αB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress †
Abstract
:1. Introduction
2. Materials and Methods
2.1. ELPs Cloning, Expression, Production, and Concentration Assessment
2.2. ELP Purity
2.3. Exact Mass Determination
2.4. Rhodamine Labeling
2.5. Transition Temperature
2.6. Dynamic Light Scattering
2.7. Chaperone Activity Assay
2.8. Cell Culture of ARPE-19 under Hydrogen Peroxide Challenge Using TUNEL, Viability, and Immunofluorescence Assays
2.9. Intracellular Localization of cry-ELPs in ARPE-19 Cells under the H2O2 Challenge
2.10. 3D Spheroid Culture
2.11. Lysosomal Trafficking of ELPs
2.12. ELP and Dextran Uptake Assays
2.13. Statistical Methods
3. Results
3.1. Purification and Characterization of ELP Constructs
3.2. Chaperone Activity of cry-ELPs
3.3. Exogenous cry-ELPs Protect ARPE-19 Cells against Oxidative Stress
3.4. cry-ELPs Are Translocated to the Nucleus under Oxidative Stress
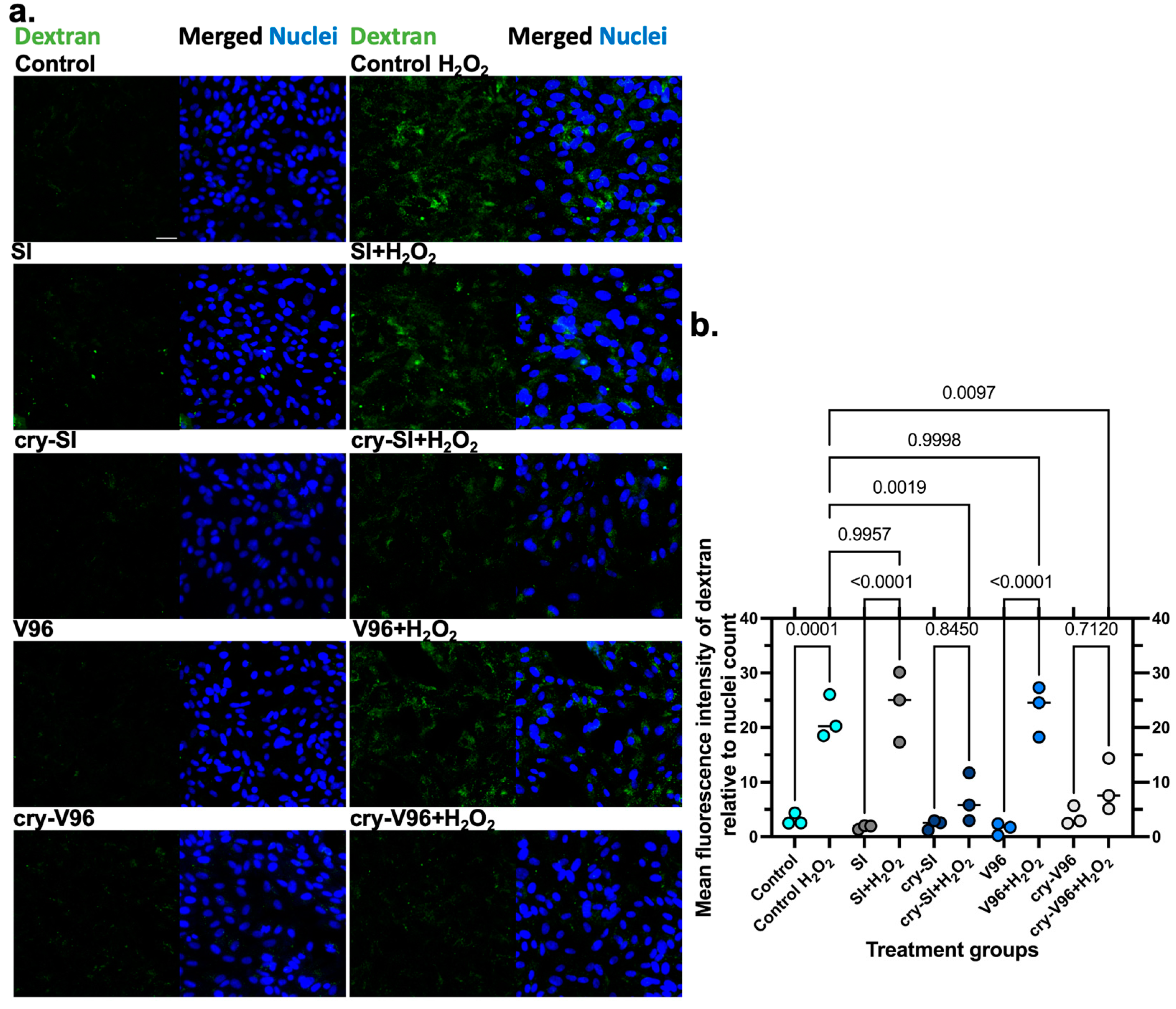
3.5. cry-ELPs Protect the ARPE-19 against Dextran Release
3.6. cry-ELP Colocalization with Lysosomes Decreases under Oxidative Stress
3.7. cry-ELPs Colocalize with Dextran
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Martin, P.M. Oxidative Stress and Inflammation in Retinal Degeneration. Antioxidants 2021, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxid. Med. Cell Longev. 2017, 2017, 9208489. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zou, H. Effects of air pollution on myopia: An update on clinical evidence and biological mechanisms. Environ. Sci. Pollut. Res. 2022, 29, 70674–70685. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.H.; Schwartz, S.S. Diabetic Retinopathy—An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front. Endocrinol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Chen, X.; Rong, S.S.; Xu, Q.; Tang, F.Y.; Liu, Y.; Gu, H.; Tam, P.O.; Chen, L.J.; Brelén, M.E.; Pang, C.P.; et al. Diabetes mellitus and risk of age-related macular degeneration: A systematic review and meta-analysis. PLoS ONE 2014, 9, e108196. [Google Scholar] [CrossRef]
- Churney, T.; Patnaik, J.; Holguin, F.; Mathias, M.; Siringo, F.; Palestine, A.G.; Lynch, A.; Mandava, N. The Relationship of Chronic Lung Disease with Age-Related Macular Degeneration in a Colorado Cohort. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4181. [Google Scholar]
- Kang, E.Y.; Liu, P.K.; Wen, Y.T.; Quinn, P.M.J.; Levi, S.R.; Wang, N.K.; Tsai, R.K. Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration. Antioxidants 2021, 10, 1948. [Google Scholar] [CrossRef]
- Shu, D.Y.; Chaudhary, S.; Cho, K.-S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Lajunen, T.; Bhattacharya, M.; Reinisalo, M.; Rilla, K.; Kidron, H.; Terasaki, T.; Urtti, A. Selective drug delivery to the retinal cells: Biological barriers and avenues. J. Control. Release 2023, 361, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.C.; Kelley, R.F.; Tesar, D.B. Protein conjugates and fusion proteins as ocular therapeutics. Drug Discov. Today 2019, 24, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sreekumar, P.G.; Valluripalli, V.; Shi, P.; Wang, J.; Lin, Y.A.; Cui, H.; Kannan, R.; Hinton, D.R.; MacKay, J.A. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J. Control. Release 2014, 191, 4–14. [Google Scholar] [CrossRef]
- Ou, K.; Li, Y.; Liu, L.; Li, H.; Cox, K.; Wu, J.; Liu, J.; Dick, A.D. Recent developments of neuroprotective agents for degenerative retinal disorders. Neural Regen. Res. 2022, 17, 1919–1928. [Google Scholar]
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Wang, K.; Spector, A. Alpha-crystallin can act as a chaperone under conditions of oxidative stress. Investig. Ophthalmol. Vis. Sci. 1995, 36, 311–321. [Google Scholar]
- Kannan, R.; Sreekumar, P.; Hinton, D. Novel roles for α-crystallins in retinal function and disease. Prog. Retin. Eye Res. 2012, 31, 576–604. [Google Scholar] [CrossRef]
- Christopher, K.L.; Pedler, M.G.; Shieh, B.; Ammar, D.A.; Petrash, J.M.; Mueller, N.H. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 309–315. [Google Scholar] [CrossRef]
- Yaung, J.; Jin, M.; Barron, E.; Spee, C.; Wawrousek, E.F.; Kannan, R.; Hinton, D.R. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol. Vis. 2007, 13, 566–577. [Google Scholar]
- Wang, T.; Yao, J.; Jia, L.; Fort, P.E.; Zacks, D.N. Loss of αA or αB-Crystallin Accelerates Photoreceptor Cell Death in a Mouse Model of P23H Autosomal Dominant Retinitis Pigmentosa. Int. J. Mol. Sci. 2022, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; MacRae, T.H. The small heat shock proteins and their role in human disease. FEBS J. 2005, 272, 2613–2627. [Google Scholar] [CrossRef]
- Phadte, A.S.; Sluzala, Z.B.; Fort, P.E. Therapeutic Potential of α-Crystallins in Retinal Neurodegenerative Diseases. Antioxidants 2021, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Smulders, R.; Carver, J.A.; Lindner, R.A.; van Boekel, M.A.; Bloemendal, H.; de Jong, W.W. Immobilization of the C-terminal extension of bovine alphaA-crystallin reduces chaperone-like activity. J. Biol. Chem. 1996, 271, 29060–29066. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Padmanabha Udupa, E.G.; Wang, J.; Sharma, K.K. Mini-αB-Crystallin: A Functional Element of αB-Crystallin with Chaperone-like Activity. Biochemistry 2006, 45, 3069–3076. [Google Scholar] [CrossRef]
- Sharma, K.K.; Kumar, R.S.; Kumar, G.S.; Quinn, P.T. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J. Biol. Chem. 2000, 275, 3767–3771. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Santhoshkumar, P.; Sharma, K.K. αA-Crystallin–Derived Mini-Chaperone Modulates Stability and Function of Cataract Causing αAG98R-Crystallin. PLoS ONE 2012, 7, e44077. [Google Scholar] [CrossRef]
- Christensen, T.; Hassouneh, W.; Trabbic-Carlson, K.; Chilkoti, A. Predicting Transition Temperatures of Elastin-Like Polypeptide Fusion Proteins. Biomacromolecules 2013, 14, 1514–1519. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Hnatuszko-Konka, K.; Gerszberg, A.; Kononowicz, A.K. Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World J. Microbiol. Biotechnol. 2014, 30, 2141–2152. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. Applications of elastin-like polypeptides in drug delivery. J. Control. Release 2014, 190, 314–330. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Li, Z.; Wang, W.; Spee, C.; Hinton, D.R.; Kannan, R.; MacKay, J.A. Intra-vitreal αB crystallin fused to elastin-like polypeptide provides neuroprotection in a mouse model of age-related macular degeneration. J. Control. Release 2018, 283, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Liu, S.; Park, R.; Pastuszka, M.K.; Shi, P.; Moses, A.S.; Orosco, M.M.; Lin, Y.A.; Cui, H.; Conti, P.S.; et al. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integr. Biol. 2013, 5, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, A.; Yao, Z.; Zheng, L.; Li, Z.; Hu, P.; Epstein, A.L.; MacKay, J.A. Generation of a Monoclonal Antibody to Detect Elastin-like Polypeptides. Biomacromolecules 2019, 20, 2942–2952. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Hsueh, P.-Y.; Janib, S.M.; Hamm-Alvarez, S.; MacKay, J.A. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J. Control. Release 2011, 155, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dabbaghizadeh, A.; Finet, S.; Morrow, G.; Moutaoufik, M.T.; Tanguay, R.M. Oligomeric structure and chaperone-like activity of Drosophila melanogaster mitochondrial small heat shock protein Hsp22 and arginine mutants in the alpha-crystallin domain. Cell Stress Chaperones 2017, 22, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Roman, S.G.; Borzova, V.A.; Eronina, T.B.; Mikhaylova, V.V.; Kurganov, B.I. Chaperone-Like Activity of HSPB5: The Effects of Quaternary Structure Dynamics and Crowding. Int. J. Mol. Sci. 2020, 21, 4940. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, S.P.; Klevit, R.E. HSPB5 engages multiple states of a destabilized client to enhance chaperone activity in a stress-dependent manner. J. Biol. Chem. 2019, 294, 3261–3270. [Google Scholar] [CrossRef]
- Michiel, M.; Duprat, E.; Skouri-Panet, F.; Lampi, J.A.; Tardieu, A.; Lampi, K.J.; Finet, S. Aggregation of deamidated human betaB2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp. Eye Res. 2010, 90, 688–698. [Google Scholar] [CrossRef]
- Cordes, C.M.; Bennett, R.G.; Siford, G.L.; Hamel, F.G. Redox regulation of insulin degradation by insulin-degrading enzyme. PLoS ONE 2011, 6, e18138. [Google Scholar] [CrossRef]
- Cia, D.; Jacquemot, N.; Cluzel, J.; Rossary, A.; Doly, M. Effects of Hydrogen Peroxide (H2O2) On Retinal Function In Vitro. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5371. [Google Scholar]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar]
- Helander, S.D.; Rogers, R.S., 3rd. The sensitivity and specificity of direct immunofluorescence testing in disorders of mucous membranes. J. Am. Acad. Dermatol. 1994, 30, 65–75. [Google Scholar] [CrossRef]
- Sorrells, S.; Toruno, C.; Stewart, R.A.; Jette, C. Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated Caspase 3. J. Vis. Exp. 2013, 82, 51060. [Google Scholar]
- Kose, S.; Furuta, M.; Imamoto, N. Hikeshi, a nuclear import carrier for Hsp70s, protects cells from heat shock-induced nuclear damage. Cell 2012, 149, 578–589. [Google Scholar] [CrossRef]
- den Engelsman, J.; van de Schootbrugge, C.; Yong, J.; Pruijn, G.J.; Boelens, W.C. Pseudophosphorylated αB-crystallin is a nuclear chaperone imported into the nucleus with help of the SMN complex. PLoS ONE 2013, 8, e73489. [Google Scholar] [CrossRef]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef]
- Boutin, M.; Hampton, C.; Quinn, R.; Ferrer, M.; Song, M.J. 3D Engineering of Ocular Tissues for Disease Modeling and Drug Testing. In Pluripotent Stem Cells in Eye Disease Therapy; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1186, pp. 171–193. [Google Scholar]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Fennell, D.F.; Whatley, R.E.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. Endothelial cells reestablish functional integrity after reversible permeabilization. Arterioscler. Thromb. 1991, 11, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Paulus, H. Studies on ribonucleoside-diphosphate reductase in permeable animal cells: I. Reversible permeabilization of mouse L cells with dextran sulfate. Arch. Biochem. Biophys. 1982, 214, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, Z.; Lou, B.; Duan, F.; Qiu, S.; Cheng, Z.; Ma, X.; Yang, Y.; Lin, X. αB-Crystallin Alleviates Endotoxin-Induced Retinal Inflammation and Inhibits Microglial Activation and Autophagy. Front. Immunol. 2021, 12, 641999. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Kesterson, R.A.; Srivastava, O.P. CRYβA3/A1-Crystallin Knockout Develops Nuclear Cataract and Causes Impaired Lysosomal Cargo Clearance and Calpain Activation. PLoS ONE 2016, 11, e0149027. [Google Scholar] [CrossRef] [PubMed]
- Barral, D.C.; Staiano, L.; Guimas Almeida, C.; Cutler, D.F.; Eden, E.R.; Futter, C.E.; Galione, A.; Marques, A.R.A.; Medina, D.L.; Napolitano, G.; et al. Current methods to analyze lysosome morphology, positioning, motility and function. Traffic 2022, 23, 238–269. [Google Scholar] [CrossRef]
- Chen, J.W.; Murphy, T.L.; Willingham, M.C.; Pastan, I.; August, J.T. Identification of two lysosomal membrane glycoproteins. J. Cell Biol. 1985, 101, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Yaung, J.; Kannan, R.; Wawrousek, E.F.; Spee, C.; Sreekumar, P.G.; Hinton, D.R. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp. Eye Res. 2008, 86, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, Y.; Kwong, J.M.K.; Caprioli, J.; Piri, N. The Role of αA- and αB-Crystallins in the Survival of Retinal Ganglion Cells after Optic Nerve Axotomy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Maity, N.; Konar, A. Healing Potency of Cryab Minipeptides Following Corneal Lamellar Flap Surgery Demonstrated, In Vitro and In Vivo In Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2023, 64, 1882. [Google Scholar]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef]
- Attia, S.A.; MacKay, J.A. Protein and polypeptide mediated delivery to the eye. Adv. Drug Deliv. Rev. 2022, 188, 114441. [Google Scholar] [CrossRef]
- Haendeler, J.; Tischler, V.; Hoffmann, J.; Zeiher, A.M.; Dimmeler, S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004, 577, 427–433. [Google Scholar] [CrossRef]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFκB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017, 12, e0171940. [Google Scholar] [CrossRef] [PubMed]
- Van Rijk, A.E.; Stege, G.J.; Bennink, E.J.; May, A.; Bloemendal, H. Nuclear staining for the small heat shock protein alphaB-crystallin colocalizes with splicing factor SC35. Eur. J. Cell Biol. 2003, 82, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Rho, J.H.; Yoon, Y.G.; Yoo, S.H.; Jeong, N.Y.; Ryu, W.Y.; Ahn, H.B.; Park, W.C.; Rho, S.H.; Yoon, H.S.; et al. Cytoplasmic and nuclear anti-apoptotic roles of αB-crystallin in retinal pigment epithelial cells. PLoS ONE 2012, 7, e45754. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.H.; Ammar, D.A.; Petrash, J.M. Cell penetration peptides for enhanced entry of αB-crystallin into lens cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Lee, S.M.; Choi, E.Y.; Lee, K.H.; Kim, S.H.; Shin, M.J.; Han, Y.S.; Kang, S.M.; Chung, J.H. Matrix metalloproteinase-1 induces cleavage of exogenous alphaB-crystallin transduced by a cell-penetrating peptide. J. Cell. Biochem. 2011, 112, 2454–2462. [Google Scholar] [CrossRef]
- Lam, L.A.; Mehta, S.; Lad, E.M.; Emerson, G.G.; Jumper, J.M.; Awh, C.C. Intravitreal Injection Therapy: Current Techniques and Supplemental Services. J. Vitreoretin. Dis. 2021, 5, 438–447. [Google Scholar] [CrossRef]
- Shah, M.; Hsueh, P.Y.; Sun, G.; Chang, H.Y.; Janib, S.M.; MacKay, J.A. Biodegradation of elastin-like polypeptide nanoparticles. Protein Sci. 2012, 21, 743–750. [Google Scholar] [CrossRef]
- Massodi, I.; Bidwell, G.L.; Raucher, D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J. Control. Release 2005, 108, 396–408. [Google Scholar] [CrossRef]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. J. Control. Release 2016, 232, 238–247. [Google Scholar] [CrossRef]
- de Vrueh, R.L.; Smith, P.L.; Lee, C.P. Transport of L-valine-acyclovir via the oligopeptide transporter in the human intestinal cell line, Caco-2. J. Pharmacol. Exp. Ther. 1998, 286, 1166–1170. [Google Scholar]
- Jain, A.; Jain, S.K. L-Valine appended PLGA nanoparticles for oral insulin delivery. Acta Diabetol. 2015, 52, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tian, C.; Wang, M.; Huang, D.; Wei, W.; Liu, Y.; Li, L.; Sun, B.; Kou, L.; Kan, Q.; et al. Dipeptide-modified nanoparticles to facilitate oral docetaxel delivery: New insights into PepT1-mediated targeting strategy. Drug Deliv. 2018, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, B.; Chemin, C.; Moreau, A.; Arnauld, T.; Baumy, P.; Cisternino, S.; Péan, J.-M.; Declèves, X. Functionalized PLA-PEG nanoparticles targeting intestinal transporter PepT1 for oral delivery of acyclovir. Int. J. Pharm. 2017, 529, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.Y.; Shim, W.S.; Chang, J.E.; Chong, S.; Kim, D.D.; Chung, S.J.; Shim, C.K. Enhanced intracellular accumulation of a non-nucleoside anti-cancer agent via increased uptake of its valine ester prodrug through amino acid transporters. Xenobiotica 2012, 42, 603–613. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Chothe, P.; Sharma, K.K.; Baid, R.; Kompella, U.; Spee, C.; Kannan, N.; Manh, C.; Ryan, S.J.; Ganapathy, V.; et al. Antiapoptotic Properties of α-Crystallin–Derived Peptide Chaperones and Characterization of Their Uptake Transporters in Human RPE Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- van Strien, J.; Escalona-Rayo, O.; Jiskoot, W.; Slütter, B.; Kros, A. Elastin-like polypeptide-based micelles as a promising platform in nanomedicine. J. Control. Release 2023, 353, 713–726. [Google Scholar] [CrossRef]
- Dzuricky, M.; Xiong, S.; Weber, P.; Chilkoti, A. Avidity and Cell Uptake of Integrin-Targeting Polypeptide Micelles is Strongly Shape-Dependent. Nano Lett. 2019, 19, 6124–6132. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid. Redox Signal. 2009, 11, 481–496. [Google Scholar] [CrossRef]
- Zigler, J.S., Jr.; Sinha, D. βA3/A1-crystallin: More than a lens protein. Prog. Retin. Eye Res. 2015, 44, 62–85. [Google Scholar] [CrossRef]
- Valapala, M.; Sergeev, Y.; Wawrousek, E.; Hose, S.; Zigler, J.S., Jr.; Sinha, D. Modulation of V-ATPase by βA3/A1-Crystallin in Retinal Pigment Epithelial Cells. Adv. Exp. Med. Biol. 2016, 854, 779–784. [Google Scholar]
- Pei, J.; Wang, G.; Feng, L.; Zhang, J.; Jiang, T.; Sun, Q.; Ouyang, L. Targeting Lysosomal Degradation Pathways: New Strategies and Techniques for Drug Discovery. J. Med. Chem. 2021, 64, 3493–3507. [Google Scholar] [CrossRef] [PubMed]






| Peptide Nomenclature | Amino Acid Sequence a | Expected MW b (kDa) | Observed MW c (kDa) | Tt, Intercept d, b, (°C) | Tt, Slope e, m, (°C/Log (μM)) |
|---|---|---|---|---|---|
SI | MG(VPGSG)48(VPGIG)48Y | 39.6 | 39.7 | 77.3 | 4.2 |
| [69.9 to 84.7] | [1.5 to 9.9] | ||||
cry-SI | MGDRFSVNLDVKHFSPEELKVK G(VPGSG)48(VPGIG)48Y | 42.1 | 42.0 | 30.1 | 3.4 |
| [26.7 to 33.5] | [0.03 to 6.8] | ||||
V96 | MG(VPGVG)96Y | 39.6 | 39.6 | 36.7 | 4.0 |
| [35.7 to 37.7] | [3.0 to 5.0] | ||||
cry-V96 | MGDRFSVNLDVKHFSPEELKVK G(VPGVG)96Y | 42.0 | 42.0 | 35.2 | 4.3 |
| [34.5 to 36] | [3.5 to 5.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, S.A.; Truong, A.T.; Phan, A.; Lee, S.-J.; Abanmai, M.; Markanovic, M.; Avila, H.; Luo, H.; Ali, A.; Sreekumar, P.G.; et al. αB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress. Antioxidants 2023, 12, 1817. https://doi.org/10.3390/antiox12101817
Attia SA, Truong AT, Phan A, Lee S-J, Abanmai M, Markanovic M, Avila H, Luo H, Ali A, Sreekumar PG, et al. αB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress. Antioxidants. 2023; 12(10):1817. https://doi.org/10.3390/antiox12101817
Chicago/Turabian StyleAttia, Sara Aly, Anh Tan Truong, Alvin Phan, Shin-Jae Lee, Manal Abanmai, Marinella Markanovic, Hugo Avila, Haozhong Luo, Atham Ali, Parameswaran G. Sreekumar, and et al. 2023. "αB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress" Antioxidants 12, no. 10: 1817. https://doi.org/10.3390/antiox12101817
APA StyleAttia, S. A., Truong, A. T., Phan, A., Lee, S.-J., Abanmai, M., Markanovic, M., Avila, H., Luo, H., Ali, A., Sreekumar, P. G., Kannan, R., & MacKay, J. A. (2023). αB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress. Antioxidants, 12(10), 1817. https://doi.org/10.3390/antiox12101817









