Anti- and Pro-Oxidant Activity of Polyphenols Extracts of Syrah and Chardonnay Grapevine Pomaces on Melanoma Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.1.1. Analytical Assays
2.1.2. Biological Assays
2.2. Sample Collection and Preparation
2.3. Hydroalcoholic Extraction of Polyphenols
2.4. Identification and Quantification of Polyphenols in the Extracts
2.5. Electrochemical Characterization and Antioxidant Activity Determination
2.6. Antioxidant Activity Determination with CUPRAC and DPPH Reference Methods
2.7. Determination of Prooxidant Activity of Polyphenols on Melanoma Cancer Cells
2.7.1. Viability Assays
2.7.2. Invasion Test
2.8. Cu Measurement
2.9. Statistical Analysis
3. Results
3.1. Chemical Characterization of Pomace Extracts
3.2. Electrochemical Characterization and Antioxidant Activity Determination
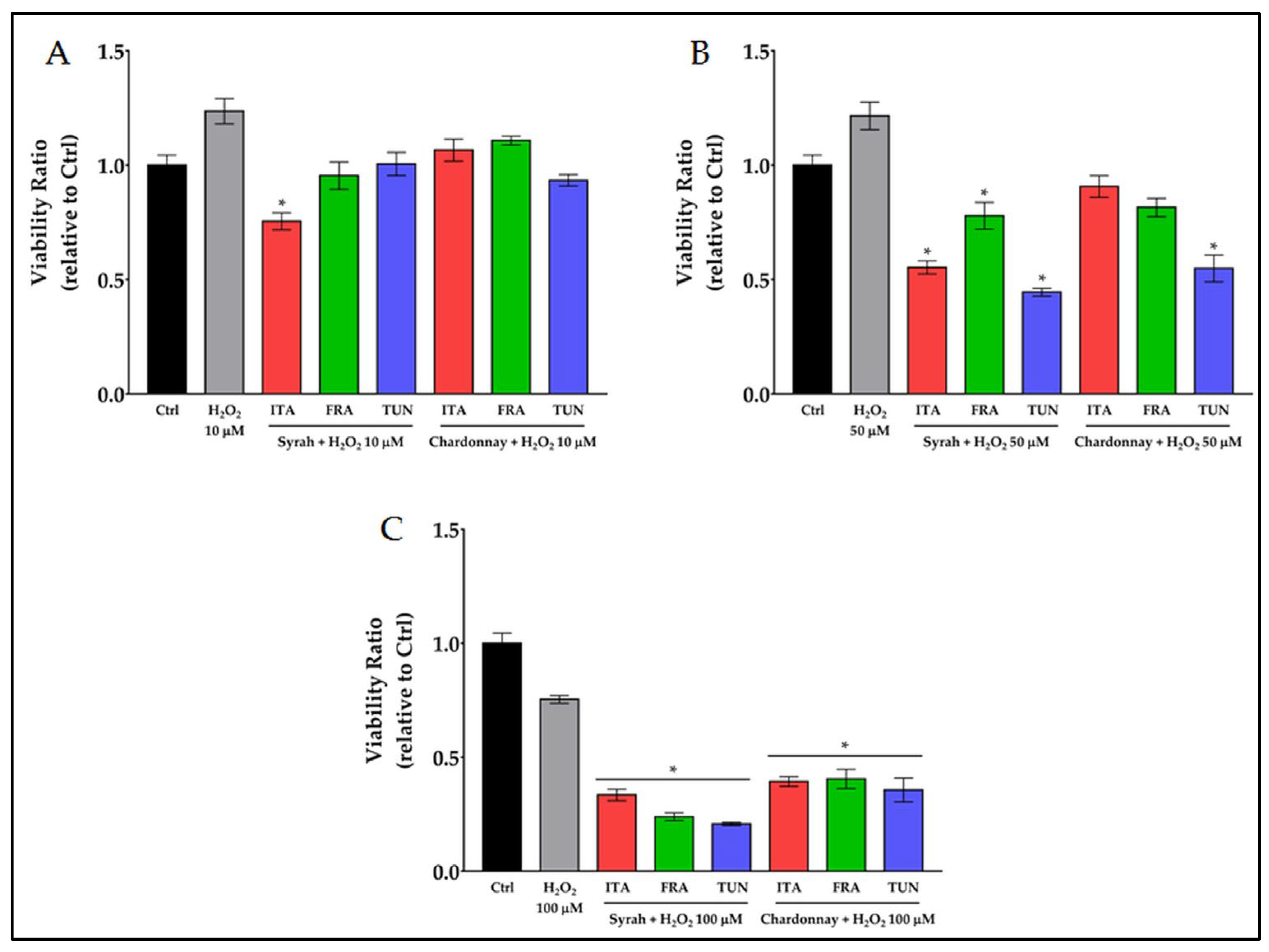
3.3. Prooxidant Activity of Polyphenols on Melanoma Cancer Cells
3.3.1. Prooxidant Activity of Combined Treatment with Polyphenols and H2O2 on Melanoma Cancer Cells
3.3.2. Quantification of Copper (Cu) in the Syrah and Chardonnay Extracts
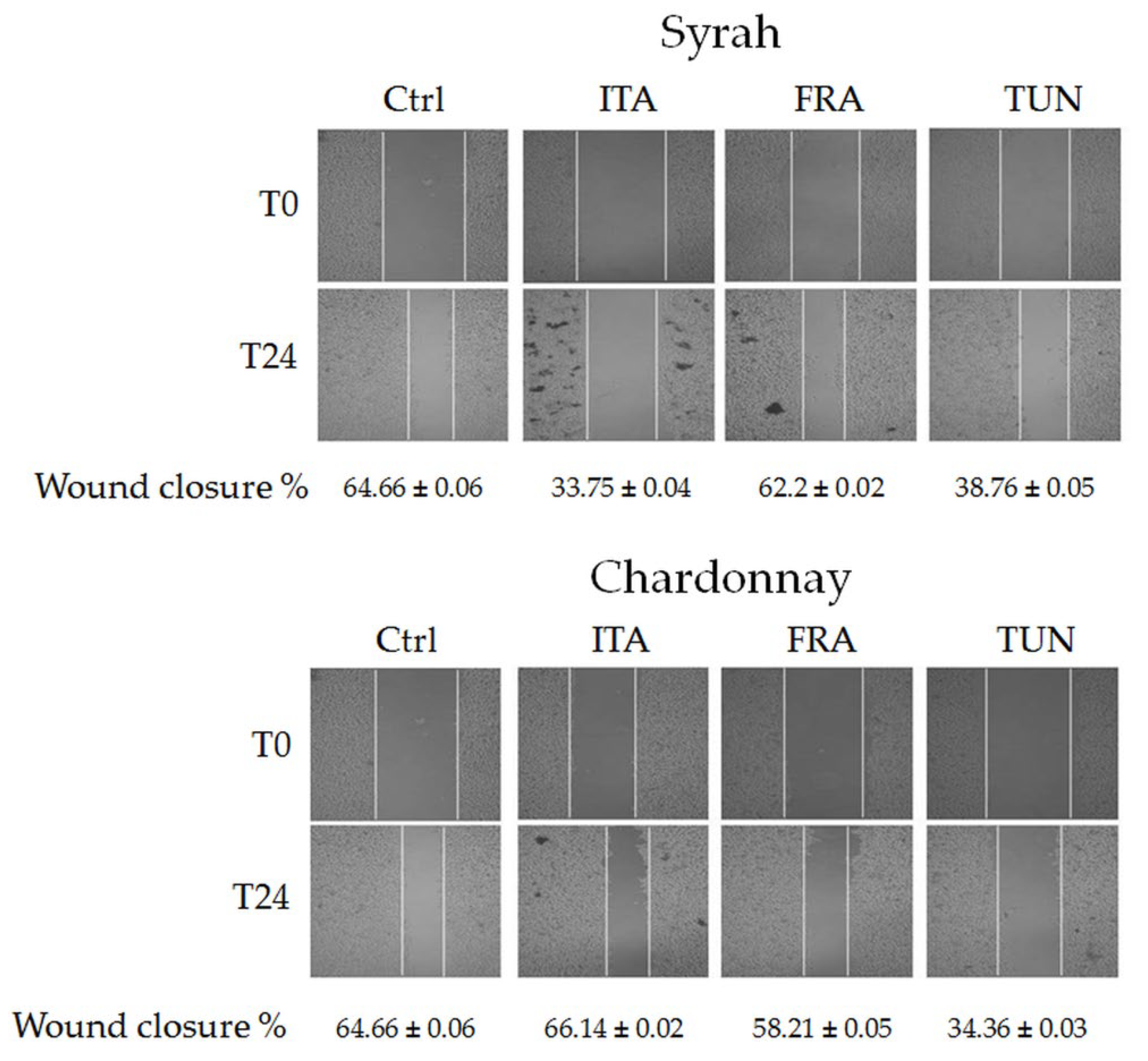
3.3.3. Effect of Syrah and Chardonnay Extracts on Invasion Capacity of Melanoma Cancer Cells
4. Discussion
4.1. Extraction and Characterization
4.2. Polyphenols and Their Antioxidant Properties
4.3. Polyphenols and Their Prooxidant Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asensi, M.; Ortega, A.; Mena, S.; Feddi, F.; Estrela, J.M. Natural Polyphenols in Cancer Therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Lizard, G.; Latruffe, N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules 2021, 26, 3483. [Google Scholar] [CrossRef] [PubMed]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starsc, A. Prooxidant activities of antioxidants and their impact on health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Hadi, S.M.; Mohammad, R.M.; Azmi, A.S. 12—Prooxidant Anticancer Activity of Plant-Derived Polyphenolic Compounds: An Underappreciated Phenomenon. In Functional Foods in Cancer Prevention and Therapy; Kabir, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 221–236. ISBN 978-0-12-816151-7. [Google Scholar]
- Yang, L.; Jia, L.; Li, X.; Zhang, K.; Wang, X.; He, Y.; Hao, M.; Rayman, M.P.; Zhang, J. Prooxidant Activity-Based Guideline for a Beneficial Combination of (−)-Epigallocatechin-3-Gallate and Chlorogenic Acid. Food Chem. 2022, 386, 132812. [Google Scholar] [CrossRef] [PubMed]
- Pasciu, V.; Posadino, A.M.; Cossu, A.; Sanna, B.; Tadolini, B.; Gaspa, L.; Marchisio, A.; Dessole, S.; Capobianco, G.; Pintus, G. Akt Downregulation by Flavin Oxidase–Induced ROS Generation Mediates Dose-Dependent Endothelial Cell Damage Elicited by Natural Antioxidants. Toxicol. Sci. 2010, 114, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Giordo, R.; Cossu, A.; Pasciu, V.; Hoa, P.T.; Posadino, A.M.; Pintus, G. Different Redox Response Elicited by Naturally Occurring Antioxidants in Human Endothelial Cells. Open Biochem. J. 2013, 7, 44–53. [Google Scholar] [CrossRef] [Green Version]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and Its Implication on Cancer Chemoprevention and Chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Radin, N.S. Designing Anticancer Drugs via the Achilles Heel: Ceramide, Allylic Ketones, and Mitochondria. Bioorganic Med. Chem. 2003, 11, 2123–2142. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin Promotes Apoptotic Cell Death via Upregulation of Nrf2 Expression by DNA Demethylase and the Interaction of Nrf2 with P53 in Human Colon Cancer Cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Vinayak, M. Long Term Effect of Curcumin in Restoration of Tumour Suppressor P53 and Phase-II Antioxidant Enzymes via Activation of Nrf2 Signalling and Modulation of Inflammation in Prevention of Cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Urueña, C.; Prieto, K.; Martinez-Usatorre, A.; Donda, A.; Barreto, A.; Romero, P.; Fiorentino, S. Immune-System-Dependent Anti-Tumor Activity of a Plant-Derived Polyphenol Rich Fraction in a Melanoma Mouse Model. Cell Death Dis. 2016, 7, e2243. [Google Scholar] [CrossRef] [Green Version]
- Heenatigala Palliyage, G.; Singh, S.; Ashby, C.R.; Tiwari, A.K.; Chauhan, H. Pharmaceutical Topical Delivery of Poorly Soluble Polyphenols: Potential Role in Prevention and Treatment of Melanoma. AAPS PharmSciTech 2019, 20, 250. [Google Scholar] [CrossRef]
- AlQathama, A.; Prieto, J.M. Natural Products with Therapeutic Potential in Melanoma Metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y.; Peng, H.-H. Antioxidant and Pro-Oxidant Effects of Various Tea Extracts. J. Agric. Food Chem. 1997, 45, 30–34. [Google Scholar] [CrossRef]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative Mechanism for Anticancer and Apoptosis-Inducing Properties of Plant-Derived Polyphenolic Compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative Breakage of Cellular DNA by Plant Polyphenols: A Putative Mechanism for Anticancer Properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical Methodologies for Investigating the Antioxidant Potential of Plant and Fruit Extracts: A Review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Zheng, Y.; Karimi-Maleh, H.; Fu, L. Evaluation of Antioxidants Using Electrochemical Sensors: A Bibliometric Analysis. Sensors 2022, 22, 3238. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Percevault, L.; Limanton, E.; Nicolas, P.; Paquin, L.; Lagrost, C. Electrochemical Determination and Antioxidant Capacity Modulation of Polyphenols in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 776–784. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Intarakamhang, S.; Leson, C.; Schuhmann, W.; Schulte, A. A Novel Automated Electrochemical Ascorbic Acid Assay in the 24-Well Microtiter Plate Format. Anal. Chim. Acta 2011, 687, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Scampicchio, M.; Giovanelli, G.; Mannino, S. A Low-Cost and Low-Tech Electrochemical Flow System for the Evaluation of Total Phenolic Content and Antioxidant Power of Tea Infusions. Talanta 2008, 75, 312–316. [Google Scholar] [CrossRef]
- Spissu, Y.; Barberis, A.; D’hallewin, G.; Orrù, G.; Scano, A.; Serra, G.R.; Pinna, M.; Pinna, C.; Marceddu, S.; Serra, P.A. An Ascorbate Bluetooth© Analyzer for Quality Control of Fresh-Cut Parsley Supply Chain. Antioxidants 2021, 10, 1485. [Google Scholar] [CrossRef]
- Spissu, Y.; Barberis, A.; Bazzu, G.; D’hallewin, G.; Rocchitta, G.; Serra, P.A.; Marceddu, S.; Vineis, C.; Garroni, S.; Culeddu, N. Functionalization of Screen-Printed Sensors with a High Reactivity Carbonaceous Material for Ascorbic Acid Detection in Fresh-Cut Fruit with Low Vitamin C Content. Chemosensors 2021, 9, 354. [Google Scholar] [CrossRef]
- Pedotti, S.; Patti, A.; Dedola, S.; Barberis, A.; Fabbri, D.; Dettori, M.A.; Serra, P.A.; Delogu, G. Synthesis of New Ferrocenyl Dehydrozingerone Derivatives and Their Effects on Viability of PC12 Cells. Polyhedron 2016, 117, 80–89. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Smith, B.G.; O’Connor, C.J.; Melton, L.D. Effect of Raw and Cooked Onion Dietary Fibre on the Antioxidant Activity of Ascorbic Acid and Quercetin. Food Chem. 2008, 111, 580–585, Berlin/Heidelberg, Germany. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical Determination of Antioxidant Capacity of Fruit Tea Infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and In Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, D.; Wiczkowski, W.; Piskula, M.K. Determination of the Relative Contribution of Quercetin and Its Glucosides to the Antioxidant Capacity of Onion by Cyclic Voltammetry and Spectrophotometric Methods. J. Agric. Food Chem. 2008, 56, 3524–3531. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. The Use of Cyclic Voltammetry for Wine Analysis: Determination of Polyphenols and Free Sulfur Dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef]
- Milia, E.; Usai, M.; Szotáková, B.; Elstnerová, M.; Králová, V.; D’hallewin, G.; Spissu, Y.; Barberis, A.; Marchetti, M.; Bortone, A.; et al. The Pharmaceutical Ability of Pistacia lentiscus L. Leaves Essential Oil against Periodontal Bacteria and Candida sp. and Its Anti-Inflammatory Potential. Antibiotics 2020, 9, 281. [Google Scholar] [CrossRef]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G.; et al. Antioxidant, Antimicrobial, and Other Biological Properties of Pompia Juice. Molecules 2020, 25, 3186. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A Pleiotropic Kinase Inhibitor against Cancer BT—Advances in Nutrition and Cancer; Zappia, V., Panico, S., Russo, G.L., Budillon, A., Della Ragione, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 185–205. [Google Scholar]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. The Polyphenol Constituents of Grape Pomace. Food Chem. 1999, 65, 1–8. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/Prooxidant Activity of a Polyphenolic Grape Seed Extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of Flavan-3-Ols in Seeds of Grape Pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- José Jara-Palacios, M.; Hernanz, D.; Luisa Escudero-Gilete, M.; Heredia, F.J. Antioxidant Potential of White Grape Pomaces: Phenolic Composition and Antioxidant Capacity Measured by Spectrophotometric and Cyclic Voltammetry Methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- BestMedGrape Project. Available online: https://www.enicbcmed.eu/projects/bestmedgrape (accessed on 23 December 2022).
- Fadda, A.; Pace, B.; Angioni, A.; Barberis, A.; Cefola, M. Suitability for Ready-to-Eat Processing and Preservation of Six Green and Red Baby Leaves Cultivars and Evaluation of Their Antioxidant Value during Storage and after the Expiration Date. J. Food Process. Preserv. 2016, 40, 550–558. [Google Scholar] [CrossRef]
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Bazzu, G.; Fadda, A.; Azara, E.; Sanna, D.; Schirra, M.; Serra, P.A. Development and Characterization of an Ascorbate Oxidase-Based Sensor–Biosensor System for Telemetric Detection of AA and Antioxidant Capacity in Fresh Orange Juice. Anal. Chem. 2014, 86, 8727–8734. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous Amperometric Detection of Ascorbic Acid and Antioxidant Capacity in Orange, Blueberry and Kiwi Juice, by a Telemetric System Coupled with a Fullerene- or Nanotubes-Modified Ascorbate Subtractive Biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef]
- Bouzabata, A.; Montoro, P.; Gil, K.A.; Piacente, S.; Youssef, F.S.; Al Musayeib, N.M.; Cordell, G.A.; Ashour, M.L.; Tuberoso, C.I.G. HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops Spinosus Organs and Evaluation of Their Antioxidant Activity. Antioxidants 2022, 11, 453. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Serreli, G.; Congiu, F.; Montoro, P.; Fenu, M.A. Characterization, Phenolic Profile, Nitrogen Compounds and Antioxidant Activity of Carignano Wines. J. Food Compos. Anal. 2017, 58, 60–68. [Google Scholar] [CrossRef]
- Floris, A.; Mazarei, M.; Yang, X.; Robinson, A.E.; Zhou, J.; Barberis, A.; D’hallewin, G.; Azara, E.; Spissu, Y.; Iglesias-Ara, A.; et al. SUMOylation Protects FASN against Proteasomal Degradation in Breast Cancer Cells Treated with Grape Leaf Extract. Biomolecules 2020, 10, 529. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. A Prooxidant Mechanism for the Anticancer and Chemopreventive Properties of Plant Polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ky, I.; Teissedre, P.-L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. From Grape to Wine: Changes in Phenolic Composition and Its Influence on Antioxidant Activity. Food Chem. 2016, 208, 228–238. [Google Scholar] [CrossRef]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic Composition and Antioxidant Capacity of Pomaces from Four Grape Varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Hollecker, L.; Pinna, M.; Filippino, G.; Scrugli, S.; Pinna, B.; Argiolas, F.; Murru, M. Simultaneous Determination of Polyphenolic Compounds in Red and White Grapes Grown in Sardinia by High Performance Liquid Chromatography–Electron Spray Ionisation-Mass Spectrometry. J. Chromatogr. A 2009, 1216, 3402–3408. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis Vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the Influence of Antioxidants on Polyphenol Oxidation in Wines Using an Electrochemical Method: Cyclic Voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Kilmartin, P.A. Electrochemistry Applied to the Analysis of Wine: A Mini-Review. Electrochem. Commun. 2016, 67, 39–42. [Google Scholar] [CrossRef]
- Piljac, J.; Martinez, S.; Stipèeviæ, T.; Petroviæ, E.; Metikoš-Hukoviæ, M. Cyclic Voltammetry Investigation of the Phenolic Content of Croatian Wines. Am. J. Enol. Vitic. 2004, 55, 417. [Google Scholar] [CrossRef]
- Chang, K.-L.; Cheng, H.-L.; Huang, L.-W.; Hsieh, B.-S.; Hu, Y.-C.; Chih, T.-T.; Shyu, H.-W.; Su, S.-J. Combined Effects of Terazosin and Genistein on a Metastatic, Hormone-Independent Human Prostate Cancer Cell Line. Cancer Lett. 2009, 276, 14–20. [Google Scholar] [CrossRef]
- Clément, M.-V.; Hirpara, J.L.; Chawdhury, S.-H.; Pervaiz, S. Chemopreventive Agent Resveratrol, a Natural Product Derived From Grapes, Triggers CD95 Signaling-Dependent Apoptosis in Human Tumor Cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef]
- He, S.; Sun, C.; Pan, Y. Red Wine Polyphenols for Cancer Prevention. Int. J. Mol. Sci. 2008, 9, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Levi, F.; Pasche, C.; Lucchini, F.; Ghidoni, R.; Ferraroni, M.; La Vecchia, C. Resveratrol and Breast Cancer Risk. Eur. J. Cancer Prev. 2005, 14, 139–142. [Google Scholar] [CrossRef]
- Khoi Ngoc, P.; Park Sun, J.; Kim Hee, J.; Xia, Y.; Kim Ho, N.; Kim Keun, K.; Jung Do, Y. (−)-Epigallocatechin-3-Gallate Blocks Nicotine-Induced Matrix Metalloproteinase-9 Expression and Invasiveness via Suppression of NF-ΚB and AP-1 in Endothelial Cells. Int. J. Oncol. 2013, 43, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Venza, I.; Venza, M.; Visalli, M.; Lentini, G.; Teti, D.; D’Alcontres, F.S. ROS as Regulators of Cellular Processes in Melanoma. Oxidative Med. Cell. Longev. 2021, 2021, 1208690. [Google Scholar] [CrossRef]
- Hyoudou, K.; Nishikawa, M.; Umeyama, Y.; Kobayashi, Y.; Yamashita, F.; Hashida, M. Inhibition of Metastatic Tumor Growth in Mouse Lung by Repeated Administration of Polyethylene Glycol-Conjugated Catalase: Quantitative Analysis with Firefly Luciferase-Expressing Melanoma Cells. Clin. Cancer Res. 2004, 10, 7685–7691. [Google Scholar] [CrossRef] [Green Version]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Nguyen, L.H.V.; Carru, C.; Pintus, G. Resveratrol Alters Human Endothelial Cells Redox State and Causes Mitochondrial-Dependent Cell Death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Dhawan, G.; Kapoor, R.; Calabrese, V.; Giordano, J. Chapter Ten—Hormesis: A Potential Strategic Approach to the Treatment of Neurodegenerative Disease. In Metabolic and Bioenergetic Drivers of Neurodegenerative Disease: Treating Neurodegenerative Diseases as Metabolic Diseases; Söderbom, G., Esterline, R., Oscarsson, J., Mattson, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 155, pp. 271–301. ISBN 0074-7742. [Google Scholar]
- Simić, A.; Manojlović, D.; Šegan, D.; Todorović, M. Electrochemical Behavior and Antioxidant and Prooxidant Activity of Natural Phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-F.; Wei, Q.-Y.; Cai, Y.-J.; Fang, J.-G.; Zhou, B.; Yang, L.; Liu, Z.-L. DNA Damage Induced by Resveratrol and Its Synthetic Analogues in the Presence of Cu (II) Ions: Mechanism and Structure-Activity Relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Shamim, U.; Ullah, M.F.; Azmi, A.S.; Bhat, S.H.; Hadi, S.M. The Anthocyanidin Delphinidin Mobilizes Endogenous Copper Ions from Human Lymphocytes Leading to Oxidative Degradation of Cellular DNA. Toxicology 2008, 249, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.T.; Ross, D.; Cohen, G.M. A Role for Metals and Free Radicals in the Induction of Apoptosis in Thymocytes. FEBS Lett. 1994, 352, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral Administration of Copper to Rats Leads to Increased Lymphocyte Cellular DNA Degradation by Dietary Polyphenols: Implications for a Cancer Preventive Mechanism. BioMetals 2011, 24, 1169–1178. [Google Scholar] [CrossRef]
- Palmieri, G.; Colombino, M.; Casula, M.; Manca, A.; Mandalà, M.; Cossu, A.; Antonio Cossu for the Italian Melanoma Intergroup (IMI). Molecular Pathways in Melanomagenesis: What We Learned from Next-Generation Sequencing Approaches. Curr. Oncol. Rep. 2018, 20, 86. [Google Scholar] [CrossRef] [Green Version]
- Crispo, A.; Corradin, M.T.; Giulioni, E.; Vecchiato, A.; Del Fiore, P.; Queirolo, P.; Spagnolo, F.; Vanella, V.; Caracò, C.; Tosti, G.; et al. Real Life Clinical Management and Survival in Advanced Cutaneous Melanoma: The Italian Clinical National Melanoma Registry Experience. Front. Oncol. 2021, 11, 672797. [Google Scholar]
- Palmieri, G.; Puzanov, I.; Massi, D.; Ascierto, P.A. Editorial: Advancements in Molecular Diagnosis and Treatment of Melanoma. Front. Oncol. 2021, 11, 728113. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2022, 41, 212–221. [Google Scholar] [CrossRef]
- Pisano, M.; Dettori, M.A.; Fabbri, D.; Delogu, G.; Palmieri, G.; Rozzo, C. Anticancer Activity of Two Novel Hydroxylated Biphenyl Compounds toward Malignant Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 5636. [Google Scholar] [CrossRef]



| ITALY | FRANCE | TUNISIA | ITALY | FRANCE | TUNISIA | |||
|---|---|---|---|---|---|---|---|---|
| Eapp | AUC (μC) | TPC (mg GAE/g dr) | ||||||
| Syrah | + 0.8 V | 5.93 b | 7.35 a | 5.05 c | Syrah | 232.2 b | 299.4 a | 217.3 b |
| Chardonnay | + 0.8 V | 5.43 b | 6.55 a | 6.47 a | Chardonnay | 321.8 a | 102.9 c | 155.9 b |
| Syrah | + 0.5 V | 2.30 b | 3.02 a | 1.80 c | DPPH (mmol TEAC/g dr) | |||
| Syrah | 1.44 b | 1.77 a | 1.39 b | |||||
| Chardonnay | 1.81 a | 0.62 c | 1.05 b | |||||
| Chardonnay | + 0.5 V | 1.89 b | 2.33 a | 2.27 a | CUPRAC (mmol Fe2+/g dr) | |||
| Syrah | 5.12 b | 8.93 a | 5.24 b | |||||
| Chardonnay | 9.78 a | 2.82 c | 3.52 b | |||||
| POLYPHENOLS (Standard) | Contribution of Polyphenols to the Total Antioxidant Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| (Equivalent Millimoles of GA) | ||||||||
| Redox Potential | Current at +0.5V | SYRAH Extracts | CHARDONNAY Extracts | |||||
| V | μA | France | Italy | Tunisia | France | Italy | Tunisia | |
| Anthocyanins | ||||||||
| Peonidin-3-O-glucoside | + 0.23 | 12.47 | 2.04 | 0.44 | 0.41 | - | - | - |
| Malvidin-3-O-glucoside * | + 0.55 | 1.22 | none | none | none | - | - | - |
| Flavonols | ||||||||
| Hydroxycinnamic acids | ||||||||
| Hydroxybenzoic acids | ||||||||
| Gallic acid | + 0.18 | 10.61 | 3.82 | 5.70 | 4.06 | 1.33 | 3.15 | 1.52 |
| Flavan 3-ols | ||||||||
| Procyanidin B1 | + 0.16 | 22.98 | 3.21 | 8.68 | 11.89 | 1.02 | 2.49 | 6.86 |
| (+)-Catechin | + 0.12 | 22.96 | 54.88 | 30.32 | 31.03 | 25.62 | 70.75 | 27.93 |
| Procyanidin B2 | + 0.16 | 21.35 | 17.79 | 11.36 | 8.24 | 6.72 | 11.44 | 8.61 |
| (−)-Epicatechin | + 0.12 | 14.26 | 28.07 | 18.49 | 12.76 | 17.47 | 40.11 | 13.78 |
| Epigallocatechin | + 0.08 | 19.56 | 6.66 | 0.14 | 0.05 | 0.19 | 3.70 | 0.28 |
| Prooxidant Activity | |||
|---|---|---|---|
| Variety | Origin | Viability (ratio vs. control) | μmoles equivalents of H2O2 |
| Italy | 0.66 ± 0.05 b | 206.00 ± 15.53 b | |
| Syrah | France | 0.74 ± 0.03 a | 172.96 ± 7.02 c |
| Tunisia | 0.51 ± 0.03 c | 272.96 ± 16.09 a | |
| Italy | 1.08 ± 0.02 a | 24.70± 0.46 c | |
| Chardonnay | France | 0.94 ± 0.05 b | 87.74 ± 4.69 b |
| Tunisia | 0.83 ± 0.07 c | 135.13 ± 11.45 a | |
| Combined Treatment | Italy | France | Tunisia | Italy | France | Tunisia | |
|---|---|---|---|---|---|---|---|
| 250 μg/mL | μM H2O2 | Reduction of Viability (%) vs. Control | μmoles of H2O2 alone | ||||
| Syrah | 50 | 44.72 | ne | 55.53 | 253.91 | ne | 300.90 |
| 100 | 66.52 | 76.10 | 79.25 | 348.71 | 390.33 | 404.03 | |
| Chardonnay | 50 | ne | ne | 45.15 | ne | ne | 255.79 |
| 100 | 60.65 | 59.48 | 64.30 | 323.19 | 318.09 | 339.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spissu, Y.; Gil, K.A.; Dore, A.; Sanna, G.; Palmieri, G.; Sanna, A.; Cossu, M.; Belhadj, F.; Gharbi, B.; Pinna, M.B.; et al. Anti- and Pro-Oxidant Activity of Polyphenols Extracts of Syrah and Chardonnay Grapevine Pomaces on Melanoma Cancer Cells. Antioxidants 2023, 12, 80. https://doi.org/10.3390/antiox12010080
Spissu Y, Gil KA, Dore A, Sanna G, Palmieri G, Sanna A, Cossu M, Belhadj F, Gharbi B, Pinna MB, et al. Anti- and Pro-Oxidant Activity of Polyphenols Extracts of Syrah and Chardonnay Grapevine Pomaces on Melanoma Cancer Cells. Antioxidants. 2023; 12(1):80. https://doi.org/10.3390/antiox12010080
Chicago/Turabian StyleSpissu, Ylenia, Katarzyna Angelika Gil, Antonio Dore, Giulia Sanna, Giuseppe Palmieri, Andrea Sanna, Maurizio Cossu, Feten Belhadj, Boutheina Gharbi, Maria Barbara Pinna, and et al. 2023. "Anti- and Pro-Oxidant Activity of Polyphenols Extracts of Syrah and Chardonnay Grapevine Pomaces on Melanoma Cancer Cells" Antioxidants 12, no. 1: 80. https://doi.org/10.3390/antiox12010080









