An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice
Abstract
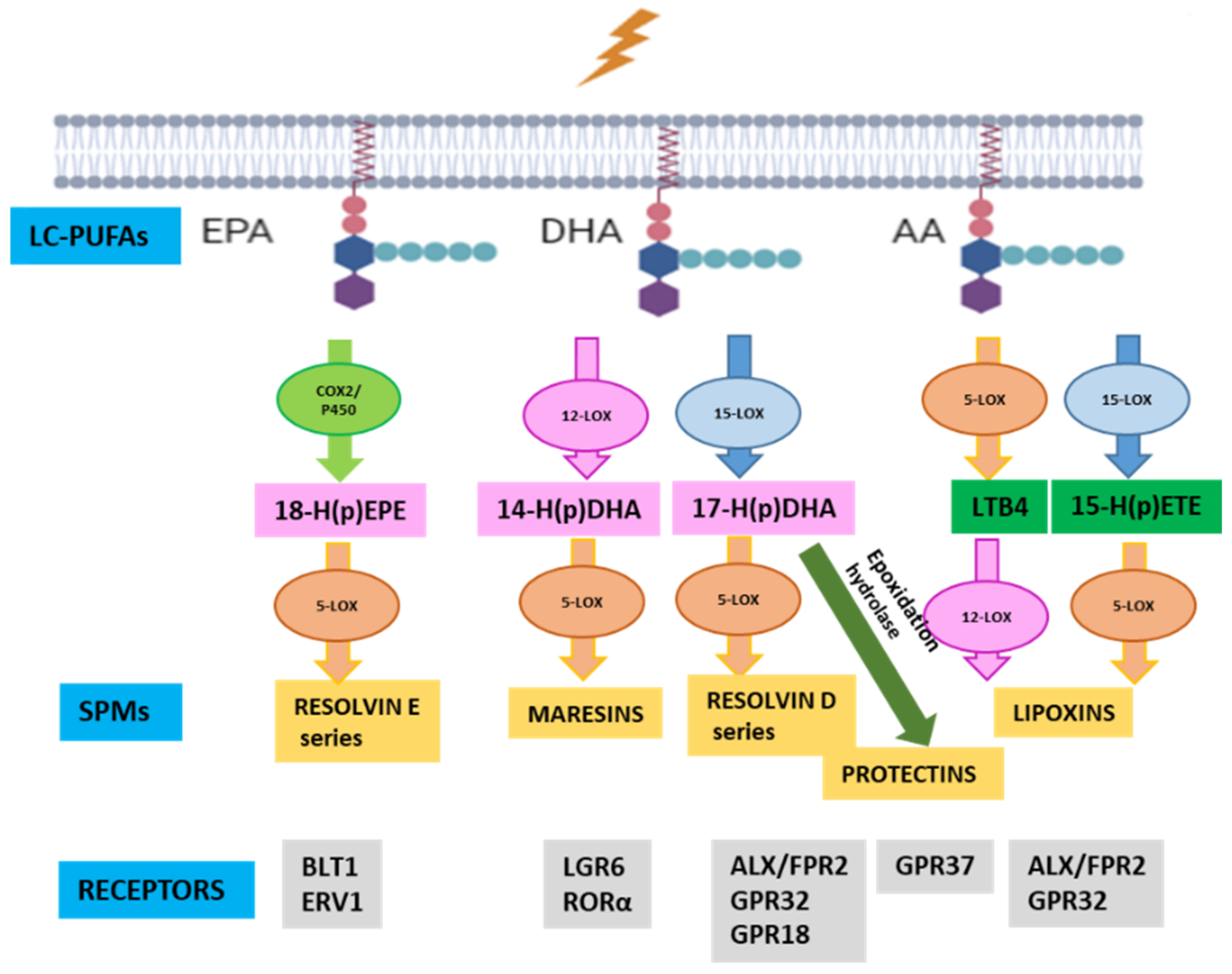
1. Introduction
2. Materials and Methods
2.1. Mechanistic Modeling of the Relevance of ELOVL4 and ALOX5 on an RP Mechanistic Map
2.2. EFA Supplement Manufacturing Process
2.3. Cell Culture of 661W and BV2
2.4. Cell Viability
2.5. Synthesis of Coumarin-6-Loaded Nanostructured Lipid Carriers (NLC) and the Internalization Study
2.6. Immnunocytochemistry
2.7. Animals and Treatment
2.8. Electroretinogram
2.9. Light/Dark Transition Test
2.10. Retinal Histology
2.11. Microscopy and Quantification
2.12. Gene Expression
2.13. Flow Cytometry of Retinal Tissue
2.14. Enzyme-Linked Immunosorbent Assay of TNFα
2.15. Determination of Redox Status
2.16. Statistical Analysis
3. Results
3.1. ALOX5 and ELOVL4 Relevance in the RP Mechanistic Map Obtained by the ML Model
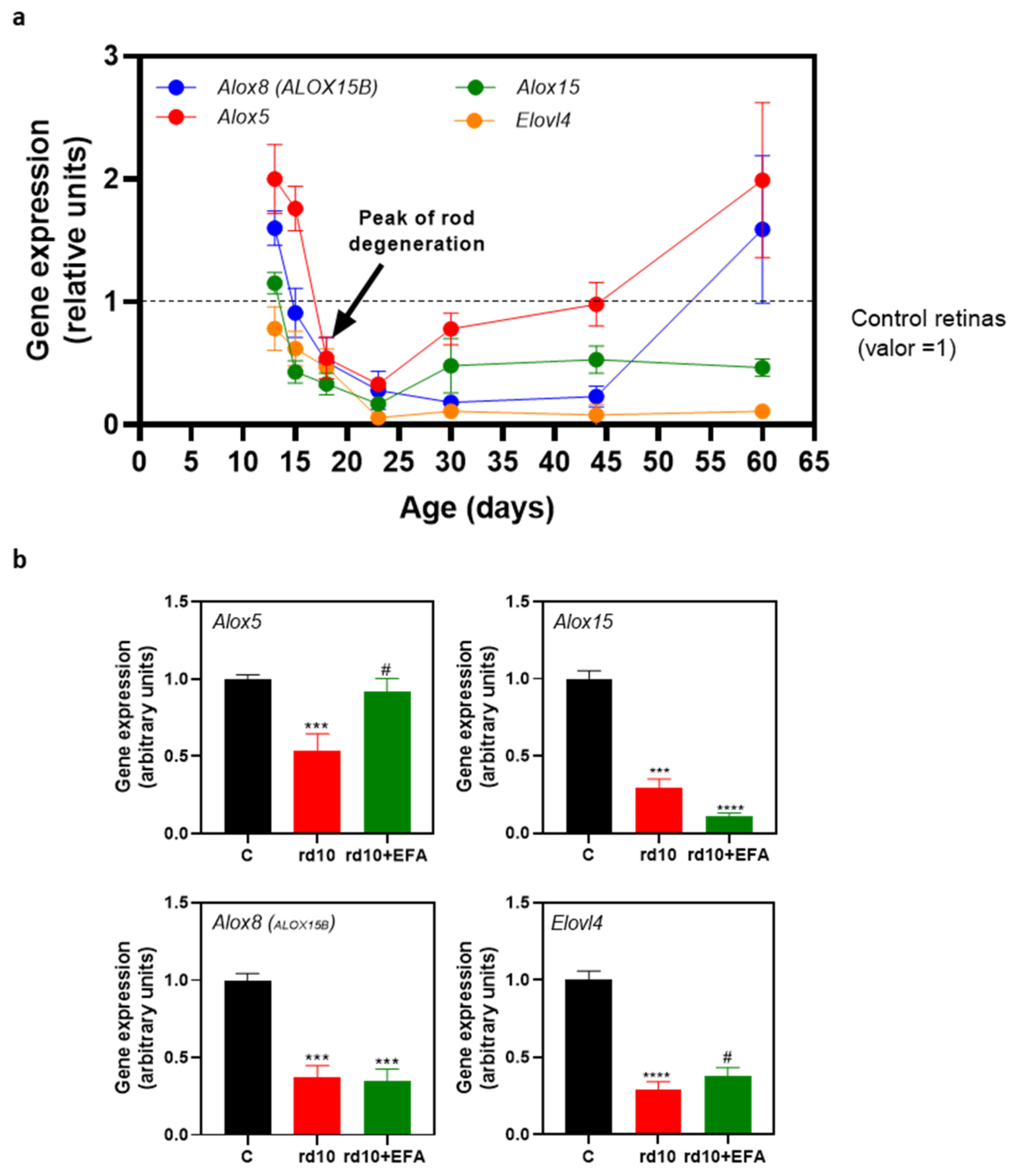
3.2. Altered Temporal Profile of Alox5, Alox8, Alox1, and Elovl4 in the Retinas of rd10 Mice
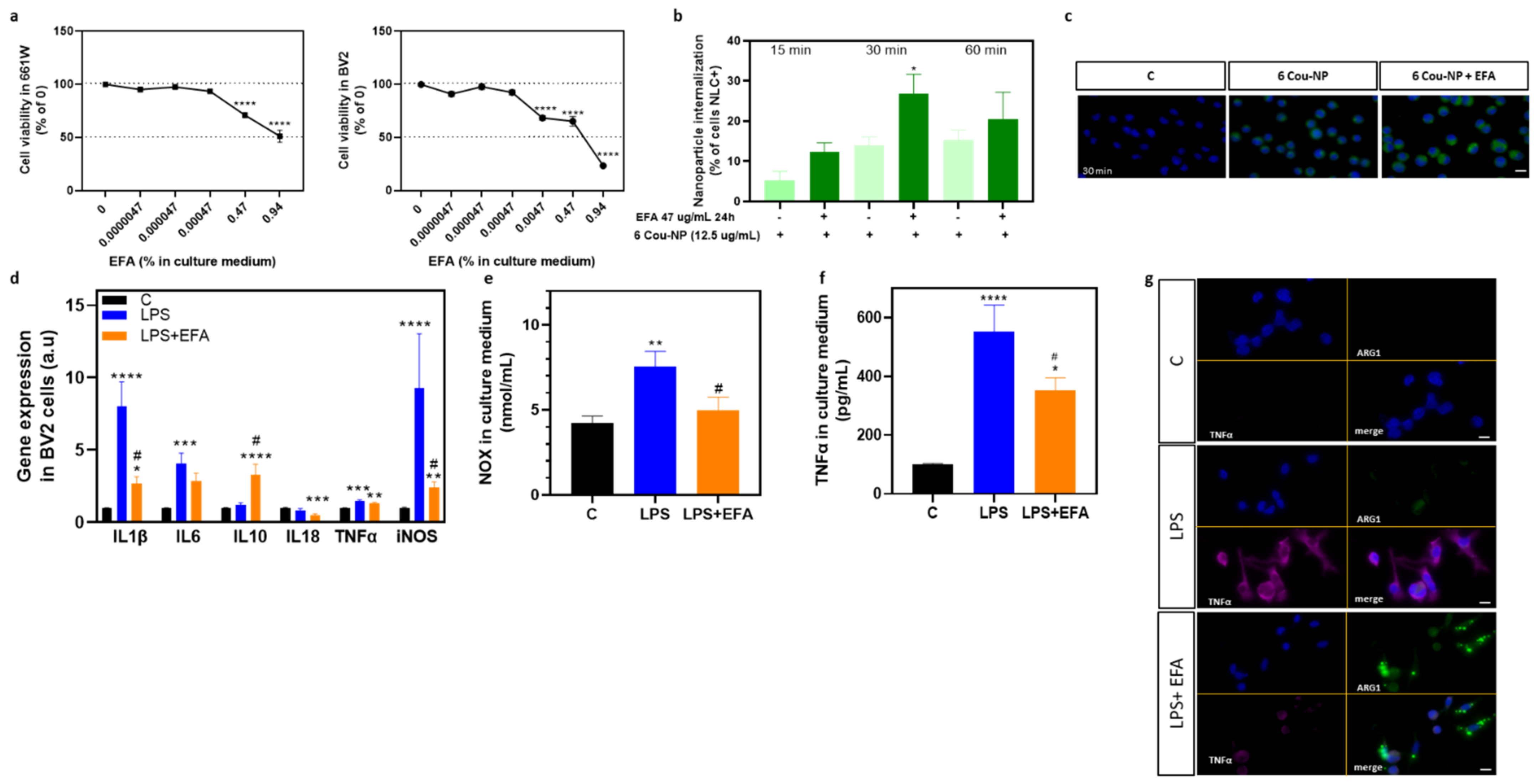
3.3. SPM Precursors Shifted M1 Microglia to M2 in LPS-Treated BV2 Cells
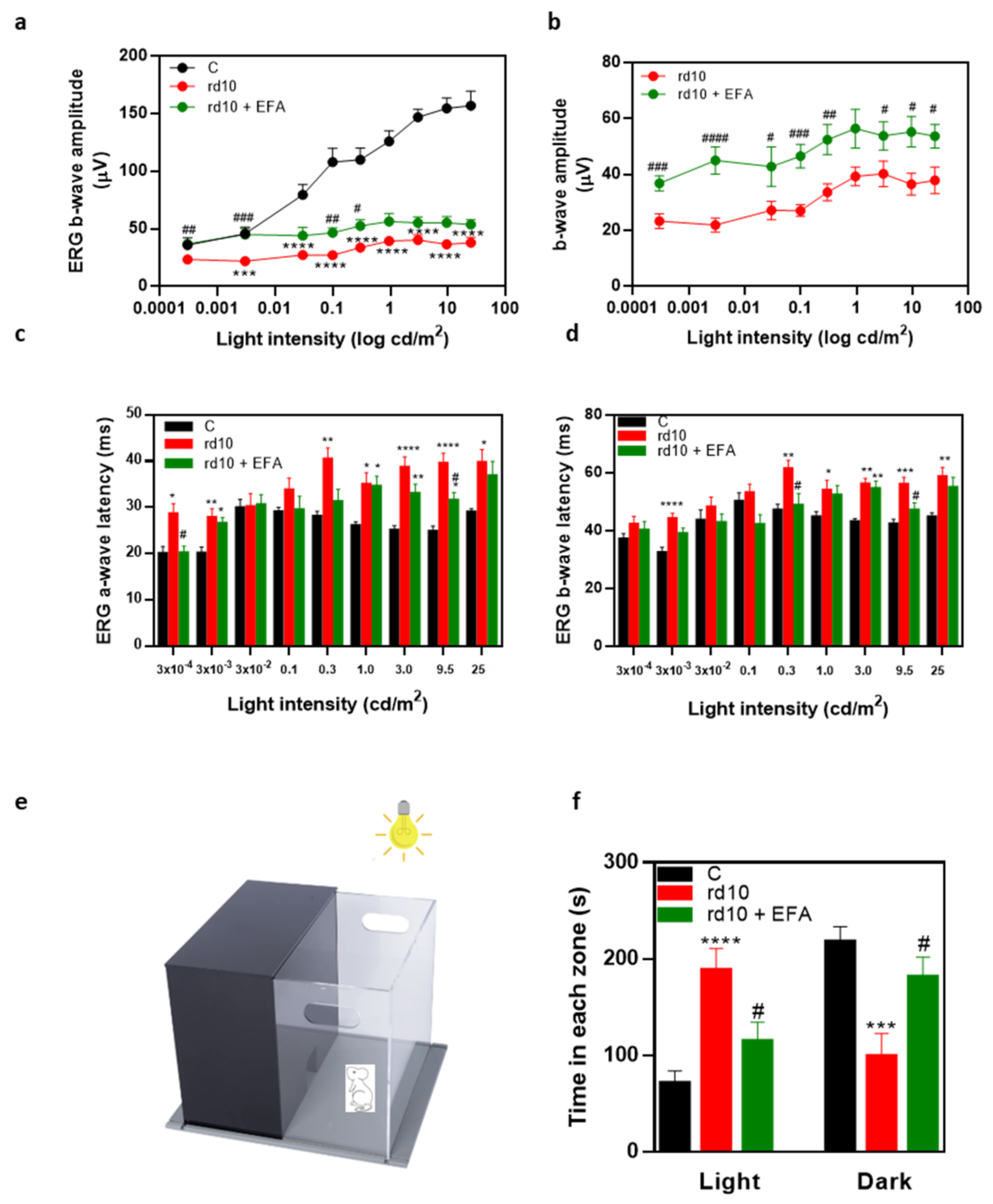
3.4. Oral Administration of SPM Precursors Ameliorated Retinal Dysfunction and Reduced PR Degeneration in rd10 Mice
3.4.1. ERG Recordings
3.4.2. Light Aversion
3.4.3. PR Degeneration
3.5. Oral Administration of SPM Precursors Reduced Reactive Gliosis, Microglia Migration, and M1-Microglia Markers and Increases M2-Microglia Markers in rd10 Mouse Retinas at P18
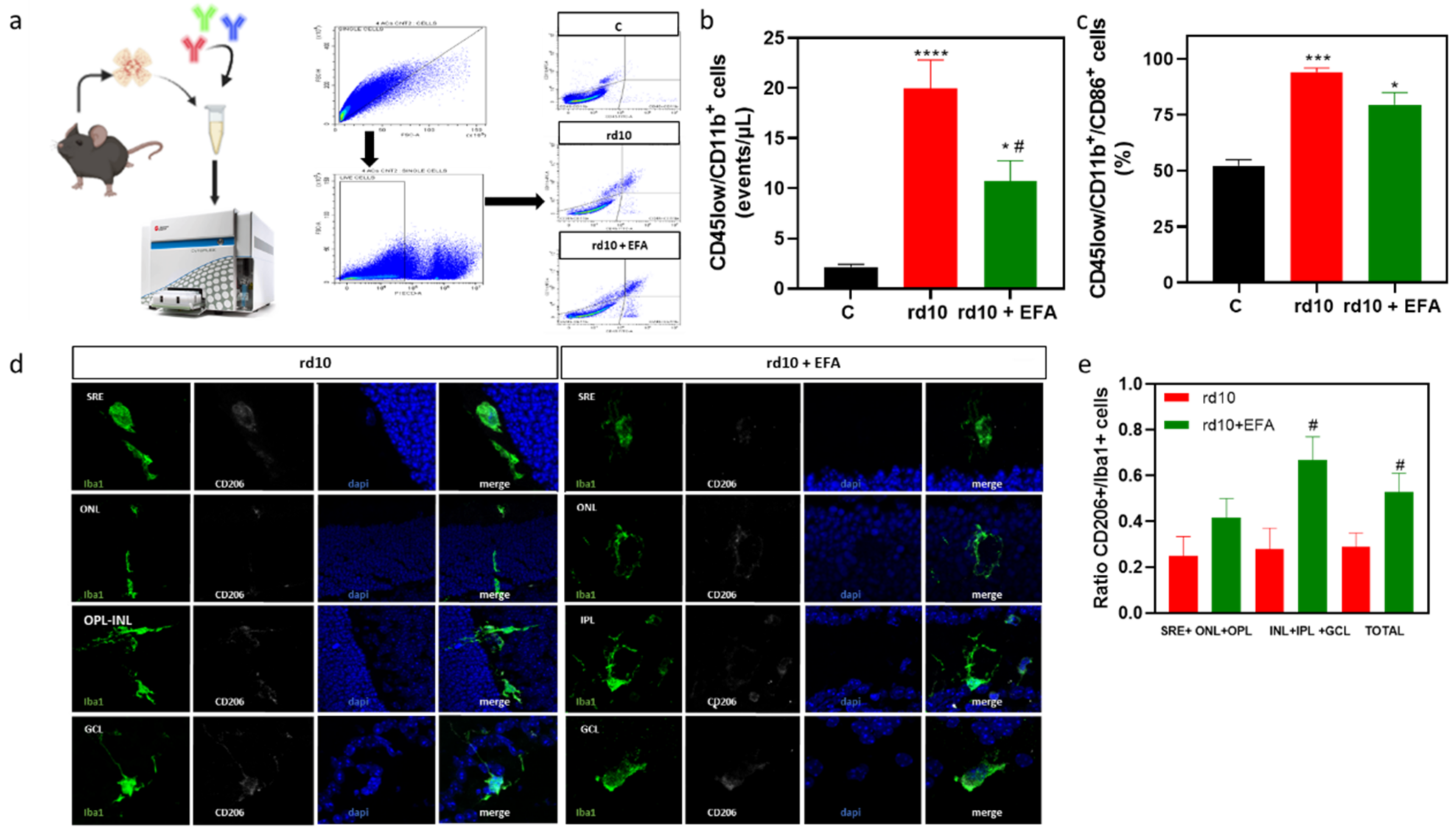
3.5.1. Reactive Gliosis and Iba1-Positive Cell Migration
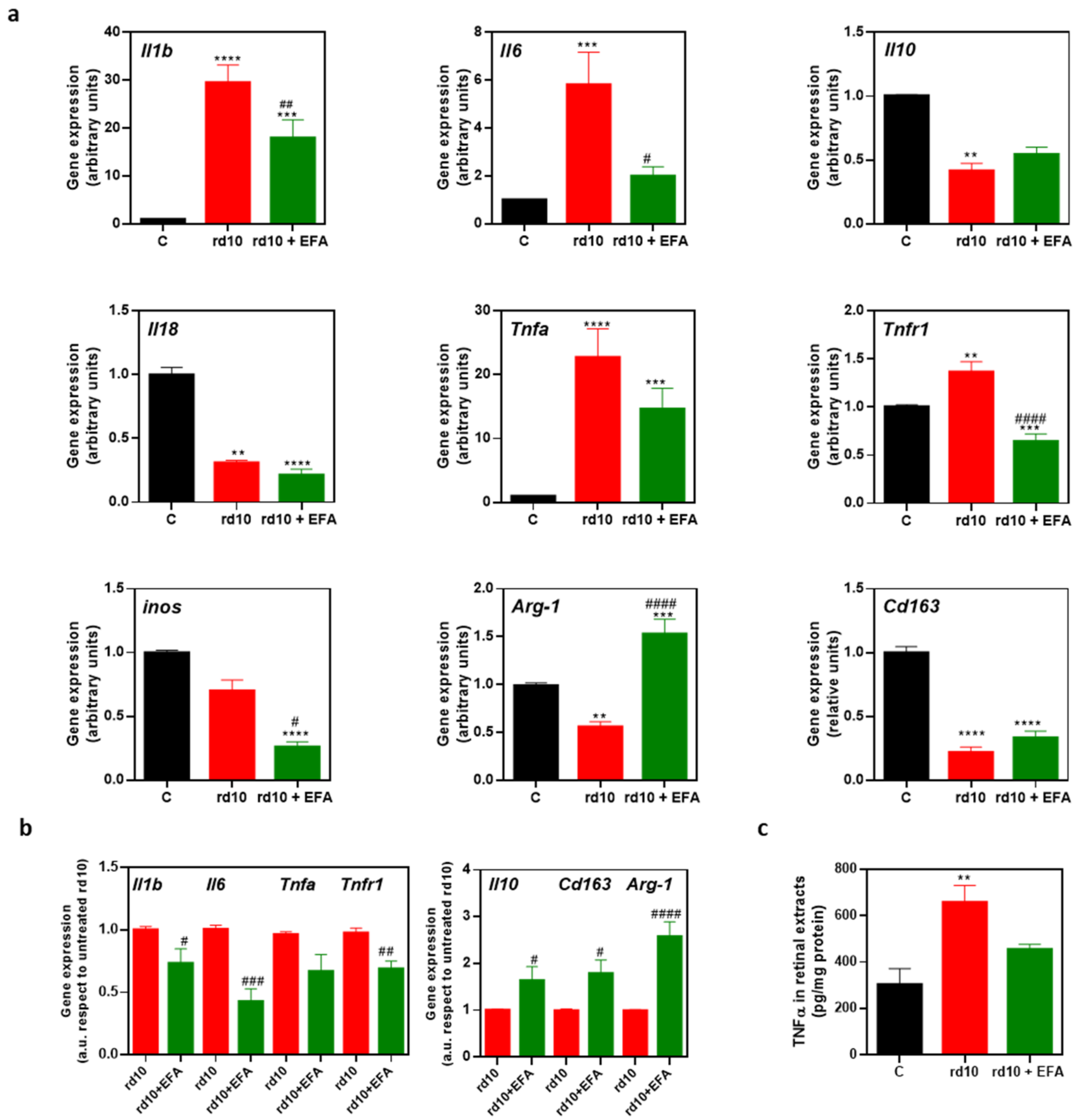
3.5.2. M1 and M2 Microglia Markers
3.6. Oral Administration of SPM Precursors Ameliorated Oxidative Stress in rd10 Mouse Retinas at P18
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, B. Animal models of retinitis pigmentosa (RP). In Animal Models of Ophthalmic Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 101–116. [Google Scholar]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ikeda, Y.; Nakatake, S.; Fujiwara, K.; Tachibana, T.; Yoshida, N.; Notomi, S.; Hisatomi, T.; Yoshida, S.; Ishibashi, T. C-Reactive protein and progression of vision loss in retinitis pigmentosa. Acta Ophthalmol. 2018, 96, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.K.; Zhao, L.; Zhang, Y.; Gonzalez, S.R.; Ma, W.; Wang, X.; Fariss, R.N.; Wong, W.T. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1-CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 2016, 64, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, M.E.; Hammer, S.S.; McCollum, G.W.; Penn, J.S. Epoxygenated Fatty Acids Inhibit Retinal Vascular Inflammation. Sci. Rep. 2016, 6, 39211. [Google Scholar] [CrossRef]
- Gubitosi-Klug, R.A.; Talahalli, R.; Du, Y.; Nadler, J.L.; Kern, T.S. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 2008, 57, 1387–1393. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef]
- Tiberi, M.; Chiurchiù, V. Specialized pro-resolving lipid mediators and glial cells: Emerging candidates for brain homeostasis and repair. Front. Cell. Neurosci. 2021, 15, 673549. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of Pro-Oxidant and Pro-Inflammatory Activities of M1 Macrophages by the Natural Dipeptide Carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef]
- Zou, T.; He, J.; Chen, X.; Sun, D.; Fan, X.; Xu, H. Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front. Mol. Neurosci. 2019, 12, 102. [Google Scholar]
- Agbaga, M.P.; Mandal, M.N.; Anderson, R.E. Retinal very long-chain PUFAs: New insights from studies on ELOVL4 protein. J. Lipid Res. 2010, 51, 1624–1642. [Google Scholar] [CrossRef]
- Ruiz-Pastor, M.J.; Kutsyr, O.; Lax, P.; Cuenca, N. Decrease in DHA and other fatty acids correlates with photoreceptor degeneration in retinitis pigmentosa. Exp. Eye Res. 2021, 209, 108667. [Google Scholar] [CrossRef]
- Callan, N.; Hanes, D.; Bradley, R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J. Transl. Med. 2020, 18, 401. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef]
- Ontoria-Oviedo, I.; Amaro-Prellezo, E.; Castellano, D.; Venegas-Venegas, E.; González-Santos, F.; Ruiz-Saurí, A. Topical Administration of a Marine Oil Rich in Pro-Resolving Lipid Mediators Accelerates Wound Healing in Diabetic db/db Mice through Angiogenesis and Macrophage Polarization. Int. J. Mol. Sci. 2022, 23, 9918. [Google Scholar] [CrossRef]
- Hidalgo, M.R.; Cubuk, C.; Amadoz, A.; Salavert, F.; Carbonell-Caballero, J.; Dopazo, J. High throughput estimation of functional cell activities reveals disease mechanisms and predicts relevant clinical outcomes. Oncotarget 2017, 8, 5160–5178. [Google Scholar] [CrossRef]
- Loucera, C.; Esteban-Medina, M.; Rian, K.; Falco, M.M.; Dopazo, J.; Peña-Chilet, M. Drug repurposing for COVID-19 using machine learning and mechanistic models of signal transduction circuits related to SARS-CoV-2 infection. Signal Transduct. Target. Ther. 2020, 5, 290. [Google Scholar] [CrossRef]
- Segal, M.; Xiao, Y. Multivariate random forests. Wiley Interdiscip.Rev. Data Min. Knowl. Discov. 2011, 1, 80–87. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.; Sechidis, K.; Brown, G. On the stability of feature selection algorithms. J. Mach. Learn. Res. 2017, 18, 6345–6398. [Google Scholar]
- Pharmacopoeia, E. Composition of Fatty Acids in Oils Rich in Omega-3 Acids, 6th ed.; European Directorate for the Quality of Medicines and Health Care of the Council of Europe: London, UK, 2017; Section 2.4.29. [Google Scholar]
- Strassburg, K.; Huijbrechts, A.M.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; van Duynhoven, J.; et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: Application in cardiac surgery. Anal. Bioanal. Chem. 2012, 404, 1413–1426. [Google Scholar] [CrossRef]
- Olivares-González, L.; Velasco, S.; Campillo, I.; Salom, D.; González-García, E.; Soriano Del Castillo, J.M.; Rodrigo, R. Nutraceutical Supplementation Ameliorates Visual Function, Retinal Degeneration, and Redox Status in rd10 Mice. Antioxidants 2021, 10, 1033. [Google Scholar] [CrossRef]
- Iura, Y.; Udo, H. Behavioral analyses of visually impaired Crx knockout mice revealed sensory compensation in exploratory activities on elevated platforms. Behav. Brain Res. 2014, 258, 1–7. [Google Scholar] [CrossRef]
- Martínez-Fernández de la Cámara, C.; Sequedo, M.D.; Gómez-Pinedo, U.; Jaijo, T.; Aller, E.; García-Tárraga, P.; García-Verdugo, J.M.; Millán, J.M.; Rodrigo, R. Phosphodiesterase inhibition induces retinal degeneration, oxidative stress and inflammation in cone-enriched cultures of porcine retina. Exp. Eye Res. 2013, 111, 122–133. [Google Scholar] [CrossRef]
- Olivares-González, L.; Martínez-Fernández de la Cámara, C.; Hervás, D.; Millán, J.M.; Rodrigo, R. HIF-1α stabilization reduces retinal degeneration in a mouse model of retinitis pigmentosa. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 2438–2451. [Google Scholar] [CrossRef]
- Martínez-Fernández de la Cámara, C.; Olivares-González, L.; Hervás, D.; Salom, D.; Millán, J.M.; Rodrigo, R. Infliximab reduces Zaprinast-induced retinal degeneration in cultures of porcine retina. J. Neuroinflammation 2014, 11, 172. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Sun, X.; Zhu, X.; Zhou, L.; Li, M.; Cheng, B.; Liu, X.; He, C. Microglia Polarization with M1/M2 Phenotype Changes in rd1 Mouse Model of Retinal Degeneration. Front. Neuroanat. 2017, 11, 77. [Google Scholar] [CrossRef]
- El-Mlili, N.; Rodrigo, R.; Naghizadeh, B.; Cauli, O.; Felipo, V. Chronic hyperammonemia reduces the activity of neuronal nitric oxide synthase in cerebellum by altering its localization and increasing its phosphorylation by calcium-calmodulin kinase II. J. Neurochem. 2008, 106, 1440–1449. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Martínez-Fernández de la Cámara, C.; Hernández-Pinto, A.M.; Olivares-González, L.; Cuevas-Martín, C.; Sánchez-Aragó, M.; Hervás, D.; Salom, D.; Cuezva, J.M.; de la Rosa, E.J.; Millán, J.M.; et al. Adalimumab Reduces Photoreceptor Cell Death in A Mouse Model of Retinal Degeneration. Sci. Rep. 2015, 5, 11764. [Google Scholar] [CrossRef]
- Gong, Y.; Fu, Z.; Liegl, R.; Chen, J.; Hellström, A.; Smith, L.E. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017, 106, 16–26. [Google Scholar] [CrossRef]
- Ren, J.; Ren, A.; Deng, X.; Huang, Z.; Jiang, Z.; Li, Z.; Gong, Y. Long-Chain Polyunsaturated Fatty Acids and Their Metabolites Regulate Inflammation in Age-Related Macular Degeneration. J. Inflamm. Res. 2022, 15, 865–880. [Google Scholar] [CrossRef]
- Hidalgo, M.R.; Amadoz, A.; Çubuk, C.; Carbonell-Caballero, J.; Dopazo, J. Models of cell signaling uncover molecular mechanisms of high-risk neuroblastoma and predict disease outcome. Biol. Direct 2018, 13, 16. [Google Scholar] [CrossRef]
- Cubuk, C.; Hidalgo, M.R.; Amadoz, A.; Pujana, M.A.; Mateo, F.; Herranz, C.; Carbonell-Caballero, J.; Dopazo, J. Gene Expression Integration into Pathway Modules Reveals a Pan-Cancer Metabolic Landscape. Cancer Res. 2018, 78, 6059–6072. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Brüne, B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10, 719. [Google Scholar] [CrossRef]
- Kutzner, L.; Goloshchapova, K.; Heydeck, D.; Stehling, S.; Kuhn, H.; Schebb, N.H. Mammalian ALOX15 orthologs exhibit pronounced dual positional specificity with docosahexaenoic acid. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2017, 1862, 666–675. [Google Scholar] [CrossRef]
- Werz, O.; Steinhilber, D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol. Ther. 2006, 112, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Mendez, E.F.; Becerra, S.P. A Novel Inhibitor of 5-Lipoxygenase (5-LOX) Prevents Oxidative Stress-Induced Cell Death of Retinal Pigment Epithelium (RPE) Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, R.P. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol. Rep. PR 2012, 64, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Chang-Chien, G.P.; Lin, S.; Hsiao, Y.T.; Kalkhoven, E.; Ke, M.C.; Chen, A.; Lin, T.K. 5-Lipoxygenase Inhibition Protects Retinal Pigment Epithelium from Sodium Iodate-Induced Ferroptosis and Prevents Retinal Degeneration. Oxidative Med. Cell. Longev. 2022, 2022, 1792894. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef]
- Häfner, A.K.; Kahnt, A.S.; Steinhilber, D. Beyond leukotriene formation-The noncanonical functions of 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2019, 142, 24–32. [Google Scholar] [CrossRef]
- Trotta, M.C.; Gesualdo, C.; Petrillo, F.; Lepre, C.C.; Della Corte, A.; Cavasso, G.; Maggiore, G.; Hermenean, A.; Simonelli, F.; D’Amico, M.; et al. Resolution of Inflammation in Retinal Disorders: Briefly the State. Int. J. Mol. Sci. 2022, 2, 4501. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Zhang, X.; Gao, Y.; Yin, Z.Q. Lipoxin A4 delays the progression of retinal degeneration via the inhibition of microglial overactivation. Biochem. Biophys. Res. Commun. 2019, 516, 900–906. [Google Scholar] [CrossRef]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef]
- Lam, B.W.S.; Yam, T.Y.A.; Chen, C.P.; Lai, M.K.P.; Ong, W.Y.; Herr, D.R. The noncanonical chronicles: Emerging roles of sphingolipid structural variants. Cell. Signal. 2021, 79, 109890. [Google Scholar] [CrossRef]
- Sánchez-Fernández, A.; Zandee, S.; Mastrogiovanni, M.; Charabati, M.; Rubbo, H.; Prat, A.; López-Vales, R. Administration of Maresin-1 ameliorates the physiopathology of experimental autoimmune encephalomyelitis. J. Neuroinflammation 2022, 19, 27. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Tiberi, M.; Matteocci, A.; Fazio, F.; Siffeti, H.; Saracini, S.; Mercuri, N.B. Lipidomics of Bioactive Lipids in Alzheimer’s and Parkinson’s Diseases: Where Are We? Int. J. Mol. Sci. 2022, 23, 6235. [Google Scholar] [CrossRef]
- Rossi, S.; Di Filippo, C.; Gesualdo, C.; Potenza, N.; Russo, A.; Trotta, M.C.; Zippo, M.V.; Maisto, R.; Ferraraccio, F.; Simonelli, F.; et al. Protection from endotoxic uveitis by intravitreal Resolvin D1: Involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediat. Inflamm. 2015, 2015, 149381. [Google Scholar] [CrossRef]
- Decker, C.; Sadhu, S.; Fredman, G. Pro-Resolving Ligands Orchestrate Phagocytosis. Front. Immunol. 2021, 12, 660865. [Google Scholar] [CrossRef]
- Simon, E.; Bardet, B.; Grégoire, S.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C.P.; Bretillon, L. Decreasing dietary linoleic acid promotes long chain omega-3 fatty acid incorporation into rat retina and modifies gene expression. Exp. Eye Res. 2011, 93, 628–635. [Google Scholar] [CrossRef]
- Schwartz, S.G.; Wang, X.; Chavis, P.; Kuriyan, A.E.; Abariga, S.A. Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst. Rev. 2020, 6, Cd008428. [Google Scholar] [CrossRef]
- Brito-García, N.; Del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Coco, R.M.; Rodríguez de la Rúa, E.; Del Cura-González, I.; Serrano-Aguilar, P. Effectiveness and safety of nutritional supplements in the treatment of hereditary retinal dystrophies: A systematic review. Eye 2017, 31, 273–285. [Google Scholar] [CrossRef]
- Querques, G.; Forte, R.; Souied, E.H. Retina and omega-3. J. Nutr. Metab. 2011, 2011, 748361. [Google Scholar] [CrossRef]
- Kishan, A.U.; Modjtahedi, B.S.; Martins, E.N.; Modjtahedi, S.P.; Morse, L.S. Lipids and age-related macular degeneration. Surv. Ophthalmol. 2011, 56, 195–213. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Kotwani, A. Implication of oxidative stress in progression of diabetic retinopathy. Surv. Ophthalmol. 2016, 61, 187–196. [Google Scholar] [CrossRef]
- Rodríguez González-Herrero, M.E.; Ruiz, M.; López Román, F.J.; Marín Sánchez, J.M.; Domingo, J.C. Supplementation with a highly concentrated docosahexaenoic acid plus xanthophyll carotenoid multivitamin in nonproliferative diabetic retinopathy: Prospective controlled study of macular function by fundus microperimetry. Clin. Ophthalmol. 2018, 12, 1011–1020. [Google Scholar] [CrossRef]
- Piñas García, P.; Hernández Martínez, F.J.; Aznárez López, N.; Castillón Torre, L.; Sempere, E.T. Supplementation with a Highly Concentrated Docosahexaenoic Acid (DHA) in Non-Proliferative Diabetic Retinopathy: A 2-Year Randomized Double-Blind Placebo-Controlled Study. Antioxidants 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Asp. Med. 2018, 64, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Rodríguez González-Herrero, M.E.; Romeo Villadóniga, S.; Domingo, J.C. Antioxidant Activity and Neuroprotective Role of Docosahexaenoic Acid (DHA) Supplementation in Eye Diseases That Can Lead to Blindness: A Narrative Review. Antioxidants 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Maccarrone, M.; Chiurchiù, V. Proresolving Lipid Mediators: Endogenous Modulators of Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 8107265. [Google Scholar] [CrossRef]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Pinilla, I.; Maneu, V.; Campello, L.; Fernández-Sánchez, L.; Martínez-Gil, N.; Kutsyr, O.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Lax, P.; Cuenca, N. Inherited Retinal Dystrophies: Role of Oxidative Stress and Inflammation in Their Physiopathology and Therapeutic Implications. Antioxidants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Wang, S.K.; Cepko, C.L. Targeting Microglia to Treat Degenerative Eye Diseases. Front. Immunol. 2022, 13, 843558. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Recent advances in arginine metabolism: Roles and regulation of the arginases. Br. J. Pharmacol. 2009, 157, 922–930. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Palma, A.; Jarrah, A.S.; Tieri, P.; Cesareni, G.; Castiglione, F. Gene Regulatory Network Modeling of Macrophage Differentiation Corroborates the Continuum Hypothesis of Polarization States. Front. Physiol. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Benarroch, E.E. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2013, 81, 1079–1088. [Google Scholar] [CrossRef]
- Loane, D.J.; Byrnes, K.R. Role of microglia in neurotrauma. Neurother. J. Am. Soc. Exp. Neuro. Ther. 2010, 7, 366–377. [Google Scholar] [CrossRef]
- Sundal, C. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2014, 82, 1846. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, Z.; Zhu, X.; Sun, X.; Liu, Y.; Cheng, B.; Li, M.; Liu, Y.; He, C.; Liu, X. Alpha-1 Antitrypsin Attenuates M1 Microglia-Mediated Neuroinflammation in Retinal Degeneration. Front. Immunol. 2018, 9, 1202. [Google Scholar] [CrossRef]
- Olivares-González, L.; Velasco, S.; Millán, J.M.; Rodrigo, R. Intravitreal administration of adalimumab delays retinal degeneration in rd10 mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 13839–13861. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Li, Y.; Wang, J.N.; Zhao, Q.X.; Wen, S.; Wang, S.C.; Sun, T. A Novel Mechanism of Specialized Proresolving Lipid Mediators Mitigating Radicular Pain: The Negative Interaction with NLRP3 Inflammasome. Neurochem. Res. 2020, 45, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A., Jr. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019, 176, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Chen, J.; Jiang, Z.X.; Zhou, Z.; Zhou, C.N. Resolvin D1 alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 signaling pathway. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Wang, Y.; Leppert, A.; Tan, S.; van der Gaag, B.; Li, N.; Schultzberg, M.; Hjorth, E. Maresin 1 attenuates pro-inflammatory activation induced by β-amyloid and stimulates its uptake. J. Cell. Mol. Med. 2021, 25, 434–447. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, F.; Wang, W.; Wang, H.; Zhang, X. Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol. Vis. 2017, 23, 242–250. [Google Scholar]
- Böttcher, C.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; Kahn, R.S.; Schulz, A.R.; et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef]
- Ford, A.L.; Goodsall, A.L.; Hickey, W.F.; Sedgwick, J.D. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1995, 154, 4309–4321. [Google Scholar]
- Zhang, G.X.; Li, J.; Ventura, E.; Rostami, A. Parenchymal microglia of naïve adult C57BL/6J mice express high levels of B7.1, B7.2, and MHC class II. Exp. Mol. Pathol. 2002, 73, 35–45. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Crotti, A.; Ransohoff, R.M. Microglial Physiology and Pathophysiology: Insights from Genome-wide Transcriptional Profiling. Immunity 2016, 44, 505–515. [Google Scholar] [CrossRef]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma. Int. J. Mol. Sci. 2018, 19, 436. [Google Scholar] [CrossRef]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef]
- Benmamar-Badel, A.; Owens, T.; Wlodarczyk, A. Protective Microglial Subset in Development, Aging, and Disease: Lessons From Transcriptomic Studies. Front. Immunol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Li, J.; Mao, M.; Li, J.; Chen, Z.; Ji, Y.; Kong, J.; Wang, Z.; Zhang, J.; Wang, Y.; Liang, W.; et al. Oral Administration of Omega-3 Fatty Acids Attenuates Lung Injury Caused by PM2.5 Respiratory Inhalation Simply and Feasibly In Vivo. Int. J. Mol. Sci. 2022, 23, 5323. [Google Scholar] [CrossRef]
- Burger, B.; Kühl, C.M.C.; Candreva, T.; Cardoso, R.D.S.; Silva, J.R.; Castelucci, B.G.; Consonni, S.R.; Fisk, H.L.; Calder, P.C.; Vinolo, M.A.R.; et al. Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice. Sci. Rep. 2019, 9, 9119. [Google Scholar] [CrossRef]
- Chacon, A.C.; Phillips, B.E.; Chacon, M.A.; Brunke-Reese, D.; Kelleher, S.L.; Soybel, D.I. Oral omega-3 fatty acids promote resolution in chemical peritonitis. J. Surg. Res. 2016, 206, 190–198. [Google Scholar] [CrossRef]
- Pisaniello, A.; Di Bartolo, B.; Liu, G.; Gibson, R.; Kim, S.; Psaltis, P.; Nicholls, S. The impact of oral omega-3 fatty acid supplementation on acute vascular inflammation in a mouse model. Atherosclerosis 2017, 263, e119. [Google Scholar] [CrossRef]
- Cutuli, D.; Landolfo, E.; Nobili, A.; De Bartolo, P.; Sacchetti, S.; Chirico, D.; Marini, F.; Pieroni, L.; Ronci, M.; D’Amelio, M.; et al. Behavioral, neuromorphological, and neurobiochemical effects induced by omega-3 fatty acids following basal forebrain cholinergic depletion in aged mice. Alzheimer’s Res. Ther. 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Prokopiou, E.; Kolovos, P.; Georgiou, C.; Kalogerou, M.; Potamiti, L.; Sokratous, K.; Kyriacou, K.; Georgiou, T. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ Open Ophthalmol. 2019, 4, e000326. [Google Scholar] [CrossRef] [PubMed]
- Gorusupudi, A.; Chang, F.Y.; Nelson, K.; Hageman, G.S.; Bernstein, P.S. n-3 PUFA Supplementation Alters Retinal Very-Long-Chain-PUFA Levels and Ratios in Diabetic Animal Models. Mol. Nutr. Food Res. 2019, 63, e1801058. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acid | g/100 g | |
|---|---|---|
| Palmitic | C16:0 | 0.47 |
| Palmitoleic | C16:1 n7 | 0.28 |
| Hexadecaenoic | C16:4 n1 | 0.09 |
| Stearic | C18:0 | 0.78 |
| Oleic | C18:1 n9 | 1.24 |
| Vaccenic | C18:1 n7 | 0.51 |
| Linoleic | C18:2 n6 | 0.19 |
| Linolenic | C18:3 n3 | 0.11 |
| Stearidonic | C18:4 n3 | 0.43 |
| Arachidic | C20:0 | 1.44 |
| Eicosenoic | C20:1 n9 | 2.78 |
| Gondonic | C20:1 n7 | 0.38 |
| Arachidonic | C20:4 n6 | 1.05 |
| Eicosatetraenoic | C20:4 n3 | 1.15 |
| Eicosapentaenoic | C20:5 n3 | 18.36 |
| Behenic | C22:0 | 1.44 |
| Erucic | C22:1 n11 | 2.78 |
| Adrenic | C22:4 n6 | 0.46 |
| Docosapentaenoic | C22:5 n6 | 0.90 |
| Docosapentaenoic | C22:5 n3 | 6.81 |
| Lignoceric | C24:0 | 0.44 |
| Docosahexaenoic | C22:6 n3 | 40.73 |
| Nervonic | C24:1 n9 | 2.17 |
| Total ω3 | 67.59 | |
| Total ω6 | 2.14 | |
| Total ω9 | 6.19 | |
| SFAs | 4.57 | |
| MUFAs | 10.14 | |
| PUFAs | 69.13 |
| Fatty Acid | mg/g of Body Weight |
|---|---|
| Palmitic | 0.013 |
| Palmitoleic | 0.008 |
| Hexadecaenoic | 0.003 |
| Stearic | 0.022 |
| Oleic | 0.035 |
| Vaccenic | 0.014 |
| Linoleic | 0.005 |
| Linolenic | 0.003 |
| Stearidonic | 0.012 |
| Arachidic | 0.041 |
| Eicosenoic | 0.078 |
| Gondonic | 0.011 |
| Arachidonic | 0.030 |
| Eicosatetraenoic | 0.032 |
| Eicosapentaenoic | 0.518 |
| Behenic | 0.041 |
| Erucic | 0.078 |
| Adrenic | 0.013 |
| Docosapentaenoic | 0.025 |
| Docosapentaenoic | 0.192 |
| Lignoceric | 0.012 |
| Docosahexaenoic | 1.149 |
| Nervonic | 0.061 |
| Total ω3 | 1.906 |
| Total ω6 | 0.060 |
| Total ω9 | 0.175 |
| SFAs | 0.129 |
| MUFAs | 0.286 |
| PUFAs | 1.949 |
| Age-Matched Comparisons 1 | Alox5 | Alox8 | Alox15 | Elovl4 |
|---|---|---|---|---|
| C-rd10 P13 | 0.0018 | 0.0013 | 0.1510 | 0.215 |
| C-rd10 P15 | 0.0006 | 0.6846 | 0.0003 | 0.0242 |
| C-rd10 P18 | 0.0315 | 0.0677 | 0.0001 | 0.0163 |
| C-rd10 P23 | <0.0001 | 0.0203 | <0.0001 | <0.0001 |
| C-rd10 P30 | 0.208 | <0.0001 | 0.4610 | <0.0001 |
| C-rd10 P44 | 0.9214 | <0.0001 | 0.0003 | <0.0001 |
| C-rd10 P60 | 0.1512 | 0.3495 | 0.0003 | <0.0001 |
| Iba1 + cells (%) | ONL Mean (SEM) | OPL Mean (SEM) | INL Mean (SEM) | IPL Mean (SEM) | GCL Mean (SEM) |
|---|---|---|---|---|---|
| C | 0.3 (0.3) | 25.5 (3.9) | 5.3 (3.8) | 44.3 (2.4) | 24.5 (2.1) |
| rd10 | 30.7 (2.8) | 18.9 (1.2) | 10.6 (1.9) | 18.8 (1.8) | 21.1 (1.0) |
| rd10 + EFA | 16.0 (3.5) | 26.7 (1.0) | 4.2 (1.5) | 26.0 (1.5) | 27.3 (3.8) |
| Marker | C Mean (SEM) | rd10 Mean (SEM) | rd10 + EFA Mean (SEM) |
|---|---|---|---|
| SOD activity | 1.93 (0.17) | 2.05 (0.2) | 1.67 (0.19) |
| CAT activity | 5.18 (0.53) | 7.22 (0.71) | 7.73 (0.43) * |
| TBARS | 0.35 (0.07) | 1.14 (0.19) *** | 0.38 (0.13) # |
| CAR | 3.78 (0.42) | 6.06 (0.29) *** | 3.55 (0.50) ## |
| Phenotypes | Stimuli | Surface Markers | Intracellular Markers | Functions |
|---|---|---|---|---|
| M1 | IFNγ, LPS, GM-CSF, TNFα | CXCL9, CD86, CD80, CD16/32 | IL6, TNFα, iNOS, IL12high/IL10low | Pro-inflammatory |
| M2a | IL4, IL13 | CD206, IL1-R | CCL17, IL10, ARG1 | Anti-inflammatory, repair and resolution |
| M2b | LPS+ immune complex, ILβ+ immune complex | CD86, CD80 | TNFα, IL6, iNOS, COX2, CCL1, IL10high/IL12low | Regulatory T cell recruitment |
| M2c | IL10, glucocorticoids | IL4R, CD206, CD163 | IL10, TGFβ, Arg1 | Immunoregulation, phagocytosis, tissue remodeling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-González, L.; Velasco, S.; Gallego, I.; Esteban-Medina, M.; Puras, G.; Loucera, C.; Martínez-Romero, A.; Peña-Chilet, M.; Pedraz, J.L.; Rodrigo, R. An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice. Antioxidants 2023, 12, 98. https://doi.org/10.3390/antiox12010098
Olivares-González L, Velasco S, Gallego I, Esteban-Medina M, Puras G, Loucera C, Martínez-Romero A, Peña-Chilet M, Pedraz JL, Rodrigo R. An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice. Antioxidants. 2023; 12(1):98. https://doi.org/10.3390/antiox12010098
Chicago/Turabian StyleOlivares-González, Lorena, Sheyla Velasco, Idoia Gallego, Marina Esteban-Medina, Gustavo Puras, Carlos Loucera, Alicia Martínez-Romero, María Peña-Chilet, José Luis Pedraz, and Regina Rodrigo. 2023. "An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice" Antioxidants 12, no. 1: 98. https://doi.org/10.3390/antiox12010098
APA StyleOlivares-González, L., Velasco, S., Gallego, I., Esteban-Medina, M., Puras, G., Loucera, C., Martínez-Romero, A., Peña-Chilet, M., Pedraz, J. L., & Rodrigo, R. (2023). An SPM-Enriched Marine Oil Supplement Shifted Microglia Polarization toward M2, Ameliorating Retinal Degeneration in rd10 Mice. Antioxidants, 12(1), 98. https://doi.org/10.3390/antiox12010098








