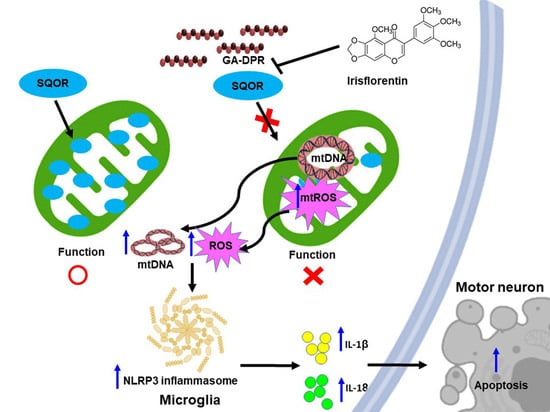
Glycine-Alanine Dipeptide Repeat Protein from C9-ALS Interacts with Sulfide Quinone Oxidoreductase (SQOR) to Induce the Activity of the NLRP3 Inflammasome in HMC3 Microglia: Irisflorentin Reverses This Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Maintenance of HMC3 Cell Lines
2.2. Construction and Transfection of Plasmid
2.3. Immunofluorescence Analysis of HMC3 Cells
2.4. Acquirements of Nuclear Extracts and Transcription Factor Assay of NF-κB p65 in HMC3 Cells
2.5. Protein Extraction and Western Blot Analysis of HMC3 Cells
2.6. Analysis of IL-1β and IL-18 in the Conditioned Media of HMC3 Cells
2.7. In Situ TUNEL Analysis of HMC3 Cells
2.8. Detection of Apoptosis in HMC3 Cells by Flow Cytometry
2.9. HMC3 Cells Were Treated with Inhibitor of NLRP3 Inflammasome
2.10. Using a Yeast Two-Hybrid Library to Screen GA-DPR-Interacting Proteins
2.11. Yeast Two-Hybrid Assay of GA50 and SQOR
2.12. Co-Immunoprecipitation Analysis of GA50 and SQOR
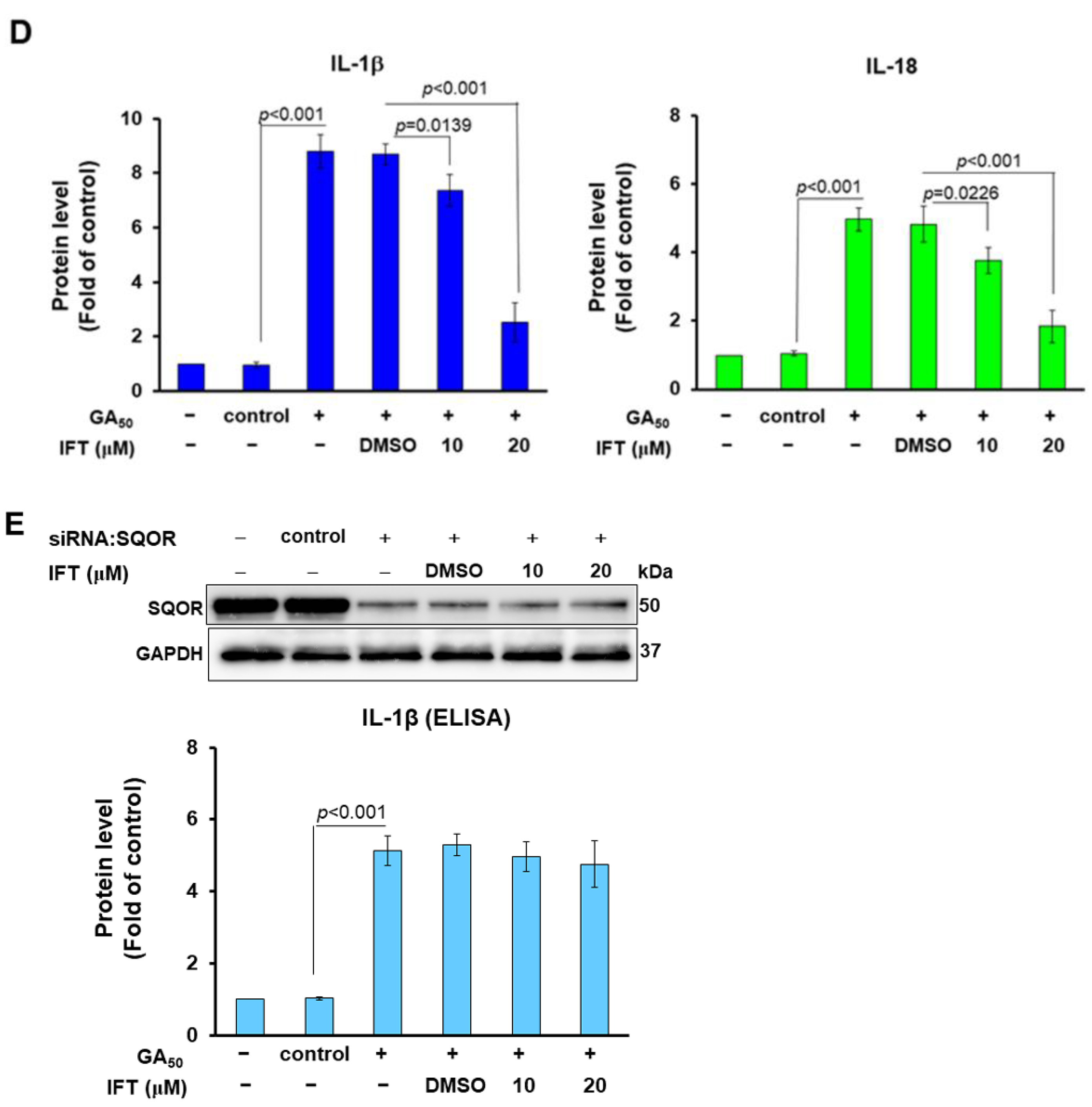
2.13. siRNA Treatment of SQOR in HMC3 Cells
2.14. Reactive Oxygen Species (ROS) Assay in HMC3 Cells
2.15. Measurement and Quantification of Cytoplasmic mtDNA in HMC3 Cells
2.16. Irisflorentin Treatment and Growth Assay of a Yeast Two-Hybrid Based on the Interaction between GA50 and SQOR
2.17. Toxicity Analysis and Treatment of Irisflorentin on HMC3 Cells
2.18. Statistical Work on the Data for This Study
3. Results
3.1. Expression of Glycine-Alanine-Dipeptide Repeat Protein (GA-DPR) Causes NLRP3 Inflammasome Activity in a Human HMC3 Microglia Cell Model
3.2. Exocytosis of Mature IL-1 β and IL-18 in GA50-Expressing HMC3 Cells Was Inhibited by Treatment with MCC950 Inhibitor of the NLRP3 Inflammasome
3.3. GA-DPR Interacts with Sulfide Quinone Oxidoreductase (SQOR) in HMC3 Microglia
3.4. Downregulation of SQOR Expression in HMC3 Microglia Activates NLRP3 Inflammasome
3.5. GA50 Expression or SQOR Knockdown Increased ROS Generation and Cytoplasmic Escape of Mitochondrial DNA in HMC Cells
3.6. The Interaction between GA50 and SQOR Can Be Inhibited by Irisflorentin in the Yeast Two-Hybrid Model
3.7. Irisflorentin (IFT) Reverses GA50-Caused Activity of NLRP3-Inflammasome in HMC3 Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, P.; Raymond, J.; Punjani, R.; Han, M.; Larson, T.; Kaye, W.; Nelson, L.M.; Topol, B.; Muravov, O.; Genson, C.; et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Front. Degener 2022, 24, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Brooks, B.R.; Silani, V. Clinical trials in amyotrophic lateral sclerosis: Why so many negative trials and how can trials be improved? Lancet. Neurol. 2014, 13, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Paez-Colasante, X.; Figueroa-Romero, C.; Sakowski, S.A.; Goutman, S.A.; Feldman, E.L. Amyotrophic lateral sclerosis: Mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 2015, 11, 266–279. [Google Scholar] [CrossRef]
- Peters, O.M.; Ghasemi, M.; Brown, R.H., Jr. Emerging mechanisms of molecular pathology in ALS. J. Clin. Investig. 2015, 125, 1767–1779. [Google Scholar] [CrossRef]
- Rohrer, J.D.; Isaacs, A.M.; Mizielinska, S.; Mead, S.; Lashley, T.; Wray, S.; Sidle, K.; Fratta, P.; Orrell, R.W.; Hardy, J.; et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol. 2015, 14, 291–301. [Google Scholar] [CrossRef]
- Prudencio, M.; Belzil, V.V.; Batra, R.; Ross, C.A.; Gendron, T.F.; Pregent, L.J.; Murray, M.E.; Overstreet, K.K.; Piazza-Johnston, A.E.; Desaro, P.; et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nat. Neurosci. 2015, 18, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Maroof, A.M.; Merkle, F.T.; Koszka, K.; Intoh, A.; Armstrong, I.; Moccia, R.; Davis-Dusenbery, B.N.; Eggan, K. The mouse C9ORF72 ortholog is enriched in neurons known to degenerate in ALS and FTD. Nat. Neurosci. 2013, 16, 1725–1727. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Frick, P.; Neumann, M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014, 127, 347–357. [Google Scholar] [CrossRef]
- Woollacott, I.O.; Mead, S. The C9ORF72 expansion mutation: Gene structure, phenotypic and diagnostic issues. Acta Neuropathol. 2014, 127, 319–332. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Shaw, P.J.; Kirby, J. The widening spectrum of C9ORF72-related disease; genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 2014, 127, 333–345. [Google Scholar] [CrossRef]
- Gendron, T.F.; Belzil, V.V.; Zhang, Y.J.; Petrucelli, L. Mechanisms of toxicity in C9FTLD/ALS. Acta Neuropathol. 2014, 127, 359–376. [Google Scholar] [CrossRef]
- Atkinson, R.A.; Fernandez-Martos, C.M.; Atkin, J.D.; Vickers, J.C.; King, A.E. C9ORF72 expression and cellular localization over mouse development. Acta Neuropathol. Commun. 2015, 3, 59. [Google Scholar] [CrossRef]
- Farg, M.A.; Sundaramoorthy, V.; Sultana, J.M.; Yang, S.; Atkinson, R.A.; Levina, V.; Halloran, M.A.; Gleeson, P.A.; Blair, I.P.; Soo, K.Y.; et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014, 23, 3579–3595. [Google Scholar] [CrossRef]
- Smeyers, J.; Banchi, E.G.; Latouche, M. C9ORF72: What It Is, What It Does, and Why It Matters. Front. Cell Neurosci. 2021, 15, 661447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, K.; Wu, Y.; Yan, G.; Zhang, C.; Li, Z.; Wang, L.; Chen, S. C9orf72 regulates the unfolded protein response and stress granule formation by interacting with eIF2alpha. Theranostics 2022, 12, 7289–7306. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; He, J.; Wang, S.; Shen, X.; Liu, Q.; Wang, S. Advances in the Structure of GGGGCC Repeat RNA Sequence and Its Interaction with Small Molecules and Protein Partners. Molecules 2023, 28, 5801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wang, S.; Mestre, A.A.; Fu, C.; Makarem, A.; Xian, F.; Hayes, L.R.; Lopez-Gonzalez, R.; Drenner, K.; Jiang, J.; et al. C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2alpha phosphorylation. Nat. Commun. 2018, 9, 51. [Google Scholar] [CrossRef]
- Gao, F.B.; Richter, J.D.; Cleveland, D.W. Rethinking Unconventional Translation in Neurodegeneration. Cell 2017, 171, 994–1000. [Google Scholar] [CrossRef]
- Green, K.M.; Glineburg, M.R.; Kearse, M.G.; Flores, B.N.; Linsalata, A.E.; Fedak, S.J.; Goldstrohm, A.C.; Barmada, S.J.; Todd, P.K. RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat. Commun. 2017, 8, 2005. [Google Scholar] [CrossRef]
- Sonobe, Y.; Ghadge, G.; Masaki, K.; Sendoel, A.; Fuchs, E.; Roos, R.P. Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol. Dis. 2018, 116, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Pinheiro Marques, J.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Jensen, B.K.; Schuldi, M.H.; McAvoy, K.; Russell, K.A.; Boehringer, A.; Curran, B.M.; Krishnamurthy, K.; Wen, X.; Westergard, T.; Ma, L.; et al. Synaptic dysfunction induced by glycine-alanine dipeptides in C9orf72-ALS/FTD is rescued by SV2 replenishment. EMBO Mol. Med. 2020, 12, e10722. [Google Scholar] [CrossRef] [PubMed]
- Schludi, M.H.; May, S.; Grasser, F.A.; Rentzsch, K.; Kremmer, E.; Kupper, C.; Klopstock, T.; German Consortium for Frontotemporal Lobar, D.; Bavarian Brain Banking, A.; Arzberger, T.; et al. Distribution of dipeptide repeat proteins in cellular models and C9orf72 mutation cases suggests link to transcriptional silencing. Acta Neuropathol. 2015, 130, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Ito, D.; Honda, T.; Kubo, K.; Noda, M.; Nakajima, K.; Suzuki, N. Characterization of the dipeptide repeat protein in the molecular pathogenesis of c9FTD/ALS. Hum. Mol. Genet. 2015, 24, 1630–1645. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Jansen-West, K.; Xu, Y.F.; Gendron, T.F.; Bieniek, K.F.; Lin, W.L.; Sasaguri, H.; Caulfield, T.; Hubbard, J.; Daughrity, L.; et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014, 128, 505–524. [Google Scholar] [CrossRef]
- Chien, H.M.; He, R.Y.; Lee, C.C.; Huang, Y.A.; Hung, I.J.; Hou, K.T.; Hsiao, J.C.; Lu, P.C.; Agnihotri, D.; Hwang, E.; et al. Nanoscopic investigation of C9orf72 poly-GA oligomers on nuclear membrane disruption by a photoinducible platform. Commun. Chem. 2021, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Baskaran, P.; Gomez, J.; Chen, H.J.; Nishimura, A.; Smith, B.; Troakes, C.; Adachi, Y.; Stepto, A.; Petrucelli, L.; et al. C9orf72 poly GA RAN-translated protein plays a key role in Amyotrophic Lateral Sclerosis via aggregation and toxicity. Hum. Mol. Genet. 2017, 26, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Masuda-Suzukake, M.; Hosokawa, M.; Shimozawa, A.; Hirai, S.; Okado, H.; Hasegawa, M. C9ORF72 dipeptide repeat poly-GA inclusions promote: Intracellular aggregation of phosphorylated TDP-43. Hum. Mol. Genet. 2018, 27, 2658–2660. [Google Scholar] [CrossRef]
- May, S.; Hornburg, D.; Schludi, M.H.; Arzberger, T.; Rentzsch, K.; Schwenk, B.M.; Grasser, F.A.; Mori, K.; Kremmer, E.; Banzhaf-Strathmann, J.; et al. C9orf72 FTLD/ALS-associated Gly-Ala dipeptide repeat proteins cause neuronal toxicity and Unc119 sequestration. Acta Neuropathol. 2014, 128, 485–503. [Google Scholar] [CrossRef]
- Sonobe, Y.; Aburas, J.; Krishnan, G.; Fleming, A.C.; Ghadge, G.; Islam, P.; Warren, E.C.; Gu, Y.; Kankel, M.W.; Brown, A.E.X.; et al. elegans model of C9orf72-associated ALS/FTD uncovers a conserved role for eIF2D in RAN translation. Nat. Commun. 2021, 12, 6025. [Google Scholar] [CrossRef]
- Masrori, P.; Beckers, J.; Gossye, H.; Van Damme, P. The role of inflammation in neurodegeneration: Novel insights into the role of the immune system in C9orf72 HRE-mediated ALS/FTD. Mol. Neurodegener. 2022, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.E.; O’Rourke, J.G.; Yanez, A.; Markman, J.L.; Ho, R.; Wang, X.; Chen, S.; Lall, D.; Jin, M.; Muhammad, A.; et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature 2020, 585, 96–101. [Google Scholar] [CrossRef]
- Pinilla, G.; Kumar, A.; Floaters, M.K.; Pardo, C.A.; Rothstein, J.; Ilieva, H. Increased synthesis of pro-inflammatory cytokines in C9ORF72 patients. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Kang, L.; Wang, Y.Z.; Yang, B.R.; Zhang, C.; Lu, Y.F.; Kang, L. Microglia in motor neuron disease: Signaling evidence from last 10 years. Dev. Neurobiol. 2022, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- LaClair, K.D.; Zhou, Q.; Michaelsen, M.; Wefers, B.; Brill, M.S.; Janjic, A.; Rathkolb, B.; Farny, D.; Cygan, M.; de Angelis, M.H.; et al. Congenic expression of poly-GA but not poly-PR in mice triggers selective neuron loss and interferon responses found in C9orf72 ALS. Acta Neuropathol. 2020, 140, 121–142. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Huang, B.; Wang, S.; Gao, Y.; Meng, F.; Chen, Y.; Zhou, F.; Guan, Y.; Wang, X. Spatiotemporal evolution of pyroptosis and canonical inflammasome pathway in hSOD1(G93A) ALS mouse model. BMC Neurosci. 2022, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Clenet, M.L.; Keaney, J.; Gillet, G.; Valadas, J.S.; Langlois, J.; Cardenas, A.; Gasser, J.; Kadiu, I. Divergent functional outcomes of NLRP3 blockade downstream of multi-inflammasome activation: Therapeutic implications for ALS. Front. Immunol. 2023, 14, 1190219. [Google Scholar] [CrossRef]
- Fu, R.H.; Tsai, C.W.; Chiu, S.C.; Liu, S.P.; Chiang, Y.T.; Kuo, Y.H.; Shyu, W.C.; Lin, S.Z. C9-ALS-Associated Proline-Arginine Dipeptide Repeat Protein Induces Activation of NLRP3 Inflammasome of HMC3 Microglia Cells by Binding of Complement Component 1 Q Subcomponent-Binding Protein (C1QBP), and Syringin Prevents This Effect. Cells 2022, 11, 3128. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Luo, R.; Lei, J.; Song, Y.; Wang, B.; Ma, L.; Liao, Z.; Ke, W.; Liu, H.; et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp. Mol. Med. 2022, 54, 129–142. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Xu, L.; Yu, S.; Fu, J.; Wang, J.; Yan, X.; Su, J. ROS-Induced mtDNA Release: The Emerging Messenger for Communication between Neurons and Innate Immune Cells during Neurodegenerative Disorder Progression. Antioxidants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Kumar, R.; Landry, A.P.; Guha, A.; Vitvitsky, V.; Lee, H.J.; Seike, K.; Reddy, P.; Lyssiotis, C.A.; Banerjee, R. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H(2)S oxidation. J. Biol. Chem. 2022, 298, 101435. [Google Scholar] [CrossRef]
- Wong, J.H.; Alfatah, M.; Sin, M.F.; Sim, H.M.; Verma, C.S.; Lane, D.P.; Arumugam, P. A yeast two-hybrid system for the screening and characterization of small-molecule inhibitors of protein-protein interactions identifies a novel putative Mdm2-binding site in p53. BMC Biol. 2017, 15, 108. [Google Scholar] [CrossRef]
- Pang, W.; Hu, F. C9ORF72 suppresses JAK-STAT mediated inflammation. iScience 2023, 26, 106579. [Google Scholar] [CrossRef]
- Lorenzini, I.; Alsop, E.; Levy, J.; Gittings, L.M.; Lall, D.; Rabichow, B.E.; Moore, S.; Pevey, R.; Bustos, L.M.; Burciu, C.; et al. Moderate intrinsic phenotypic alterations in C9orf72 ALS/FTD iPSC-microglia despite the presence of C9orf72 pathological features. Front. Cell Neurosci. 2023, 17, 1179796. [Google Scholar] [CrossRef] [PubMed]
- Trageser, K.J.; Yang, E.J.; Smith, C.; Iban-Arias, R.; Oguchi, T.; Sebastian-Valverde, M.; Iqbal, U.H.; Wu, H.; Estill, M.; Al Rahim, M.; et al. Inflammasome-Mediated Neuronal-Microglial Crosstalk: A Therapeutic Substrate for the Familial C9orf72 Variant of Frontotemporal Dementia/Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2023, 60, 4004–4016. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Munoz-Planillo, R.; Kuffa, P.; Martinez-Colon, G.; Smith, B.L.; Rajendiran, T.M.; Nunez, G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Qiu, M.; Lv, S.; Zheng, H.; Niu, B.; Liu, H. Hydrogen Sulfide Plays an Important Role by Influencing NLRP3 inflammasome. Int. J. Biol. Sci. 2020, 16, 2752–2760. [Google Scholar] [CrossRef]
- Chiarini, A.; Gui, L.; Viviani, C.; Armato, U.; Dal Pra, I. NLRP3 Inflammasome’s Activation in Acute and Chronic Brain Diseases-An Update on Pathogenetic Mechanisms and Therapeutic Perspectives with Respect to Other Inflammasomes. Biomedicines 2023, 11, 999. [Google Scholar] [CrossRef]
- Van Schoor, E.; Ospitalieri, S.; Moonen, S.; Tome, S.O.; Ronisz, A.; Ok, O.; Weishaupt, J.; Ludolph, A.C.; Van Damme, P.; Van Den Bosch, L.; et al. Increased pyroptosis activation in white matter microglia is associated with neuronal loss in ALS motor cortex. Acta Neuropathol. 2022, 144, 393–411. [Google Scholar] [CrossRef]
- Deora, V.; Lee, J.D.; Albornoz, E.A.; McAlary, L.; Jagaraj, C.J.; Robertson, A.A.B.; Atkin, J.D.; Cooper, M.A.; Schroder, K.; Yerbury, J.J.; et al. The microglial NLRP3 inflammasome is activated by amyotrophic lateral sclerosis proteins. Glia 2020, 68, 407–421. [Google Scholar] [CrossRef]
- Van Zeller, M.; Dias, D.; Sebastiao, A.M.; Valente, C.A. NLRP3 Inflammasome: A Starring Role in Amyloid-beta- and Tau-Driven Pathological Events in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef]
- Han, Q.Q.; Le, W. NLRP3 Inflammasome-Mediated Neuroinflammation and Related Mitochondrial Impairment in Parkinson’s Disease. Neurosci. Bull. 2023, 39, 832–844. [Google Scholar] [CrossRef]
- Shu, X.; Wei, C.; Tu, W.Y.; Zhong, K.; Qi, S.; Wang, A.; Bai, L.; Zhang, S.X.; Luo, B.; Xu, Z.Z.; et al. Negative regulation of TREM2-mediated C9orf72 poly-GA clearance by the NLRP3 inflammasome. Cell Rep. 2023, 42, 112133. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Kubitza, M.; Maier, K.; Brawanski, A.; Hauska, G.; Pina, A.L. The vertebrate homolog of sulfide-quinone reductase is expressed in mitochondria of neuronal tissues. Neuroscience 2011, 199, 1–12. [Google Scholar] [CrossRef]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. H2S oxidation by nanodisc-embedded human sulfide quinone oxidoreductase. J. Biol. Chem. 2017, 292, 11641–11649. [Google Scholar] [CrossRef]
- Marutani, E.; Morita, M.; Hirai, S.; Kai, S.; Grange, R.M.H.; Miyazaki, Y.; Nagashima, F.; Traeger, L.; Magliocca, A.; Ida, T.; et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 2021, 12, 3108. [Google Scholar] [CrossRef]
- Landry, A.P.; Ballou, D.P.; Banerjee, R. Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase. Chembiochem 2021, 22, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, G.; Barca, E.; Ziosi, M.; Emmanuele, V.; Xu, Y.; Hidalgo-Gutierrez, A.; Qiao, C.; Tadesse, S.; Area-Gomez, E.; Lopez, L.C.; et al. CoQ(10) supplementation rescues nephrotic syndrome through normalization of H(2)S oxidation pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3708–3722. [Google Scholar] [CrossRef]
- Rottenberg, H.; Hoek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 2017, 16, 943–955. [Google Scholar] [CrossRef]
- Lawrence, G.; Holley, C.L.; Schroder, K. Come on mtDNA, light my fire. Immunity 2022, 55, 1331–1333. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.T.; Huynh, T.D.; Wang, C.S.; Lai, K.H.; Lin, Z.C.; Lin, W.N.; Chen, Y.L.; Peng, T.Y.; Wu, H.C.; Lee, I.T. The Potential Implications of Hydrogen Sulfide in Aging and Age-Related Diseases through the Lens of Mitohormesis. Antioxidants 2022, 11, 1619. [Google Scholar] [CrossRef]
- Ji, Y.; Li, Y.; Zhao, Z.; Li, P.; Xie, Y. Hydrogen Sulfide Overproduction Is Involved in Acute Ischemic Cerebral Injury Under Hyperhomocysteinemia. Front. Neurosci. 2020, 14, 582851. [Google Scholar] [CrossRef] [PubMed]
- Davoli, A.; Greco, V.; Spalloni, A.; Guatteo, E.; Neri, C.; Rizzo, G.R.; Cordella, A.; Romigi, A.; Cortese, C.; Bernardini, S.; et al. Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann. Neurol. 2015, 77, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Dai, J.; Xia, Y.; He, K.; Xue, H.; Guo, Q.; Tian, D.; Xiao, L.; Zhang, X.; Teng, X.; et al. Hydrogen Sulfide Ameliorated High Choline-Induced Cardiac Dysfunction by Inhibiting cGAS-STING-NLRP3 Inflammasome Pathway. Oxid. Med. Cell Longev. 2022, 2022, 1392896. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, S.; Chen, Y.; Shen, J.; Zheng, Y.; Liu, X.; Zhu, M.; Meng, G. Protective role of hydrogen sulfide against diabetic cardiomyopathy via alleviating necroptosis. Free Radic. Biol. Med. 2022, 181, 29–42. [Google Scholar] [CrossRef]
- Basic, A.; Alizadehgharib, S.; Dahlen, G.; Dahlgren, U. Hydrogen sulfide exposure induces NLRP3 inflammasome-dependent IL-1beta and IL-18 secretion in human mononuclear leukocytes in vitro. Clin. Exp. Dent. Res. 2017, 3, 115–120. [Google Scholar] [CrossRef]
- Hu, X.; Chi, Q.; Liu, Q.; Wang, D.; Zhang, Y.; Li, S. Atmospheric H2S triggers immune damage by activating the TLR-7/MyD88/NF-kappaB pathway and NLRP3 inflammasome in broiler thymus. Chemosphere 2019, 237, 124427. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, G.X.; Zhao, G.F.; Zhao, S.F. Characterization of the metabolites of irisflorentin by using ultra-high performance liquid chromatography combined with quadrupole/orbitrap tandem mass spectrometry. J. Pharm. Biomed. Anal. 2021, 203, 114222. [Google Scholar] [CrossRef]
- Noh, D.; Choi, J.G.; Lee, Y.B.; Jang, Y.P.; Oh, M.S. Protective effects of Belamcandae Rhizoma against skin damage by ameliorating ultraviolet-B-induced apoptosis and collagen degradation in keratinocytes. Environ. Toxicol. 2019, 34, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, S.P.; Lin, H.L.; Chan, M.C.; Chen, Y.C.; Huang, Y.L.; Tsai, M.C.; Fu, R.H. Irisflorentin improves alpha-synuclein accumulation and attenuates 6-OHDA-induced dopaminergic neuron degeneration, implication for Parkinson’s disease therapy. Biomedicine 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.H.; Tsai, C.W.; Tsai, R.T.; Liu, S.P.; Chan, T.M.; Ho, Y.C.; Lin, H.L.; Chen, Y.M.; Hung, H.S.; Chiu, S.C.; et al. Irisflorentin modifies properties of mouse bone marrow-derived dendritic cells and reduces the allergic contact hypersensitivity responses. Cell Transpl. 2015, 24, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, L.; Liu, F.; Zong, C.; Cai, R.; Chen, X.; Qi, Y. Suppressive effects of irisflorentin on LPS-induced inflammatory responses in RAW 264.7 macrophages. Exp. Biol. Med. 2014, 239, 1018–1024. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.-H.; Chen, H.-J.; Hong, S.-Y. Glycine-Alanine Dipeptide Repeat Protein from C9-ALS Interacts with Sulfide Quinone Oxidoreductase (SQOR) to Induce the Activity of the NLRP3 Inflammasome in HMC3 Microglia: Irisflorentin Reverses This Interaction. Antioxidants 2023, 12, 1896. https://doi.org/10.3390/antiox12101896
Fu R-H, Chen H-J, Hong S-Y. Glycine-Alanine Dipeptide Repeat Protein from C9-ALS Interacts with Sulfide Quinone Oxidoreductase (SQOR) to Induce the Activity of the NLRP3 Inflammasome in HMC3 Microglia: Irisflorentin Reverses This Interaction. Antioxidants. 2023; 12(10):1896. https://doi.org/10.3390/antiox12101896
Chicago/Turabian StyleFu, Ru-Huei, Hui-Jye Chen, and Syuan-Yu Hong. 2023. "Glycine-Alanine Dipeptide Repeat Protein from C9-ALS Interacts with Sulfide Quinone Oxidoreductase (SQOR) to Induce the Activity of the NLRP3 Inflammasome in HMC3 Microglia: Irisflorentin Reverses This Interaction" Antioxidants 12, no. 10: 1896. https://doi.org/10.3390/antiox12101896