Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Feed Intake and Growth Performance
2.3. Diarrhea Incidence
2.4. Blood Sampling and Analysis
2.5. Rumen Sampling and Analysis
2.6. Statistical Analysis
3. Results
3.1. Intake and Growth Performance
3.2. Diarrhea Incidence
3.3. Serum Antioxidant and Immune Indicators
3.4. Rumen Fermentation Parameters
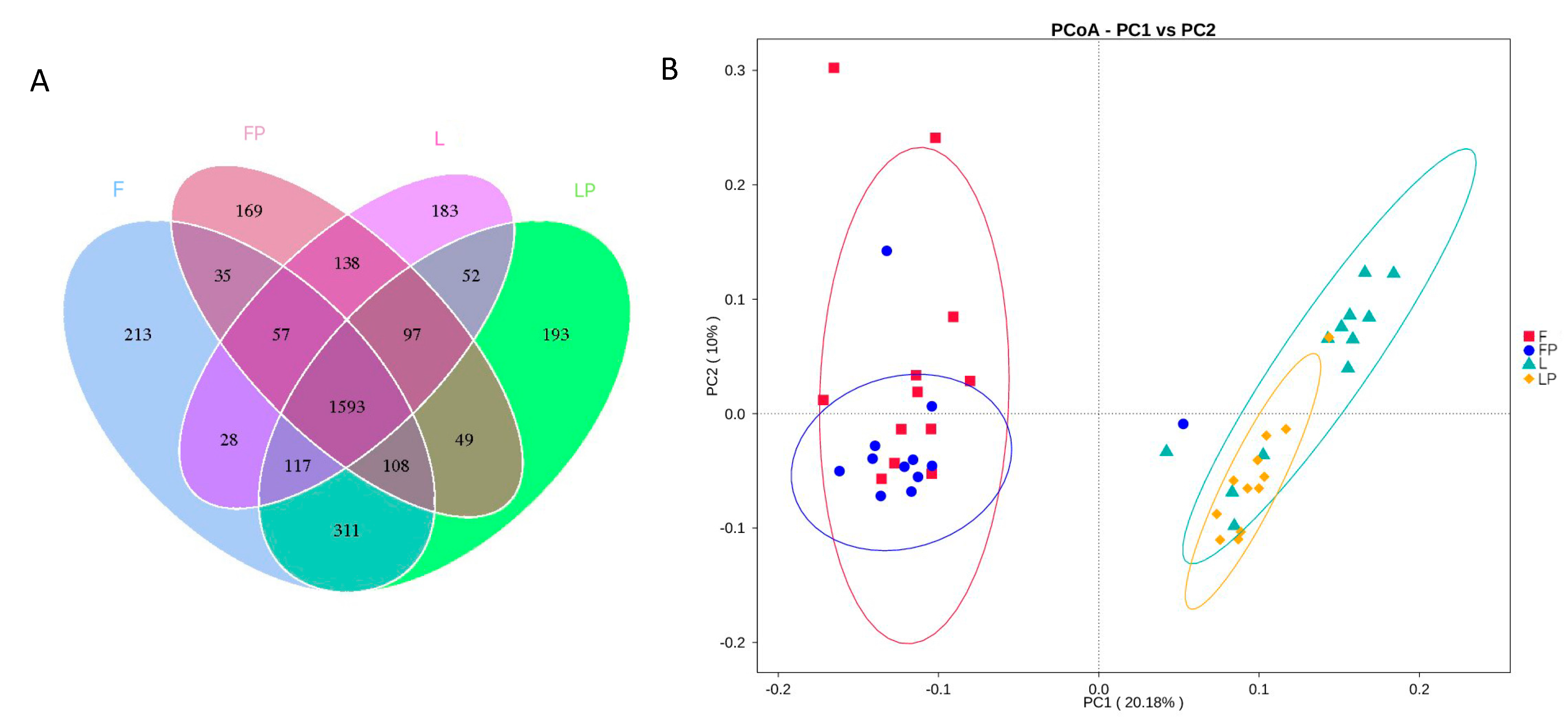
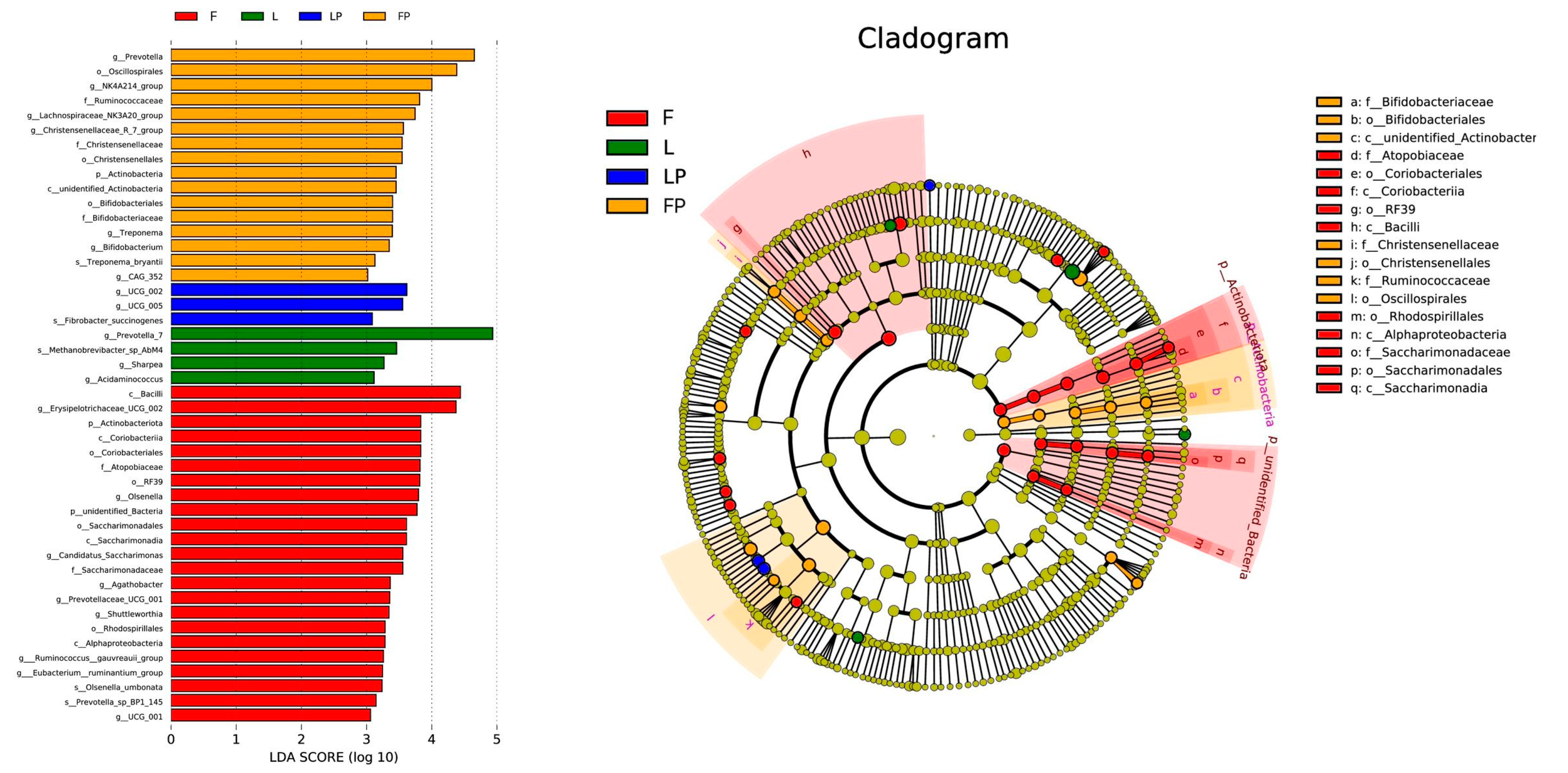
3.5. Rumen Bacteria Community
4. Discussion
4.1. Dry Matter Intake and Growth Performance
4.2. Diarrhea Incidence
4.3. Serum Antioxidant and Immune Indicators
4.4. Rumen Fermentation Parameters
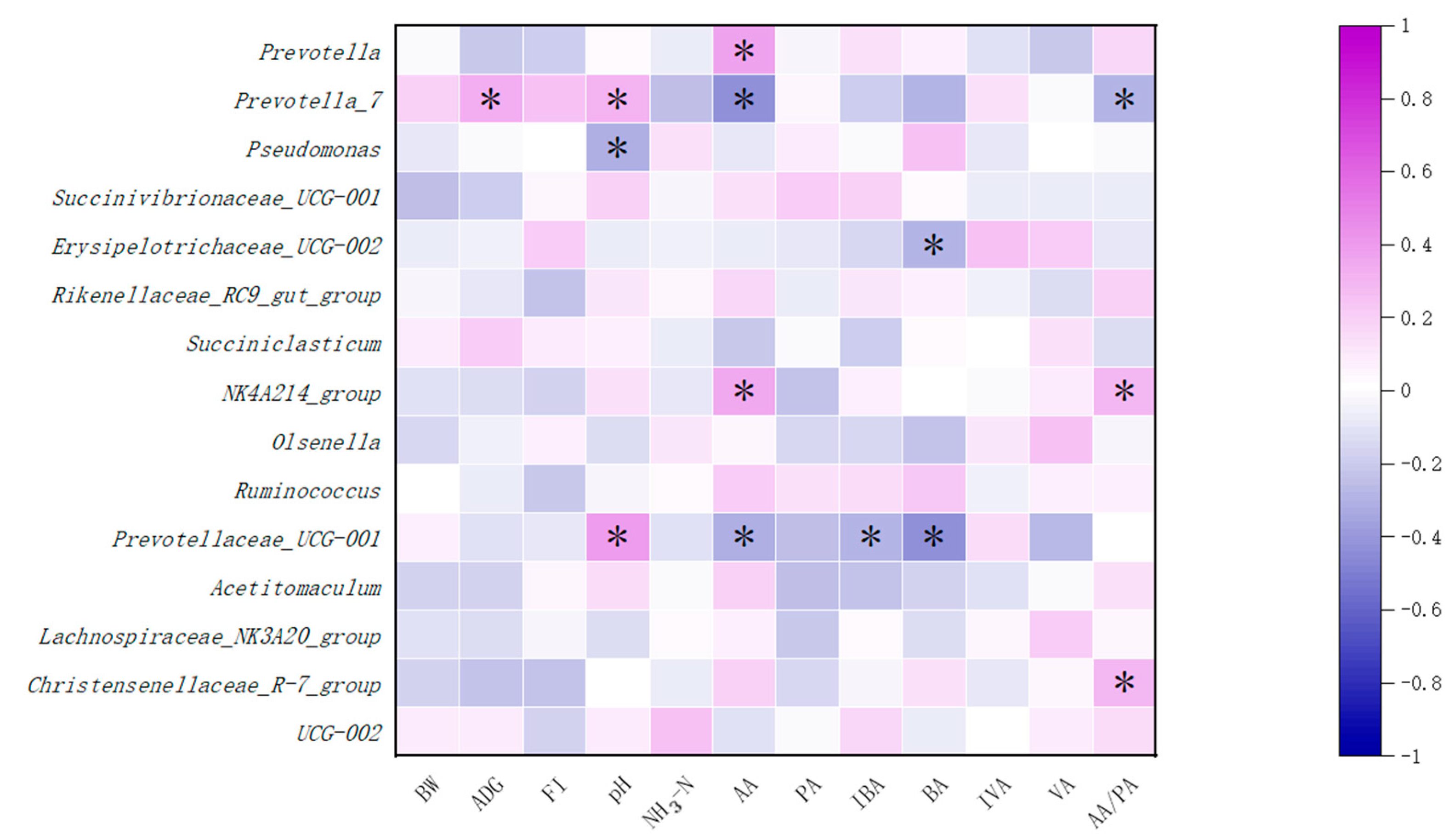
4.5. Rumen Bacteria Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Kertz, A.F.; Hill, T.M.; Quigley Iii, J.D.; Heinrichs, A.J.; Linn, J.G.; Drackley, J.K. A 100-Year Review: Calf nutrition and management. J. Dairy Sci. 2017, 100, 10151–10172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Alugongo, G.M.; Li, J.; Wang, Y.; Li, S.; Cao, Z. How forage feeding early in life influences the growth rate, ruminal environment, and the establishment of feeding behavior in pre-weaned calves. Animals 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nagata, R.; Ohtani, N.; Ichijo, T.; Ikuta, K.; Sato, S. Effects of dietary forage and calf starter diet on ruminal pH and bacteria in Holstein calves during weaning transition. Front. Microbiol. 2016, 7, 1575. [Google Scholar] [CrossRef]
- Laarman, A.H.; Oba, M. Effect of calf starter on rumen pH of Holstein dairy calves at weaning. J. Dairy. Sci. 2011, 94, 5661–5664. [Google Scholar] [CrossRef]
- Diao, Q.; Zhang, R.; Fu, T. Review of strategies to promote rumen development in calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef]
- Nemati, M.; Amanlou, H.; Khorvash, M.; Mirzaei, M.; Moshiri, B.; Ghaffari, M.H. Effect of different alfalfa hay levels on growth performance, rumen fermentation, and structural growth of Holstein dairy calves. J. Anim. Sci. 2016, 94, 1141–1148. [Google Scholar] [CrossRef]
- Imani, M.; Mirzaei, M.; Baghbanzadeh-Nobari, B.; Ghaffari, M.H. Effects of forage provision to dairy calves on growth performance and rumen fermentation: A meta-analysis and meta-regression. J. Dairy Sci. 2017, 100, 1136–1150. [Google Scholar] [CrossRef]
- O’Hara, E.; Neves, A.L.; Song, Y.; Guan, L.L. The role of the gut microbiome in cattle production and health: Driver or passenger? Annu. Rev. Anim. Biosci. 2020, 8, 199–220. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef]
- Andremont, A.; Cervesi, J.; Bandinelli, P.; Vitry, F.; de Gunzburg, J. Spare and repair the gut microbiota from antibiotic-induced dysbiosis: State-of-the-art. Drug Discov. Today 2021, 26, 2159–2163. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Vi, R.B.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen development, intestinal growth and hepatic metabolism in the pre-and postweaning ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar]
- Cangiano, L.R.; Yohe, T.T.; Steele, M.A.; Renaud, D.L. Invited Review: Strategic use of microbial-based probiotics and prebiotics in dairy calf rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar] [CrossRef]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics—A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysiss, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Rose, C.L.; Omi, S.K.; Forry, K.R.; Durall, D.M.; Bigg, W.L. Starch determination by perchloric acid vs enzymes: Evaluating the accuracy and precision of six colorimetric methods. J. Agric. Food Chem. 1991, 39, 2–11. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Li, J.; Meng, Q.S.; Wu, D.L.; Xu, M. Effects of butyric acid supplementation of acidified milk on digestive function and weaning stress of cattle calves. Livest. Sci. 2019, 225, 78–84. [Google Scholar] [CrossRef]
- Marcondes, M.I.; Pereira, T.R.; Chagas, J.; Filgueiras, E.A.; Castro, M.; Costa, G.P.; Sguizzato, A.; Sainz, R.D. Performance and health of Holstein calves fed different levels of milk fortified with symbiotic complex containing pre-and probiotics. Trop. Anim. Health Prod. 2016, 48, 1555–1560. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Parente, E.; Zotta, T.; Ercolini, D. A comparison of bioinformatic approaches for 16S rRNA gene profiling of food bacterial microbiota. Int. J. Food Microbiol. 2018, 265, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Alikhani, M.; Khorvash, M.; Hashemzadeh, F.; Sadeghi-Sefidmazgi, A.; Rafiee, H.; Ferraretto, L.F. Effects of different forage to concentrate ratios on performance, plasma metabolites, and feeding behaviour of weaned dairy calves from 70 to 120 days of age. Ital. J. Anim. Sci. 2021, 20, 1317–1327. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Aris, A.; Terré, M. Effects of forage provision to young calves on rumen fermentation and development of the gastrointestinal tract. J. Dairy Sci. 2013, 96, 5226–5236. [Google Scholar] [CrossRef]
- Castells, L.; Bach, A.; Araujo, G.; Montoro, C.; Terré, M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J. Dairy Sci. 2012, 95, 286–293. [Google Scholar] [CrossRef]
- Maamouri, O.; Ben Salem, M. The effect of live yeast Saccharomyces cerevisiae as probiotic supply on growth performance, feed intake, ruminal pH and fermentation in fattening calves. Vet. Med. Sci. 2022, 8, 398–404. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Tu, Y.; Zhang, N.F.; Deng, K.D.; Ma, T.; Diao, Q.Y. Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress-related indicators in Holstein calves. J. Anim. Physiol. Anim. Nutr. 2016, 100, 33–38. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Gao, Z.; Li, Q.; Qiu, X.; Wu, F.; Guan, T.; Cao, B.; Su, H. Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J. Dairy Sci. 2022, 105, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Galarza, C.; LeBlanc, S.J.; Jones-Bitton, A.; DeVries, T.J.; Rushen, J.; de Passillé, A.M.; Endres, M.I.; Haley, D.B. Associations between management practices and within-pen prevalence of calf diarrhea and respiratory disease on dairy farms using automated milk feeders. J. Dairy Sci. 2018, 101, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh-Dehaghani, A.; Towhidi, A.; Zhandi, M.; Mojgani, N.; Fouladi-Nashta, A. Combined effect of probiotics and specific immunoglobulin Y directed against Escherichia coli on growth performance, diarrhea incidence, and immune system in calves. Animal 2021, 15, 100124. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, B.; Sroka, J.; Katzer, F.; Goliński, P.; Nowak, W. The effect of probiotics, phytobiotics and their combination as feed additives in the diet of dairy calves on performance, rumen fermentation and blood metabolites during the preweaning period. Anim. Feed. Sci. Technol. 2021, 272, 114738. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Salisu, I.B.; Shahid, A.A.; Ali, Q.; Rao, A.Q.; Husnain, T. Nutritional assessment of dietary Bt and CP4EPSPS proteins on the serum biochemical changes of rabbits at different developmental stages. Front. Nutr. 2018, 5, 49. [Google Scholar] [CrossRef]
- Jia, F.; Dou, W.; Hu, F.; Wang, J. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 956–963. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Z.; Deng, M.; Li, Y.; Liu, G.; Liu, D.; Liu, Q.; Liu, Q.; Sun, B. Effects of a multi-strain probiotic on growth, health, and fecal bacterial flora of neonatal dairy calves. Anim. Biosci. 2022, 35, 204. [Google Scholar] [CrossRef]
- Jiang, Z.; Lin, Y.; Zhou, G.; Luo, L.; Jiang, S.; Chen, F. Effects of dietary selenomethionine supplementation on growth performance, meat quality and antioxidant property in yellow broilers. J. Agric. Food Chem. 2009, 57, 9769–9772. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Luo, R.; Chen, H.; Nie, C.; Niu, J.; Chen, C.; Xu, Y.; Li, X.; Zhang, W. Effect of a multispecies probiotic mixture on the growth and incidence of diarrhea, immune function, and fecal microbiota of pre-weaning dairy calves. Front. Microbiol. 2021, 12, 681014. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Lamas, G.; Combs, D.K. Effect of forage to concentrate ratio and intake level on utilization of early vegetative alfalfa silage by dairy cows. J. Dairy Sci. 1991, 74, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Erdman, R.A. Dietary buffering requirements of the lactating dairy cow: A review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.J.; Cui, Z.Q.; Zhang, Y.G. Effects of supplementation with Lactobacillus plantarum 299v on the performance, blood metabolites, rumen fermentation and bacterial communities of preweaning calves. Livest. Sci. 2020, 239, 104120. [Google Scholar] [CrossRef]
- Sutton, J.D. Altering milk composition by feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.; Noel, S.J.; Lund, P.; Larsen, M.; Weisbjerg, M.R.; Børsting, C.F. Feeding up to 91% concentrate to Holstein and Jersey dairy cows: Effects on enteric methane emission, rumen fermentation and bacterial community, digestibility, production, and feeding behavior. J. Dairy Sci. 2022, 105, 9523–9541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, P.; Wang, L.; Zhao, Z.; Chen, Y.; Yang, Y. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl. Microbiol. Biotechnol. 2017, 101, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Parmar, N.R.; Solanki, J.V.; Patel, A.B.; Shah, T.M.; Patel, A.K.; Parnerkar, S.; Kumar JI, N.; Joshi, C.G. Metagenome of Mehsani buffalo rumen microbiota: An assessment of variation in feed-dependent phylogenetic and functional classification. J. Mol. Microb. Biotech. 2014, 24, 249–261. [Google Scholar] [CrossRef]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. ISME J. 2022, 16, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Daghio, M.; Ciucci, F.; Buccioni, A.; Cappucci, A.; Casarosa, L.; Serra, A.; Conte, G.; Viti, C.; McAmmond, B.M.; Van Hamme, J.D. Correlation of breed, growth performance, and rumen microbiota in two rustic cattle breeds reared under different conditions. Front. Microbiol. 2021, 12, 652031. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Mizrahi, I. The road not taken: The rumen microbiome, functional groups, and community states. Trends Microbiol. 2019, 27, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Nocek, J.E.; Kautz, W.P.; Leedle, J.; Allman, J.G. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 2002, 85, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.H.; Shan, A.S.; Ma, N.; Ma, Q.Q.; Sun, Z.W. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 429–436. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Eugène, M.; Ferlay, A.; Popova, M.; Morgavi, D.P. Bacterial direct-fed microbials fail to reduce methane emissions in primiparous lactating dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C.; Du, M.; Yan, P.; Long, R.; Han, J. Effects of dietary energy levels on rumen fermentation, microbial diversity, and feed efficiency of yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Hofer, U. Pro-inflammatory prevotella? Nat. Rev. Microbiol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]

| Nutrient Composition | Pelleted Starter b | Oat Hay |
|---|---|---|
| Dry matter | 90.56 | 89.45 |
| NEL, MCal/kg | 1.86 | 1.10 |
| Crude protein | 21.94 | 6.20 |
| Starch | 28.50 | 0.40 |
| Ether extract | 3.86 | 2.16 |
| Neutral detergent fiber | 21.82 | 63.93 |
| Acid detergent fiber | 8.15 | 32.71 |
| Calcium | 1.13 | 0.44 |
| Phosphorus | 0.52 | 0.15 |
| Item a | Treatment b | SEM | p-Value c | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| Pelleted starter intake (kg of DM/d) | ||||||||
| 1–30 d | 3.22 | 3.45 | 3.32 | 3.23 | 0.03 | 0.25 | 0.16 | <0.01 |
| 31–60 d | 4.24 | 4.43 | 4.61 | 4.52 | 0.03 | <0.01 | 0.31 | <0.01 |
| 61–84 d | 4.99 | 4.72 | 5.13 | 4.98 | 0.03 | <0.01 | <0.01 | 0.22 |
| 1–84 d | 4.15 | 4.20 | 4.38 | 4.24 | 0.03 | 0.04 | 0.51 | 0.17 |
| Oat hay intake (kg of DM/d) | ||||||||
| 1–30 d | 0.56 | 0.65 | 0.58 | 0.58 | 0.01 | 0.29 | 0.05 | 0.05 |
| 31–60 d | 1.01 | 1.02 | 0.85 | 0.89 | 0.02 | <0.01 | 0.42 | 0.54 |
| 61–84 d | 1.33 | 1.34 | 1.03 | 1.01 | 0.03 | <0.01 | 0.74 | 0.42 |
| 1–84 d | 0.97 | 1.01 | 0.82 | 0.85 | 0.02 | <0.01 | 0.35 | 0.9 |
| Total feed intake (kg of DM/d) | ||||||||
| 1–30 d | 3.78 | 4.10 | 3.90 | 3.81 | 0.03 | 0.17 | 0.05 | <0.01 |
| 31–60 d | 5.25 | 5.44 | 5.46 | 5.41 | 0.03 | 0.16 | 0.27 | 0.06 |
| 61–84 d | 6.32 | 6.06 | 6.16 | 5.99 | 0.04 | 0.11 | <0.01 | 0.51 |
| 1–84 d | 5.12 | 5.20 | 5.20 | 5.09 | 0.04 | 0.86 | 0.91 | 0.28 |
| Protein intake (g/d) | ||||||||
| 1–30 d | 741.02 | 796.14 | 763.67 | 744.96 | 6.31 | 0.21 | 0.11 | <0.01 |
| 31–60 d | 992.60 | 1034.53 | 1064.55 | 1046.70 | 6.58 | <0.01 | 0.28 | 0.01 |
| 61–84 d | 1177.65 | 1117.84 | 1189.39 | 1155.93 | 7.27 | 0.05 | <0.01 | 0.30 |
| 1–84 d | 970.53 | 983.10 | 1010.88 | 983.83 | 7.59 | 0.18 | 0.63 | 0.19 |
| Starch intake (g/d) | ||||||||
| 1–30 d | 919.56 | 984.44 | 947.39 | 923.09 | 7.85 | 0.24 | 0.16 | <0.01 |
| 31–60 d | 1212.14 | 1266.15 | 1317.76 | 1291.26 | 8.57 | <0.01 | 0.30 | <0.01 |
| 61–84 d | 1427.86 | 1349.32 | 1465.82 | 1424.42 | 9.60 | <0.01 | <0.01 | 0.23 |
| 1–84 d | 1186.60 | 1200.08 | 1250.32 | 1213.01 | 9.44 | 0.04 | 0.52 | 0.17 |
| NDF intake (g/d) | ||||||||
| 1–30 d | 1061.62 | 1167.26 | 1096.21 | 1077.60 | 10.95 | 0.16 | 0.03 | <0.01 |
| 31–60 d | 1570.18 | 1615.33 | 1550.03 | 1557.25 | 12.68 | 0.13 | 0.30 | 0.45 |
| 61–84 d | 1940.23 | 1887.20 | 1780.53 | 1731.76 | 16.70 | <0.01 | 0.04 | 0.93 |
| 1–84 d | 1524.54 | 1558.58 | 1497.42 | 1468.88 | 15.99 | 0.04 | 0.71 | 0.48 |
| Body weight (kg) | ||||||||
| 0 d | 93.67 | 93.58 | 93.5 | 94.08 | 0.78 | 0.92 | 0.88 | 0.84 |
| 30 d | 132.67 | 135.58 | 133.67 | 135.17 | 1.14 | 0.90 | 0.35 | 0.76 |
| 60 d | 162.5 | 168.0 | 167.42 | 166.42 | 1.29 | 0.53 | 0.39 | 0.21 |
| 84 d | 189.33 | 191.92 | 193.75 | 195.42 | 1.36 | 0.15 | 0.44 | 0.87 |
| ADG (kg/d) | ||||||||
| 1–30 d | 1.30 | 1.40 | 1.34 | 1.37 | 0.03 | 0.94 | 0.22 | 0.52 |
| 31–60 d | 0.99 | 1.08 | 1.13 | 1.04 | 0.02 | 0.28 | 0.97 | 0.05 |
| 61–84 d | 1.11 | 1.00 | 1.09 | 1.20 | 0.02 | 0.02 | 0.93 | 0.01 |
| 1–84 d | 1.14 | 1.17 | 1.19 | 1.21 | 0.01 | 0.10 | 0.41 | 0.72 |
| Item | Treatment a | SEM | p-Value b | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| Diarrhea rate, % | 20.06 | 19.14 | 20.99 | 18.21 | 0.01 | 1.00 | 0.18 | 0.50 |
| Fecal index | 2.07 | 2.02 | 2.10 | 2.01 | 0.01 | 0.62 | <0.01 | 0.23 |
| Item a | Treatment b | SEM | p-Value c | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| GSH-Px (nmol/L) | 864.76 | 844.76 | 792.38 | 770.48 | 20.82 | 0.08 | 0.62 | 0.98 |
| SOD (U/mL) | 42.86 | 38.23 | 39.88 | 42.10 | 0.77 | 0.77 | 0.43 | 0.03 |
| T-AOC (mmol/L) | 0.86 | 0.80 | 0.88 | 0.86 | 0.02 | 0.31 | 0.22 | 0.54 |
| CAT (U/mL) | 1.95 | 2.24 | 1.85 | 2.69 | 0.08 | 0.21 | <0.01 | 0.06 |
| MDA (nmol/L) | 5.76 | 3.84 | 4.60 | 4.80 | 0.18 | 0.74 | <0.01 | 0.01 |
| IgA (μg/mL) | 1493.34 | 2029.98 | 1867.55 | 2395.00 | 67.21 | <0.01 | <0.01 | 0.96 |
| IgG (mg/mL) | 4.13 | 4.56 | 5.15 | 5.85 | 0.15 | <0.01 | 0.02 | 0.56 |
| IgM (μg/mL) | 632.28 | 840.41 | 904.67 | 1063.90 | 37.15 | <0.01 | <0.01 | 0.69 |
| Item a | Treatment b | SEM | p-Value c | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| pH | 6.49 | 6.46 | 6.33 | 6.27 | 0.49 | 0.08 | 0.66 | 0.92 |
| NH3-N (mg/dL) | 10.94 | 11.62 | 11.95 | 12.10 | 0.58 | 0.54 | 0.73 | 0.83 |
| TVFA (mmol/L) | 84.00 | 88.38 | 87.02 | 85.85 | 1.91 | 0.94 | 0.60 | 0.37 |
| Acetic acid (%) | 67.38 | 65.88 | 62.50 | 62.95 | 0.72 | 0.01 | 0.70 | 0.48 |
| propionic acid (%) | 17.92 | 18.77 | 20.72 | 21.59 | 0.69 | 0.04 | 0.53 | 0.99 |
| Butyric acid (%) | 10.49 | 10.29 | 11.14 | 10.25 | 0.46 | 0.75 | 0.57 | 0.72 |
| Isobutyric acid(%) | 1.00 | 1.15 | 1.13 | 1.30 | 0.06 | 0.23 | 0.15 | 0.93 |
| Valeric acid (%) | 1.88 | 2.23 | 2.01 | 2.15 | 0.13 | 0.91 | 0.36 | 0.69 |
| Isovaleric acid(%) | 1.34 | 1.67 | 2.51 | 1.76 | 0.18 | 0.08 | 0.56 | 0.13 |
| Acetic acid/propionic acid | 3.96 | 3.74 | 3.15 | 2.89 | 0.18 | <0.01 | 0.44 | 0.94 |
| Item | Treatment a | SEM | p-Value b | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| Ace | 1288.57 | 1380.35 | 1146.94 | 1224.26 | 21.99 | <0.01 | 0.03 | 0.85 |
| Chao1 | 1270.24 | 1367.42 | 1121.99 | 1206.28 | 22.57 | <0.01 | 0.02 | 0.87 |
| Simpson | 0.96 | 0.94 | 0.90 | 0.95 | 0.01 | 0.08 | 0.40 | 0.03 |
| Shannon | 6.72 | 6.76 | 5.67 | 6.52 | 0.12 | <0.01 | 0.03 | 0.05 |
| Item | Treatment a | SEM | p-Value b | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| Firmicutes | 41.09 | 38.15 | 36.03 | 32.71 | 1.30 | 0.04 | 0.22 | 0.94 |
| Bacteroidota | 43.41 | 43.21 | 41.88 | 48.21 | 1.54 | 0.58 | 0.33 | 0.30 |
| Proteobacteria | 7.99 | 10.76 | 15.84 | 12.11 | 1.78 | 0.20 | 0.89 | 0.37 |
| Unidentified-Bacteria | 2.14 | 1.77 | 0.72 | 1.50 | 0.16 | <0.01 | 0.49 | 0.06 |
| Actinobacteria | 0.25 | 0.78 | 0.19 | 0.05 | 0.10 | 0.05 | 0.31 | 0.10 |
| Actinobacteriota | 1.91 | 1.62 | 1.09 | 0.66 | 0.13 | <0.01 | 0.12 | 0.77 |
| Euryarchaeota | 0.46 | 0.35 | 0.81 | 0.53 | 0.11 | 0.26 | 0.40 | 0.72 |
| Spirochaetota | 0.61 | 0.76 | 0.47 | 0.87 | 0.10 | 0.94 | 0.18 | 0.51 |
| Fibrobacterota | 0.08 | 0.14 | 0.07 | 0.31 | 0.04 | 0.37 | 0.08 | 0.32 |
| Desulfobacterota | 0.34 | 0.25 | 0.43 | 0.31 | 0.02 | 0.10 | 0.02 | 0.77 |
| Item | Treatment a | SEM | p-Value b | |||||
|---|---|---|---|---|---|---|---|---|
| F | FP | L | LP | OH | CMP | OH × CMP | ||
| Prevotella | 15.81 | 18.06 | 7.97 | 15.91 | 1.35 | 0.05 | 0.05 | 0.27 |
| Prevotella-7 | 13.87 | 4.49 | 22.58 | 10.16 | 1.78 | 0.02 | <0.01 | 0.63 |
| Pseudomonas | 3.08 | 5.38 | 10.62 | 5.97 | 1.66 | 0.23 | 0.73 | 0.30 |
| Succinivibrionaceae-UCG-001 | 4.23 | 4.80 | 4.97 | 5.92 | 1.00 | 0.65 | 0.71 | 0.93 |
| Erysipelotrichaceae-UCG-002 | 6.61 | 4.92 | 6.29 | 1.97 | 0.63 | 0.18 | 0.02 | 0.28 |
| Rikenellaceae-RC9-gut-group | 3.79 | 4.89 | 2.78 | 6.66 | 0.46 | 0.66 | <0.01 | 0.11 |
| Succiniclasticum | 4.89 | 3.11 | 4.41 | 4.15 | 0.35 | 0.69 | 0.15 | 0.28 |
| NK4A214-group | 1.48 | 2.63 | 0.63 | 1.45 | 0.18 | <0.01 | <0.01 | 0.60 |
| Olsenella | 1.79 | 1.46 | 1.06 | 0.63 | 0.13 | <0.01 | 0.10 | 0.83 |
| Ruminococcus | 0.74 | 1.32 | 0.64 | 0.98 | 0.07 | 0.10 | <0.01 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-Q.; Hu, Y.-R.; Liu, S.-R.; Wang, M.; Xian, Z.-Y.; Liu, D.-W.; Sun, B.-L.; Li, Y.-K.; Liu, G.-B.; Deng, M.; et al. Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves. Antioxidants 2023, 12, 1851. https://doi.org/10.3390/antiox12101851
Guo Y-Q, Hu Y-R, Liu S-R, Wang M, Xian Z-Y, Liu D-W, Sun B-L, Li Y-K, Liu G-B, Deng M, et al. Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves. Antioxidants. 2023; 12(10):1851. https://doi.org/10.3390/antiox12101851
Chicago/Turabian StyleGuo, Yong-Qing, Ya-Ru Hu, Su-Ran Liu, Meng Wang, Zhen-Yu Xian, De-Wu Liu, Bao-Li Sun, Yao-Kun Li, Guang-Bin Liu, Ming Deng, and et al. 2023. "Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves" Antioxidants 12, no. 10: 1851. https://doi.org/10.3390/antiox12101851
APA StyleGuo, Y.-Q., Hu, Y.-R., Liu, S.-R., Wang, M., Xian, Z.-Y., Liu, D.-W., Sun, B.-L., Li, Y.-K., Liu, G.-B., Deng, M., Hu, W.-F., & Liu, Q.-S. (2023). Effects of the Oat Hay Feeding Method and Compound Probiotic Supplementation on the Growth, Antioxidant Capacity, Immunity, and Rumen Bacteria Community of Dairy Calves. Antioxidants, 12(10), 1851. https://doi.org/10.3390/antiox12101851






