogenesis and blood flow by targeting NOX: Interplay of NOX1, IL-6, and SASP in propagating cell senescence. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Yu, P.; Zuo, L.; Zhou, Q.; Zhou, X.; Zhu, H. MicroRNA-126 Modulates Palmitate-Induced Migration in HUVECs by Downregulating Myosin Light Chain Kinase via the ERK/MAPK Pathway. Front. Bioeng Biotechnol. 2020, 8, 913. [Google Scholar] [CrossRef] [PubMed]
- Alegria, J.R.; Miller, T.D.; Gibbons, R.J.; Yi, Q.L.; Yusuf, S.; Collaborative Organization of RheothRx Evaluation Trial, I. Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am. Heart J. 2007, 154, 743–750. [Google Scholar] [CrossRef]
- Lejay, A.; Fang, F.; John, R.; Van, J.A.; Barr, M.; Thaveau, F.; Chakfe, N.; Geny, B.; Scholey, J.W. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell Cardiol. 2016, 91, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, R.; Xia, R.; Xia, Z.; Wu, Z.; Wang, T. Amelioration of myocardial ischemia/reperfusion injury in diabetes: A narrative review of the mechanisms and clinical applications of dexmedetomidine. Front. Pharmacol. 2022, 13, 949754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, J.; Yang, L. Insights for Oxidative Stress and mTOR Signaling in Myocardial Ischemia/Reperfusion Injury under Diabetes. Oxid Med. Cell Longev. 2017, 2017, 6437467. [Google Scholar] [CrossRef]
- Tan, L.; Liu, L.; Yao, J.; Piao, C. miR-145-5p attenuates inflammatory response and apoptosis in myocardial ischemia-reperfusion injury by inhibiting (NADPH) oxidase homolog 1. Exp Anim 2021, 70, 311–321. [Google Scholar] [CrossRef]
- Jae, N.; Dimmeler, S. Noncoding RNAs in Vascular Diseases. Circ Res 2020, 126, 1127–1145. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Q.; Yu, X.; He, Y.; Guo, W. FENDRR: A pivotal, cancer-related, long non-coding RNA. Biomed Pharmacother 2021, 137, 111390. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Liu, M.; Meng, F.; Liu, K. LncRNA FENDRR Servers as a Possible Marker of Essential Hypertension and Regulates Human Umbilical Vein Endothelial Cells Dysfunction via miR-423-5p/Nox4 Axis. Int. J. Gen Med. 2022, 15, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lei, Z.; Zhang, H.; Xu, Z.; Cui, Q.; Xu, Z.C. Toll-like receptor 4 is associated with seizures following ischemia with hyperglycemia. Brain Res. 2014, 1590, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rehni, A.K.; Nautiyal, N.; Perez-Pinzon, M.A.; Dave, K.R. Hyperglycemia/hypoglycemia-induced mitochondrial dysfunction and cerebral ischemic damage in diabetics. Metab. Brain Dis. 2015, 30, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.; Xing, X.; Wang, Q.; Li, W. Dexmedetomidine Protects against Transient Global Cerebral Ischemia/Reperfusion Induced Oxidative Stress and Inflammation in Diabetic Rats. PLoS ONE 2016, 11, e0151620. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, J.; Wang, Y.; Tang, J.-Y.; Yang, S.-L.; Xiang, H.-G.; Wu, S.-X.; Li, X.-J. Inhibition of MiRNA-125b Decreases Cerebral Ischemia/Reperfusion Injury by Targeting CK2alpha/NADPH Oxidase Signaling. Cell Physiol. Biochem. 2018, 45, 1818–1826. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, F.; Yang, D.; Zhang, X.; Zeng, M.; Wan, L. MicroRNA-126a-5p Exerts Neuroprotective Effects on Ischemic Stroke via Targeting NADPH Oxidase 2. Neuropsychiatr. Dis. Treat 2021, 17, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Han, H.; Zhou, Y.; Liu, Z.; Ma, T.; Cao, X. MicroRNA-454 modulates the oxidative stress and neuronal apoptosis after cerebral ischemia/reperfusion injury via targeting NADPH oxidase 4 (NOX4). J. Biochem. Mol. Toxicol. 2022, 36, e23153. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Zuo, M.L.; Wang, A.P.; Tian, Y.; Dong, L.C.; Li, T.M.; Kuang, D.B.; Song, G.L.; Yang, Z.B. Low expression of miR5323p contributes to cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Mol. Med. Rep. 2020, 22, 2415–2423. [Google Scholar] [CrossRef]
- Zuo, M.L.; Wang, A.P.; Song, G.L.; Yang, Z.B. miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed. Pharmacother. 2020, 124, 109860. [Google Scholar] [CrossRef]
- Liu, Z.; Tuo, Y.H.; Chen, J.W.; Wang, Q.Y.; Li, S.; Li, M.C.; Dai, G.; Wang, J.S.; Zhang, Y.L.; Feng, L.; et al. NADPH oxidase inhibitor regulates microRNAs with improved outcome after mechanical reperfusion. J. Neurointerv. Surg. 2017, 9, 702–706. [Google Scholar] [CrossRef]
- Tao, W.; Yu, L.; Shu, S.; Liu, Y.; Zhuang, Z.; Xu, S.; Bao, X.; Gu, Y.; Cai, F.; Song, W.; et al. miR-204-3p/Nox4 Mediates Memory Deficits in a Mouse Model of Alzheimer’s Disease. Mol. Ther. 2021, 29, 396–408. [Google Scholar] [CrossRef]
- Aroor, A.R.; DeMarco, V.G. Oxidative stress and obesity: The chicken or the egg? Diabetes 2014, 63, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity (Silver Spring) 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, E.; Saha, J.; Csanyi, G.; Ghouleh, I.A.; Sahoo, S.; Rodriguez, A.; Wipf, P.; Pagano, P.J.; Skoda, E.M. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox2) inhibitors. Medchemcomm 2013, 4, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Pagano, M.E.; Meijles, D.N.; Pagano, P.J. Nox Inhibitors & Therapies: Rational Design of Peptidic and Small Molecule Inhibitors. Curr. Pharm. Des. 2015, 21, 6023–6035. [Google Scholar] [CrossRef]
- Fan, L.M.; Liu, F.; Du, J.; Geng, L.; Li, J.M. Inhibition of endothelial Nox2 activation by LMH001 protects mice from angiotensin II-induced vascular oxidative stress, hypertension and aortic aneurysm. Redox Biol. 2022, 51, 102269. [Google Scholar] [CrossRef]
- Hirano, K.; Chen, W.S.; Chueng, A.L.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G.; et al. Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Li, Y.; Cifuentes-Pagano, E.; DeVallance, E.R.; de Jesus, D.S.; Sahoo, S.; Meijles, D.N.; Koes, D.; Camacho, C.J.; Ross, M.; St Croix, C.; et al. NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow. Redox Biol. 2019, 22, 101143. [Google Scholar] [CrossRef]
- Pagano, P.J.; Cifuentes-Pagano, E. The Enigmatic Vascular NOX: From Artifact to Double Agent of Change: Arthur C. Corcoran Memorial Lecture—2019. Hypertension 2021, 77, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, J.; Li, X.; Long, X.; Huang, Y.; Zhang, X. miR-92b-3p Exerts Neuroprotective Effects on Ischemia/Reperfusion-Induced Cerebral Injury via Targeting NOX4 in a Rat Model. Oxidative Med. Cell. Longev. 2022, 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Pathology | Model/Patients | miRNA Identified | NOX Regulation | Ref. |
|---|---|---|---|---|
| Atherosclerosis | apoE−/− Mouse, HFD (21% fat, 0.15% CHL), 12 wks; miR-155 administered | ↑ miR-155 | ↓ NOXA1 | [47] |
| Atherosclerosis | apoE−/− Mouse HFD (45% fat; 0.5 mg/g butyrate), 10 wks | ↑ miR-181b | ↓ NOX2 | [49] |
| T1DM-Nephropathy | Rat, STZ (50 mg/kg QD), 5 d; DM = FG ≥ 15 mM | ↓ miR-25 | ↑ NOX4 | [40] |
| T1DM-Nephropathy | Mouse, STZ (50 mg/kg QD), 5 d DM = FG > 16.65 mM | ↓ miR-25 | ↑ NOX4 | [41] |
| T1DM-Nephropathy | Mouse, STZ (55 mg/kg QD), 5 d | ↓ miR-146a | ↑ NOX4 | [36] |
| T2DM-Retinopathy | BKS.Cg-m+/+Leprdb/J (db/db) Mouse DM = FG > 15 mM | ↓ miR-182 | ↑ NOX4 | [44] |
| T2DM | Mouse, HFD (60% fat, 20% carbohydrates), 8 wks STZ (120 mg/kg x1 at 4wks) DM = FG ≥ 13.9 mM. Brown fat transplantation. | ↑ miR-99a | ↓ NOX4 | [45] |
| Dyslipidemia | Rat, HFD (2% CHL, 0.25% cholate), 12 wks | ↓ miR-25 | ↑ NOX4 | [50] |
| Atherosclerosis | Mouse, HFD (1.25 g/kg Vit D3, 2% CHL, 0.5% cholate, 3% lard, 0.2% PTU), 3 mos; 3 g/kg Vit D3 Inj. 1x/mos | ↓ miR-363-3p | ↑ NOX4 | [51] |
| T2DM | Patients aged 45–60 y with foot wounds Circulating Serum Exosomes harvested | ↑ miR-15a-3p | ↓ NOX5 | [46] |
| T2DM-Nephropathy | Adult T2DM patients Renal Fibroblasts harvested | ↓ miR-423-5p | ↑ NOX4 | [42] |
| Cell Line | Model/Patients | miRNA Identified | NOX Regulation | Ref. |
|---|---|---|---|---|
| MPC5 | NG vs HG (5 mM/30 mM D-glucose) miR-423-5p administered | ↑ miR-423-5p | ↓ NOX4 | [42] |
| HAECs | NG vs HG (25 mM L-glucose/25 mM D-glucose) + Thrombin (2 U/mL) | ↓ miR-146a | ↑ NOX4 | [35] |
| HMCs | NG vs NG + Mannitol vs HG (5.5 mM D-glucose/5.5 mM D-glucose + 24.5 mM Mannitol/30 mM D-glucose) | ↓ miR-485 | ↑ NOX5 | [43] |
| CAECs | Homocysteine (0.25 mM), 24 h | ↓ miR-363-3p | ↑ NOX4 | [51] |
| SH-SY5Y | SH-SY5YOGD/R Hypoxia (N2/CO2 95:5), 10 min Hypoxia + Glucose-free medium, 2 h Normoxia and NG, 24 h | ↓ miR-126a-5p | ↑ NOX2 | [66] |
| SH-SY5Y | OGD/R Hypoxia, 10 min Hypoxia + Glucose-free medium, 2 h Normoxia and NG, 24 h | ↓ miR-454 | ↑ NOX4 | [67] |
| SH-SY5Y | H/R Hypoxia (N2/CO2 95:5) + PBS, 5 h Normoxia, 20 h | ↓ miR-652 | ↑ NOX2 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, S.R.; Pagano, P.J.; Kračun, D. MicroRNAs in the Regulation of NADPH Oxidases in Vascular Diabetic and Ischemic Pathologies: A Case for Alternate Inhibitory Strategies? Antioxidants 2023, 12, 70. https://doi.org/10.3390/antiox12010070
Wallace SR, Pagano PJ, Kračun D. MicroRNAs in the Regulation of NADPH Oxidases in Vascular Diabetic and Ischemic Pathologies: A Case for Alternate Inhibitory Strategies? Antioxidants. 2023; 12(1):70. https://doi.org/10.3390/antiox12010070
Chicago/Turabian StyleWallace, Sean R., Patrick J. Pagano, and Damir Kračun. 2023. "MicroRNAs in the Regulation of NADPH Oxidases in Vascular Diabetic and Ischemic Pathologies: A Case for Alternate Inhibitory Strategies?" Antioxidants 12, no. 1: 70. https://doi.org/10.3390/antiox12010070
APA StyleWallace, S. R., Pagano, P. J., & Kračun, D. (2023). MicroRNAs in the Regulation of NADPH Oxidases in Vascular Diabetic and Ischemic Pathologies: A Case for Alternate Inhibitory Strategies? Antioxidants, 12(1), 70. https://doi.org/10.3390/antiox12010070





