The Effects of Flavonol and Flavone Glucuronides from Potentilla chinensis Leaves on TNF-α-Exposed Normal Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability
2.3. Intracellular ROS Generation Assay
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Western Blotting
2.6. Statistical Analysis
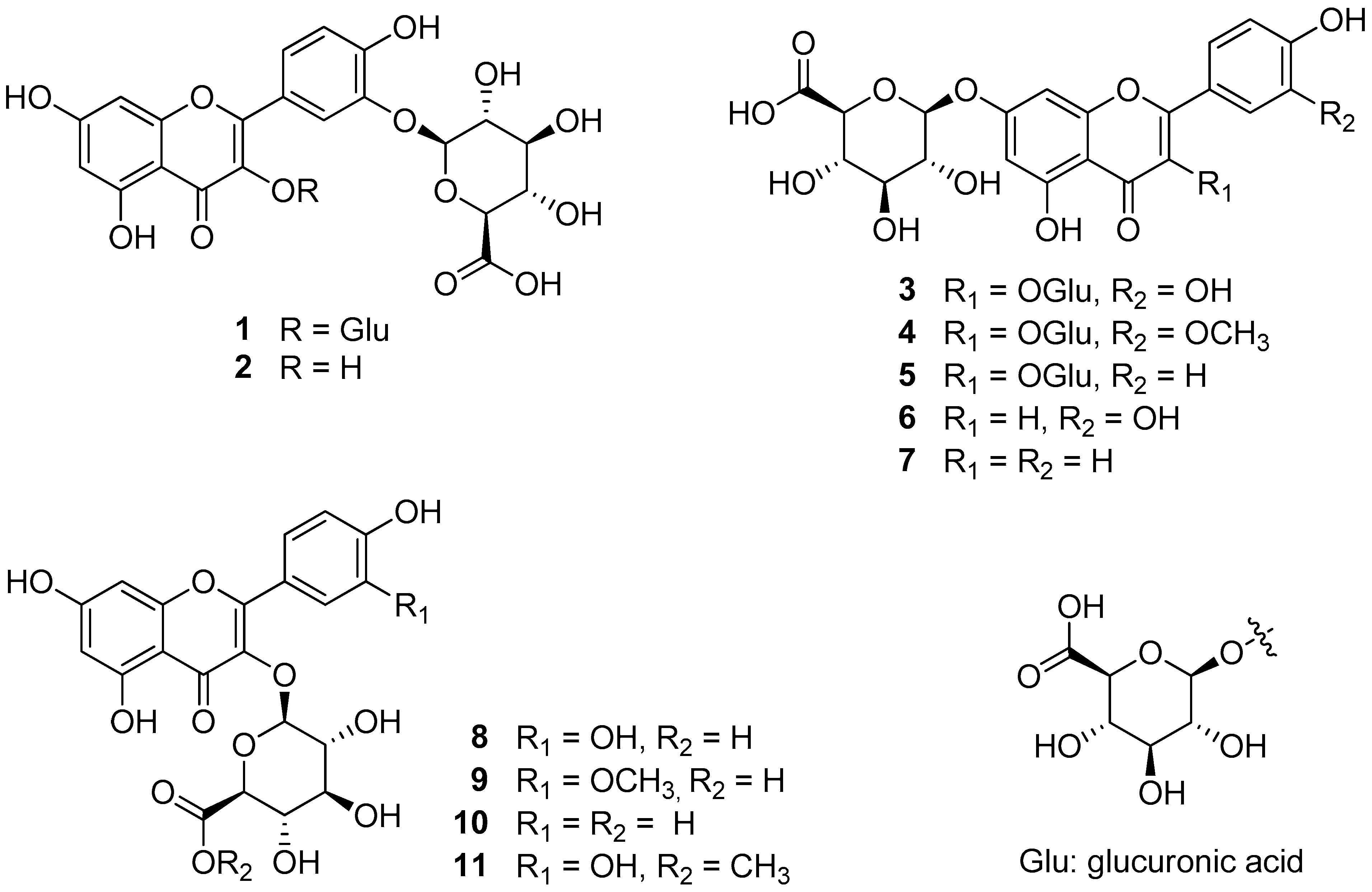
2.7. Sample Preparation
3. Results
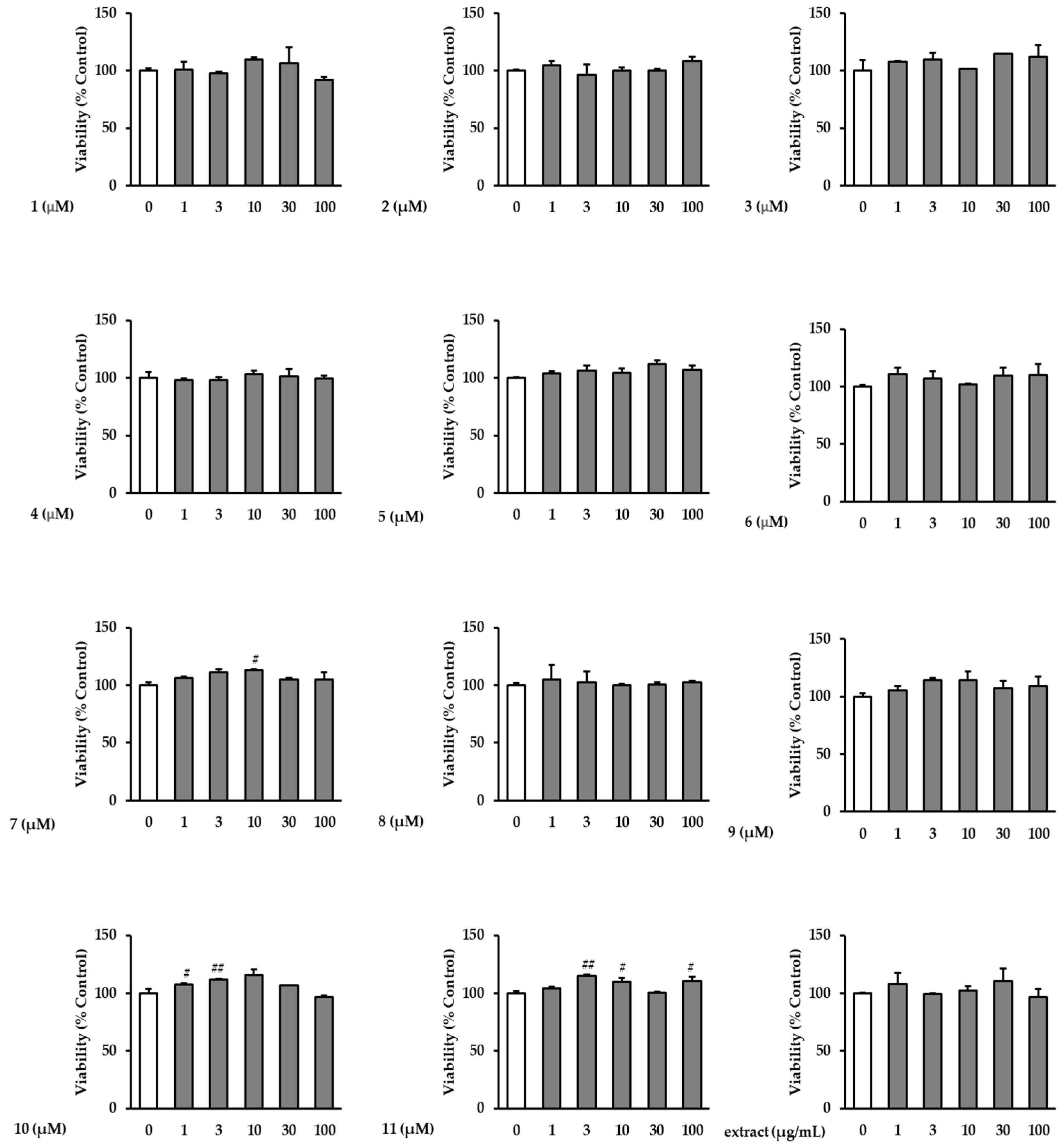
3.1. Effects of the Extract and Isolates on NHDF Viability
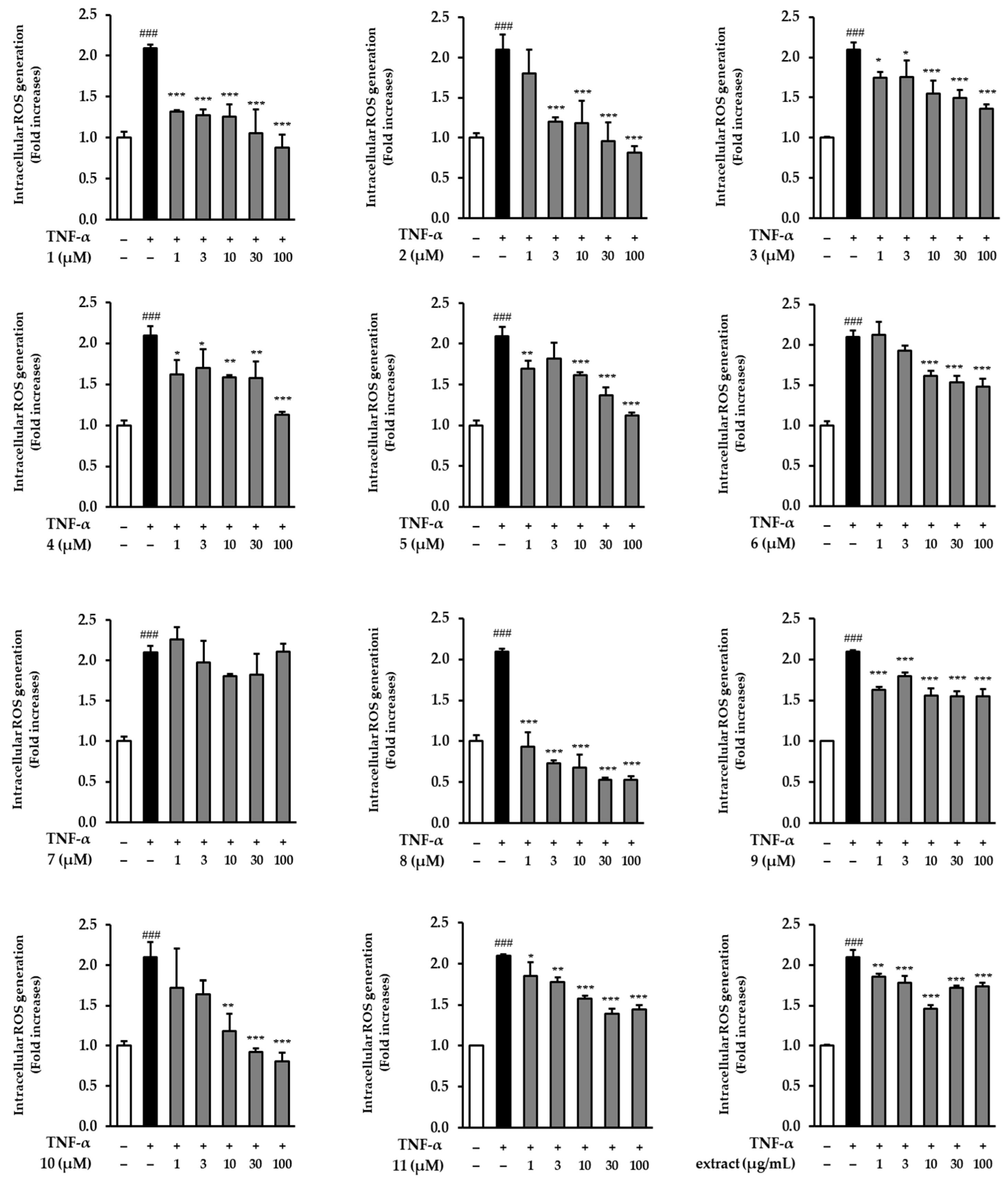
3.2. Effects of the Extract and Isolates on Intracellular ROS Generation in TNF-α-Stimulated NHDFs
3.3. Effects of the Extract and Isolates on MMP-1 Secretion in TNF-α-Stimulated NHDFs
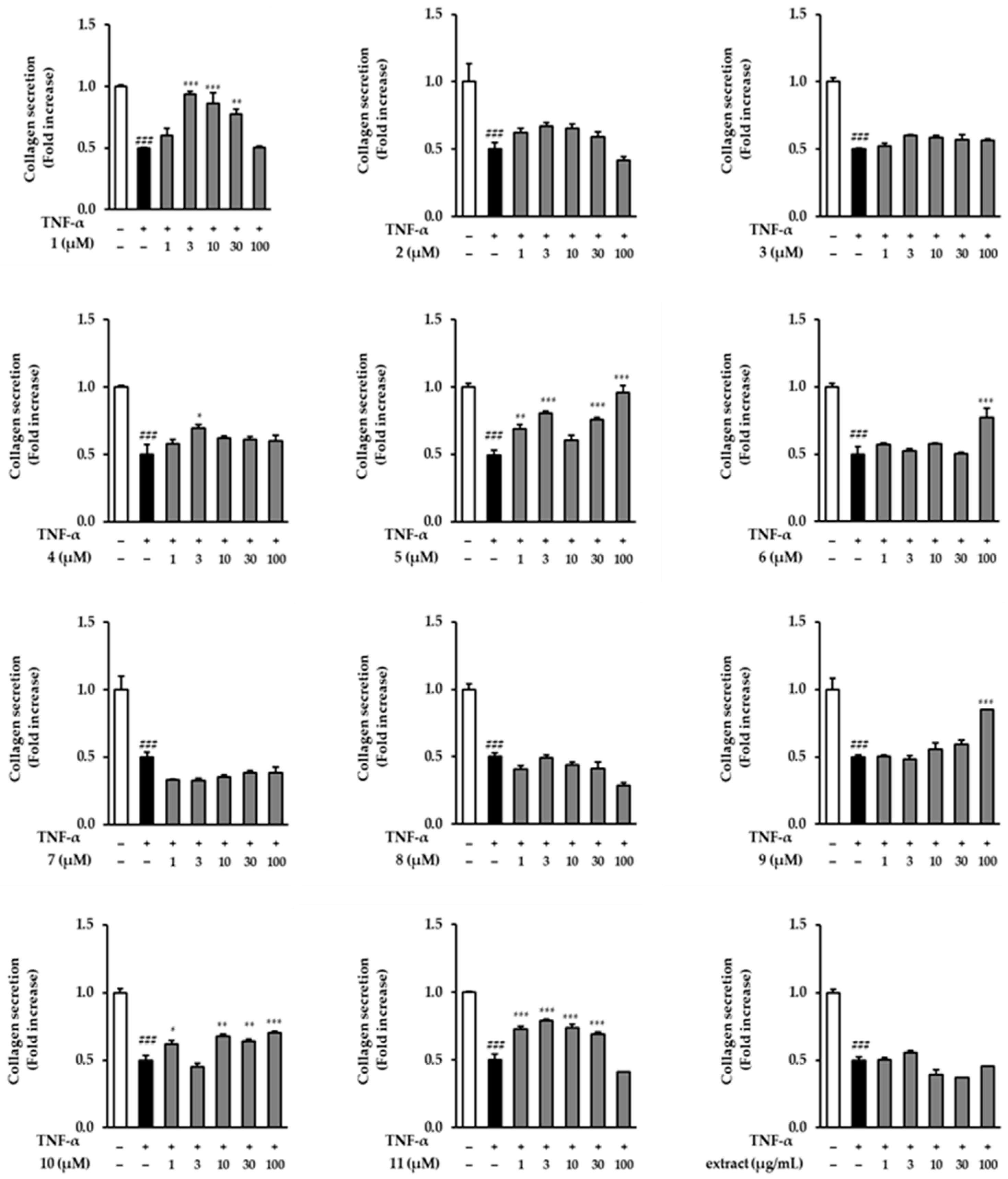
3.4. Effects of the Extract and Isolates on COLIA1 Secretion in TNF-α-Stimulated NHDFs
3.5. Spider Chart for Efficiency Comparison of the Extract and Isolates in TNF-α-Stimulated NHDFs
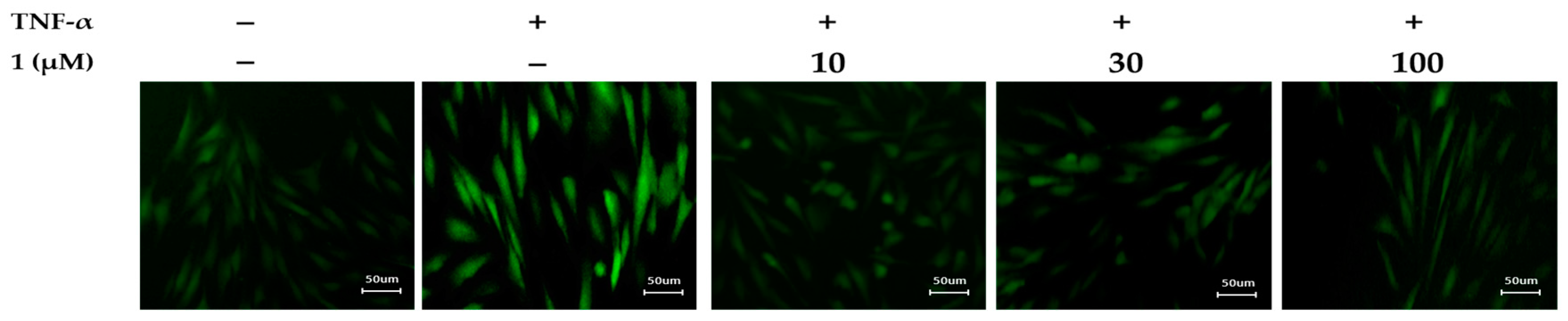
3.6. Effects of Potentilloside A (1) on Intracellular ROS Generation in TNF-α-Stimulated NHDFs
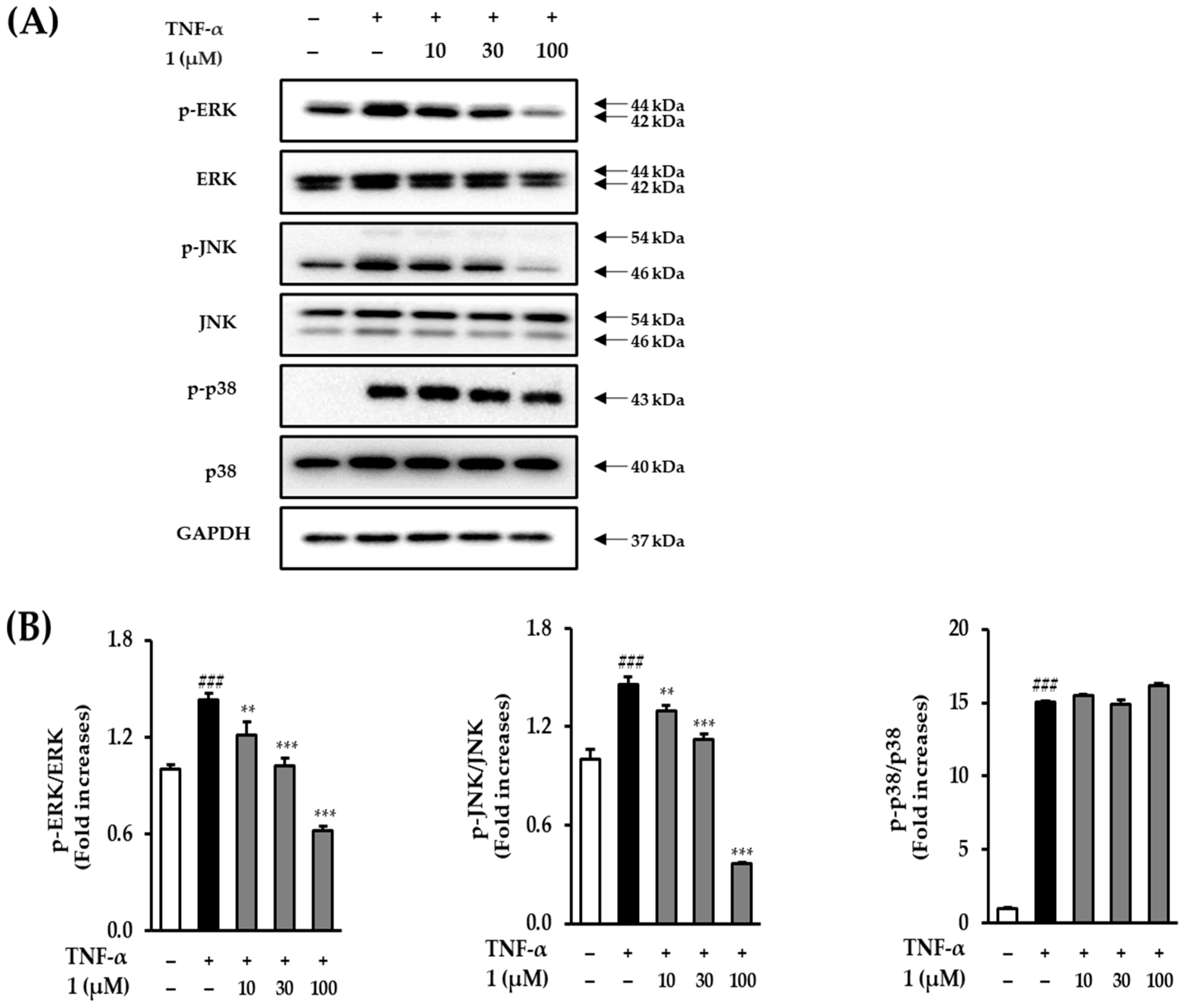
3.7. Effect of Potentilloside A (1) on MAPK Phosphorylation in TNF-α-Stimulated NHDFs
3.8. Effect of Potentilloside A (1) on NF-κB and c-Jun Phosphorylation in TNF-α-Stimulated NHDFs
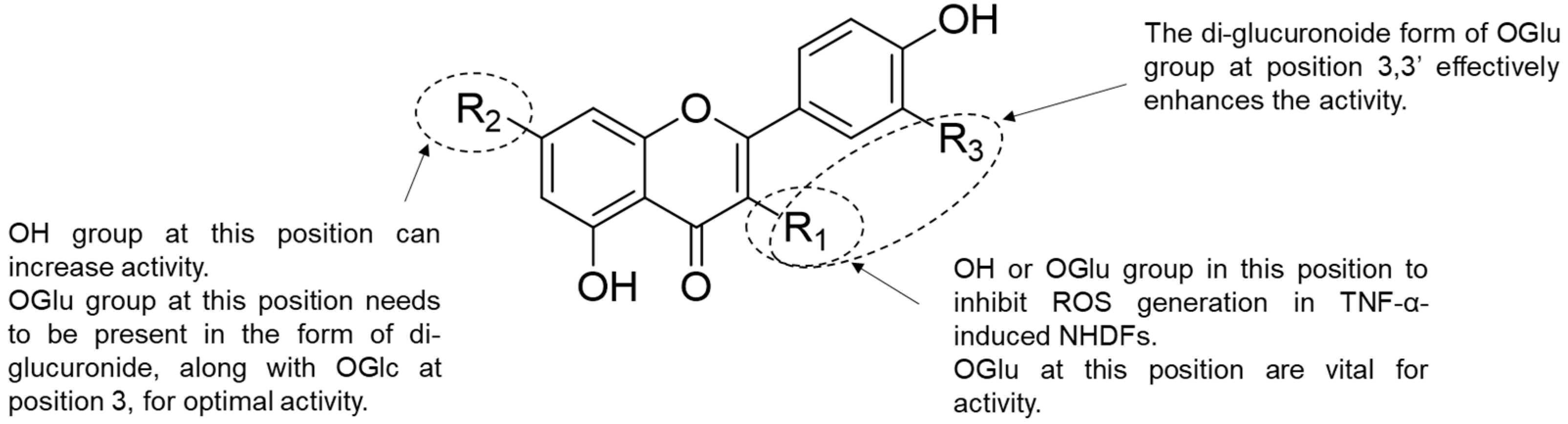
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keaney, T.C. Aging in the male face: Intrinsic and extrinsic factors. Dermatol. Surg. 2016, 42, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Sjerobabski-Masnec, I.; Situm, M. Skin aging. Acta Clin. Croat. 2010, 49, 515–518. [Google Scholar] [PubMed]
- Lee, K.-J.; Park, K.H.; Hahn, J.-H. Alleviation of ultraviolet-B radiation-induced photoaging by a TNFR antagonistic peptide, TNFR2-SKE. Mol. Cells 2019, 42, 151. [Google Scholar] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.J.; Lee, S.; Kang, K.S.; Jang, T.S.; Kim, K.H. Protective effects of withagenin A diglucoside from Indian ginseng (withania somnifera) against human dermal fibroblast damaged by TNF-α stimulation. Antioxidants 2022, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Leirós, G.J.; Kusinsky, A.G.; Balañá, M.E.; Hagelin, K. Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2017, 85, 124–130. [Google Scholar] [CrossRef]
- Starodubtseva, N.; Sobolev, V.; Soboleva, A.; Nikolaev, A.; Bruskin, S. Genes expression of metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) associated with psoriasis. Russ. J. Genet. 2011, 47, 1117–1123. [Google Scholar] [CrossRef]
- Ozkanli, S.; Karadag, A.S.; Ozlu, E.; Uzuncakmak, T.K.; Takci, Z.; Zemheri, E.; Zindancı, I.; Akdeniz, N. A comparative study of MMP-1, MMP-2, and TNF-α expression in different acne vulgaris lesions. Int. J. Dermatol. 2016, 55, 1402–1407. [Google Scholar] [CrossRef]
- Harper, J.; Godwin, H.; Green, A.; Wilkes, L.; Holden, N.; Moffatt, M.; Cookson, W.; Layton, G.; Chandler, S. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br. J. Dermatol. 2010, 162, 397–403. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.-S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.-H. Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-κβ and stimulation of TGF-β/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 185, 241–253. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.K.; Kim, J.K.; Nam, J.I.; So, U.-K.; Oh, C.H.; Jeon, H. Antioxidant and anti-inflammatory effect of the methanolic extracts of Potentilla chinensis. Orient. Pharm. Exp. Med. 2011, 11, 137–142. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, Y.J.; Son, S.-R.; Yoon, Y.-S.; Lee, S.-H.; Lee, K.-T.; Lee, S.; Jang, D.S. Potentilloside A, a New Flavonol-Bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion. Plants 2022, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Schauber, J. Skin: Architecture and function. In Dermal Replacements in General, Burn, and Plastic Surgery: Tissue Engineering in Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–11. [Google Scholar]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.; Watkinson, A.C. Skin structure, function, and permeation. In Topical and Transdermal Drug Delivery: Principles and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–22. [Google Scholar]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Zheng, P.; Dong, G. Aged skin: A study by light, transmission electron, and scanning electron microscopy. J. Investig. Dermatol. 1987, 88, 44–51. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360. [Google Scholar] [CrossRef]
- English, J.; Dawe, R.; Ferguson, J. Environmental effects and skin disease. Br. Med. Bull. 2003, 68, 129–142. [Google Scholar] [CrossRef]
- Flament, F.; Bazin, R.; Laquieze, S.; Rubert, V.; Simonpietri, E.; Piot, B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.P.; Lee, T.K. Adverse effects of ultraviolet radiation: A brief review. Prog. Biophys. Mol. Biol. 2006, 92, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, Y.J.; Jang, D.S.; Lee, S. 2-O-β-d-Glucopyranosyl-4, 6-dihydroxybenzaldehyde Isolated from Morus alba (Mulberry) Fruits Suppresses Damage by Regulating Oxidative and Inflammatory Responses in TNF-α-Induced Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 14802. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Brincat, S.; Camilleri, G.; Schembri-Wismayer, P.; Brincat, M.; Calleja-Agius, J. The role of cytokines in skin aging. Climacteric 2013, 16, 514–521. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, D.; Patel, S.; Singh, M.R. Role of enzymatic free radical scavengers in management of oxidative stress in autoimmune disorders. Int. J. Biol. Macromol. 2017, 101, 502–517. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, J.; Karadeniz, F.; Kim, H.R.; Park, S.Y.; Seo, Y.; Kong, C.-S. Santamarine shows anti-photoaging properties via inhibition of MAPK/AP-1 and stimulation of TGF-β/smad signaling in UVA-irradiated HDFs. Molecules 2021, 26, 3585. [Google Scholar] [CrossRef]
- Novotná, R.; Škařupová, D.; Hanyk, J.; Ulrichová, J.; Křen, V.; Bojarová, P.; Brodsky, K.; Vostálová, J.; Franková, J. Hesperidin, Hesperetin, Rutinose, and Rhamnose Act as Skin Anti-Aging Agents. Molecules 2023, 28, 1728. [Google Scholar] [CrossRef]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lin, Y.-T.; Kuo, H.-C.; Chiou, W.-F.; Lee, M.-H. Compounds isolated from Eriobotrya deflexa leaves protect against ultraviolet radiation B-induced photoaging in human fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 175, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective effect of polymethoxyflavones isolated from Kaempferia parviflora against TNF-α-induced human dermal fibroblast damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.; Yi, T.-H. Prunella vulgaris L. exerts a protective effect against extrinsic aging through NF-κB, MAPKs, AP-1, and TGF-β/Smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits UVB and Advanced Glycation End Products-Induced MMP-1 Expression by Suppressing the MAPK and NF-κB Pathways in HaCaT Cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Anti-inflammatory, antioxidant, moisturizing, and antimelanogenesis effects of quercetin 3-O-β-D-glucuronide in human keratinocytes and melanoma cells via activation of NF-κB and AP-1 pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef]
- Lee, S.; Hoang, G.D.; Kim, D.; Song, H.S.; Choi, S.; Lee, D.; Kang, K.S. Efficacy of alpinumisoflavone isolated from maclura tricuspidata fruit in tumor necrosis factor-α-induced damage of human dermal fibroblasts. Antioxidants 2021, 10, 514. [Google Scholar] [CrossRef]
- Nisar, M.F.; Liu, T.; Wang, M.; Chen, S.; Chang, L.; Karisma, V.W.; Diao, Q.; Xue, M.; Tang, X.; Pourzand, C. Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B: Biol. 2022, 226, 112350. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Lee, S.Y.; Son, S.-R.; Park, J.Y.; Jang, D.S.; Lee, S. The Effects of Flavonol and Flavone Glucuronides from Potentilla chinensis Leaves on TNF-α-Exposed Normal Human Dermal Fibroblasts. Antioxidants 2023, 12, 1803. https://doi.org/10.3390/antiox12101803
Choi YJ, Lee SY, Son S-R, Park JY, Jang DS, Lee S. The Effects of Flavonol and Flavone Glucuronides from Potentilla chinensis Leaves on TNF-α-Exposed Normal Human Dermal Fibroblasts. Antioxidants. 2023; 12(10):1803. https://doi.org/10.3390/antiox12101803
Chicago/Turabian StyleChoi, Yea Jung, So Young Lee, So-Ri Son, Jun Yeon Park, Dae Sik Jang, and Sullim Lee. 2023. "The Effects of Flavonol and Flavone Glucuronides from Potentilla chinensis Leaves on TNF-α-Exposed Normal Human Dermal Fibroblasts" Antioxidants 12, no. 10: 1803. https://doi.org/10.3390/antiox12101803
APA StyleChoi, Y. J., Lee, S. Y., Son, S.-R., Park, J. Y., Jang, D. S., & Lee, S. (2023). The Effects of Flavonol and Flavone Glucuronides from Potentilla chinensis Leaves on TNF-α-Exposed Normal Human Dermal Fibroblasts. Antioxidants, 12(10), 1803. https://doi.org/10.3390/antiox12101803






