Food-Derived Extracellular Vesicles as Multi-Bioactive Complex and Their Versatile Health Effects
Abstract
:1. Introduction
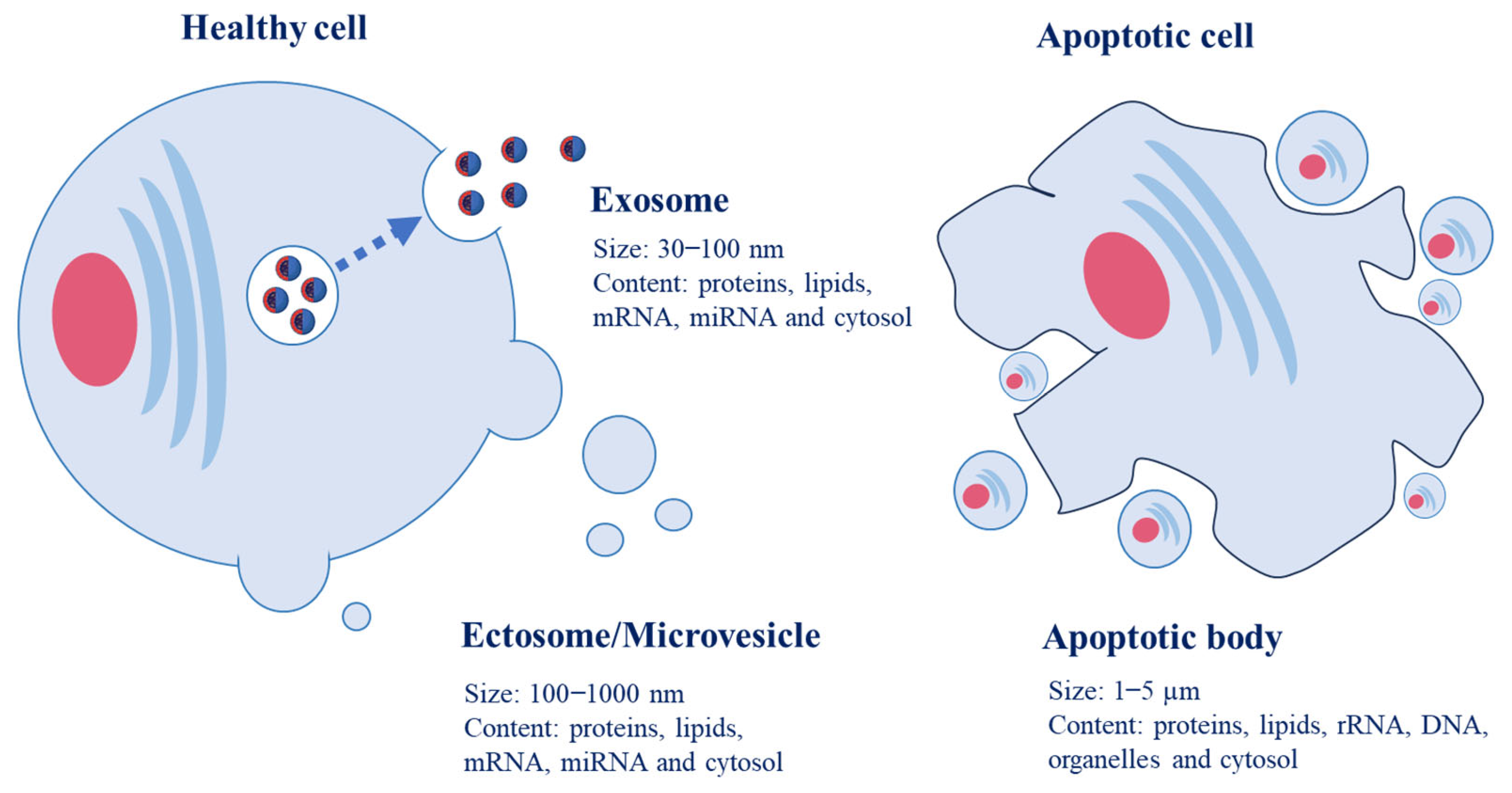
2. Physiological Roles of EVs in Animal Cells
3. Physiological Roles of EVs in Plant Cells
4. Structural Stability of Food-Derived EVs
5. Absorption and Uptake of Food-Derived EVs
6. Health Benefits of Food-Derived EVs
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Effect
6.3. Anticarcinogenic Activity
6.4. Gut Microbiome Modulation and Intestinal Barrier Enhancement
6.5. Inhibition of the Effect of COVID-19
6.6. Suppression of Alcoholic Liver Disease
6.7. Improvement in the Growth of Probiotics
6.8. Enhancement in Immune Systems
7. Other Bioactivities of Food-Derived EVs
8. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Cho, W.C.; Yee, B.K.; Kwan, Y.W.; Tai, W.C. Exosomes in inflammation and inflammatory disease. Proteomics 2019, 19, e1800149. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly everything—The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, 12144. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Möller, A.; Lobb, R.J. The evolving translational potential of small extracellular vesicles in cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like nanovesicles isolated from Citrus limon L. exert antioxidative effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of exosome-like nanoparticles and analysis of microRNAs derived from coconut water based on small RNA high-throughput sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, M.; de la Canal, L.; Lousa, C.d.M. A call for Rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Burgelman, M.; Vandendriessche, C.; Vandenbroucke, R.E. Extracellular vesicles: A double-edged sword in sepsis. Pharmaceuticals 2021, 14, 829. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, V.; Berglund, M.; Skogberg, G.; Lindgren, S.; Lundqvist, C.; Gudmundsdottir, J.; Thörn, K.; Telemo, E.; Ekwall, O. Thymic exosomes promote the final maturation of thymocytes. Sci. Rep. 2016, 6, 36479. [Google Scholar] [CrossRef]
- Ayre, D.C.; Elstner, M.; Smith, N.C.; Moores, E.S.; Hogan, A.M.; Christian, S.L. Dynamic regulation of CD24 expression and release of CD24-containing microvesicles in immature B cells in response to CD24 engagement. Immunology 2015, 146, 217–233. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadewall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Johnston, E.L.; Heras, B.; Kufer, T.A.; Kaparakis-Liaskos, M. Detection of bacterial membrane vesicles by NOD-like receptors. Int. J. Mol. Sci. 2021, 22, 1005. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.B.; Zhou, Z.R.; Peng, Y.Q.; Liu, X.Q.; He, B.X.; Chen, D.H.; Chen, D.; Fu, Q.L. Plasma EVs display antigen-presenting characteristics in patients with allergic rhinitis and promote differentiation of th2 cells. Front. Immunol. 2021, 12, 710372. [Google Scholar] [CrossRef] [PubMed]
- Katz-Kiriakos, E.; Steinberg, D.F.; Kluender, C.E.; Osorio, O.A.; Newsom-Stewart, C.; Baronia, A.; Byers, D.E.; Holtzman, M.; Katafiasz, D.; Bailey, K.L.; et al. Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease. JCI Insight 2021, 6, e136166. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Q.; Tang, W.; Wu, Y.; Lv, J.; Sun, L.; Shi, G.; Wu, M.; Qu, J.; Di, C.; et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J. Allergy Clin. Immunol. 2021, 148, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Sarkar, F.H.; Ramasamy, T.S. The versatile role of exosomes in cancer progression: Diagnostic and therapeutic implications. Cell. Oncol. 2018, 41, 223–252. [Google Scholar] [CrossRef]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 2015, 72, 659–671. [Google Scholar] [CrossRef]
- Yadaly, G.; Alexandre, F. Emerging roles of extracellular vesicles in the central nervous system: Physiology, pathology, and therapeutic perspectives. Front. Cell. Neurosci. 2021, 15, 626043. [Google Scholar]
- Liu, L.; Chen, X. Intercellular and systemic trafficking of RNAs in plants. Nat. Plants 2018, 4, 869–878. [Google Scholar] [CrossRef]
- Maizel, A.; Markmann, K.; Timmermans, M.; Wachter, A. To move or not to move: Roles and specificity of plant RNA mobility. Curr. Opin. Plant Biol. 2020, 57, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Dora, S.; Terrett, O.M.; Sanchez-Rodriguez, C. Plant-microbe interactions in the apoplast: Communication at the plant cell wall. Plant Cell 2022, 34, 1532–1550. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- De la Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.S.; Jang, G. Isolation of high-purity and high-stability exosomes from ginseng. Front. Plant Sci. 2023, 13, 1064412. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyaprakash, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential detergent sensitivity of extracellular vesicle subpopulations. Org. Biomol. Chem. 2015, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics 2013, 13, 22. [Google Scholar] [CrossRef]
- Ghiasi, M.; Rahimi, E.; Amirkhani, Z.; Salehi, R. Leucinerich repeat-containing G-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv. Biomed. Res. 2018, 7, 125. [Google Scholar]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- López de Las Hazas, M.C.; Del Pozo-Acebo, L.; Hansen, M.S.; Gil-Zamorano, J.; Mantilla-Escalante, D.C.; Gómez-Coronado, D.; Marín, F.; Garcia-Ruiz, A.; Rasmussen, J.T.; Dávalos, A. Dietary bovine milk miRNAs transported in extracellular vesicles are partially stable during GI digestion, are bioavailable and reach target tissues but need a minimum dose to impact on gene expression. Eur. J. Nutr. 2022, 61, 1043–1056. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Zech, D.; Rana, S.; Büchler, M.W.; Zöller, M. Tumor-exosomes and leukocyte activation: An ambivalent crosstalk. Cell Commun. Signal. 2012, 10, 37. [Google Scholar] [CrossRef]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Lentz, T.L.; Burrage, T.G.; Smith, A.L.; Crick, J.; Tignor, G.H. Is the acetylcholine receptor a rabies virus receptor? Science 1982, 215, 182–184. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.F.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Hazan-Halevy, I.; Rosenblum, D.; Weinstein, S.; Bairey, O.; Raanani, P.; Peer, D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015, 364, 59–69. [Google Scholar] [CrossRef]
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J. Funct. Foods 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma caco-2 cells and rat small intestinal iec-6 cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological signaling functions of reactive oxygen species in stem cells: From flies to man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of plant exosome vesicles from grapefruit (Citrus × paradisi) and tomato (Solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Wang, R.; Luo, J.Y.; He, J.J.; Ye, R.S.; Xie, M.Y.; Xi, Q.Y.; Jiang, Q.Y.; Sun, J.J.; et al. Plant MIR156 regulates intestinal growth in mammals by targeting the Wnt/β-catenin pathway. Am. J. Physiol. 2019, 317, 434–448. [Google Scholar] [CrossRef]
- Subha, D.; Harshnii, K.; Madhikiruba, K.G.; Nandhini, M.; Tamilselvi, K.S. Plant derived exosome- like Nanovesicles: An updated overview. Plant Nano Biol. 2023, 3, 100022. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef]
- Zhao, W.J.; Bian, Y.P.; Wang, Q.H.; Yin, F.; Yin, L.; Zhang, Y.L.; Liu, J.H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B.S.B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. Green nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]
- Wang, B.S.; Huang, G.J.; Lu, Y.H.; Chang, L.W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2013, 138, 751–756. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the microRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal Caco-2 cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef] [PubMed]
- Arntz, O.J.; Pieters, B.C.; Oliveira, M.C.; Broeren, M.G.; Bennink, M.B.; de Vries, M.; van Lent, P.L.; Koenders, M.I.; van den Berg, W.B.; van der Kraan, P.M.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.K.; Tian, Y.; Qureshi, R.A.; Gormley, M.; Ertel, A.; Gao, R.; Aradillas Lopez, E.; Alexander, G.M.; Sacan, A.; Fortina, P.; et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 2014, 155, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Shah, S.H.; Tamayo, A.; Robbins, P.D.; Golberg, R.B.; Mendez, A.J.; Ricordi, C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 2017, 7, 5998. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Z.; Lai, M.F.; Li, Y.P.; Xu, C.H.; Zhang, H.R.; Kuang, J.G. Human marrow stromal cells secrete microRNA-375-containing exosomes to regulate glioma progression. Cancer Gene Ther. 2020, 27, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ingenito, F.; Roscigno, G.; Affinito, A.; Nuzzo, S.; Scognamiglio, I.; Quintavalle, C.; Condorelli, G. The Role of exo-miRNAs in cancer: A focus on therapeutic and diagnostic applications. Int. J. Mol. Sci. 2019, 20, 4687–4703. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Wang, Y.; Luo, J.; Yu, W. MicroRNA-148a suppresses epithelial-to-mesenchymal transition by targeting ROCK1 in non-small cell lung cancer cells. Mol. Cell. Biochem. 2013, 380, 277–282. [Google Scholar] [CrossRef]
- Long, X.R.; He, Y.; Huang, C.; Li, J. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in hepatocellular carcinogenesis. Int. J. Oncol. 2014, 44, 1915–1922. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021, 12, 3950–3965. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Rodosthenous, R.S.; Jara, C.; Brennan, K.J.; Wright, R.O.; Baccarelli, A.A.; Wright, R.J. Detection of long non-coding RNAs in human breastmilk extracellular vesicles: Implications for early child development. Epigenetics 2016, 11, 721–729. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Cristaldi, M.; Monteleone, F.; Fontana, S.; Alessandro, R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J. Proteomics 2018, 173, 1–11. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Diez-Sainz, E.; Lorente-Cebrian, S.; Aranaz, P.; Riezu-Boj, J.I.; Martinez, J.A.; Milagro, F.I. Potential mechanisms linking foodderived microRNAs, gut microbiota and intestinal barrier functions in the context of nutrition and human health. Front. Nutr. 2021, 8, 586564–586583. [Google Scholar] [CrossRef]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, 618–624. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, M.L.; Zhu, W.J.; Cao, Y.N.; Peng, L.X.; Yan, Z.Y.; Zhao, G. In vitro effects of Tartary buckwheat-derived nanovesicles on gut microbiota. J. Agric. Food Chem. 2022, 70, 2616–2629. [Google Scholar] [CrossRef]
- Konig, J.; Wells, J.; Cani, P.D.; Garcia-Rodenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, 196–208. [Google Scholar] [CrossRef]
- Yun, B.; Kim, Y.; Park, D.J.; Oh, S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J. Anim. Sci. Technol. 2021, 63, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Du, X.; Li, J.; Lonnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082–1700113. [Google Scholar] [CrossRef]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, Z.; Xu, X.; Li, M.; Li, P. Characterization of three different types of extracellular vesicles and their impact on bacterial growth. Food Chem. 2019, 272, 372–378. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. IScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Matic, S.; D’Souza, D.H.; Wu, T.; Pangloli, P.; Dia, V.P. Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol. Investig. 2020, 49, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Ascanius, S.R.; Hansen, M.S.; Ostenfeld, M.S.; Rasmussen, J.T. Milk-derived extracellular vesicles suppress inflammatory cytokine expression and nuclear factor-κb activation in lipopolysaccharide-stimulated macrophages. Dairy 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Ito, S.; Aizawa, S.; Namiki, T.; Hayakawa, S. Cow milk exosomes activate NK cells and γδT cells in human PBMCs in vitro. Immunol. Med. 2020, 43, 161–170. [Google Scholar] [CrossRef]
- Lei, C.; Mu, J.; Teng, Y.; He, L.; Xu, F.; Zhang, X.; Sundaram, K.; Kumar, A.; Sriwastva, M.K.; Lawrenz, M.B.; et al. Lemon exosome-like nanoparticles-manipulated probiotics protect mice from C. diff infection. IScience 2020, 23, 101571. [Google Scholar] [CrossRef]
- Luo, S.; Sun, X.; Huang, M.; Ma, Q.; Du, L.; Cui, Y. Enhanced neuroprotective effects of epicatechin gallate encapsulated by bovine milk-derived exosomes against parkinson’s disease through antiapoptosis and antimitophagy. J. Agric. Food Chem. 2021, 69, 5134–5143. [Google Scholar] [CrossRef]
- Xie, M.Y.; Hou, L.J.; Sun, J.J.; Zeng, B.; Xi, Q.Y.; Luo, J.Y.; Chen, T.; Zhang, Y.L. Porcine milk exosome miRNAs attenuate LPS-induced apoptosis through inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. J. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Neal, M.D.; Sodhi, C.P.; Jia, H.; Dyer, M.; Egan, C.E.; Yazji, I.; Good, M.; Afrazi, A.; Marino, R.; Slagle, D.; et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J. Biol. Chem. 2012, 287, 37296–37308. [Google Scholar] [CrossRef]
- Sahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, J. Food-Derived Extracellular Vesicles as Multi-Bioactive Complex and Their Versatile Health Effects. Antioxidants 2023, 12, 1862. https://doi.org/10.3390/antiox12101862
Yeo J. Food-Derived Extracellular Vesicles as Multi-Bioactive Complex and Their Versatile Health Effects. Antioxidants. 2023; 12(10):1862. https://doi.org/10.3390/antiox12101862
Chicago/Turabian StyleYeo, JuDong. 2023. "Food-Derived Extracellular Vesicles as Multi-Bioactive Complex and Their Versatile Health Effects" Antioxidants 12, no. 10: 1862. https://doi.org/10.3390/antiox12101862




