How Antioxidants, Osmoregulation, Genes and Metabolites Regulate the Late Seeding Tolerance of Rapeseeds (Brassica napus L.) during Wintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Materials and Experimental Design
2.1.1. Conditions of Experiment Site
2.1.2. Plant Materials
2.1.3. Experimental Design
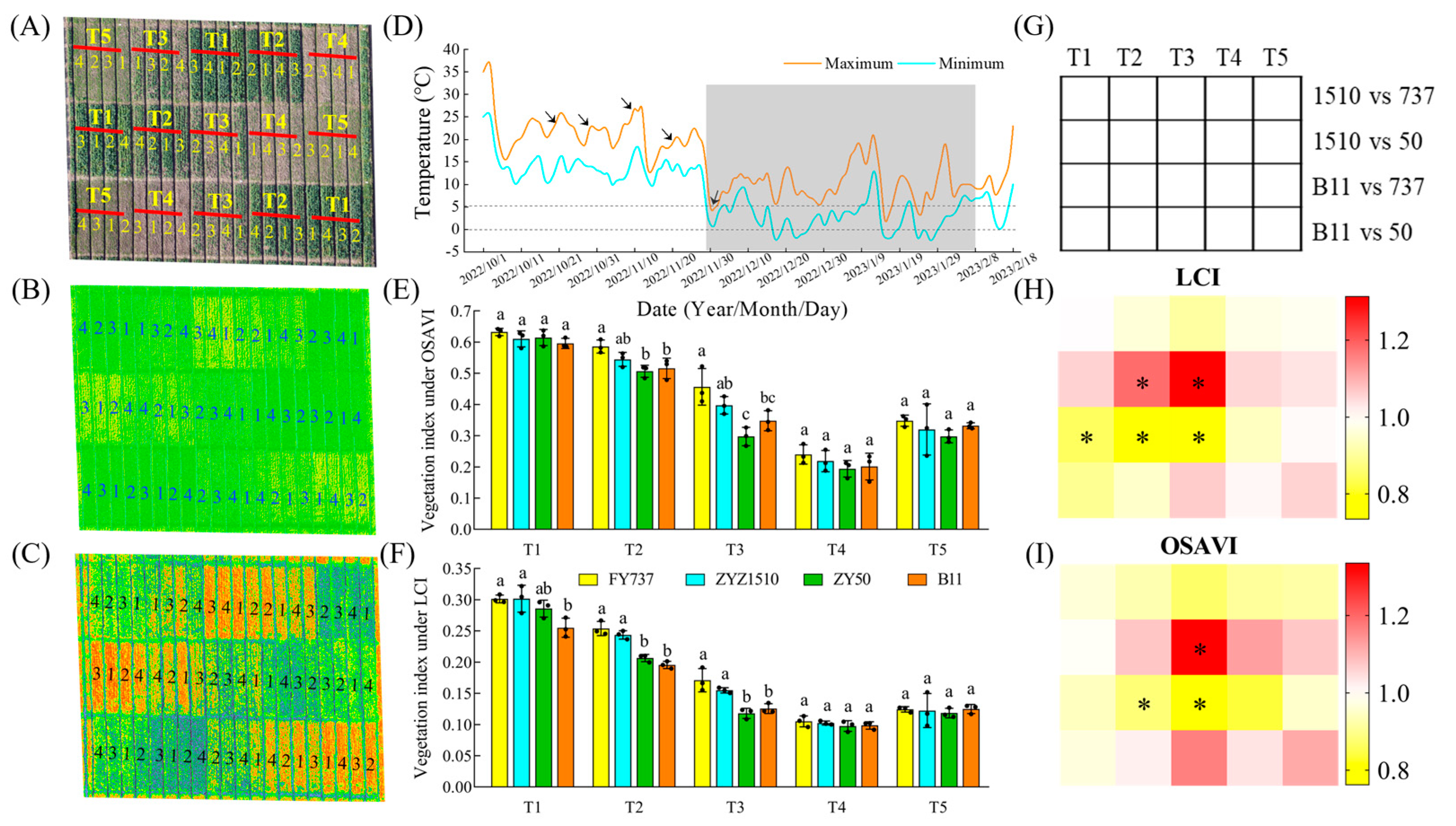
2.2. Unmanned Aerial Vehicle (UAV) Aerial Photography and Vegetation Index Measurement
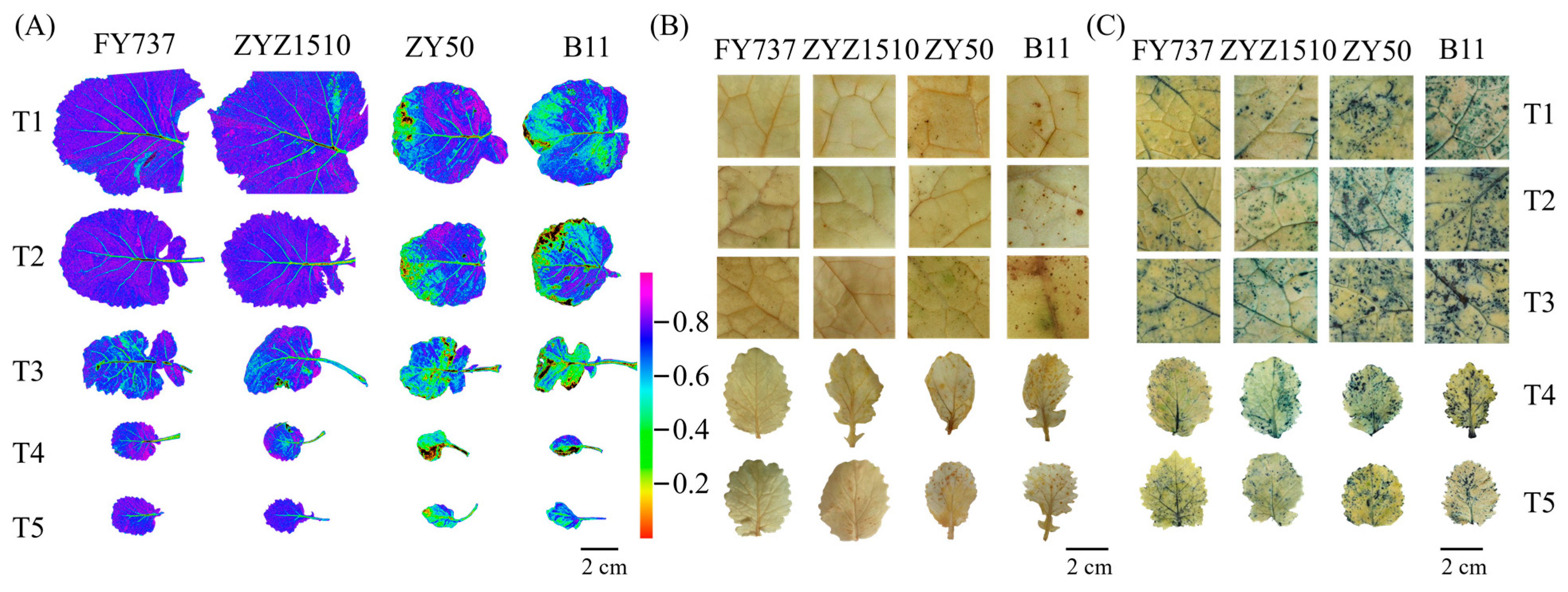
2.3. Chlorophyll Fluorescence Imaging and Histochemical Staining with DAB and NBT
2.4. Quantification of Osmoregulation Substances and Antioxidase Activities
2.5. Transcriptomic and Metabolomic Analysis
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA) of Transcriptome and Metabolome Data
2.7. Statistical Analysis
3. Results
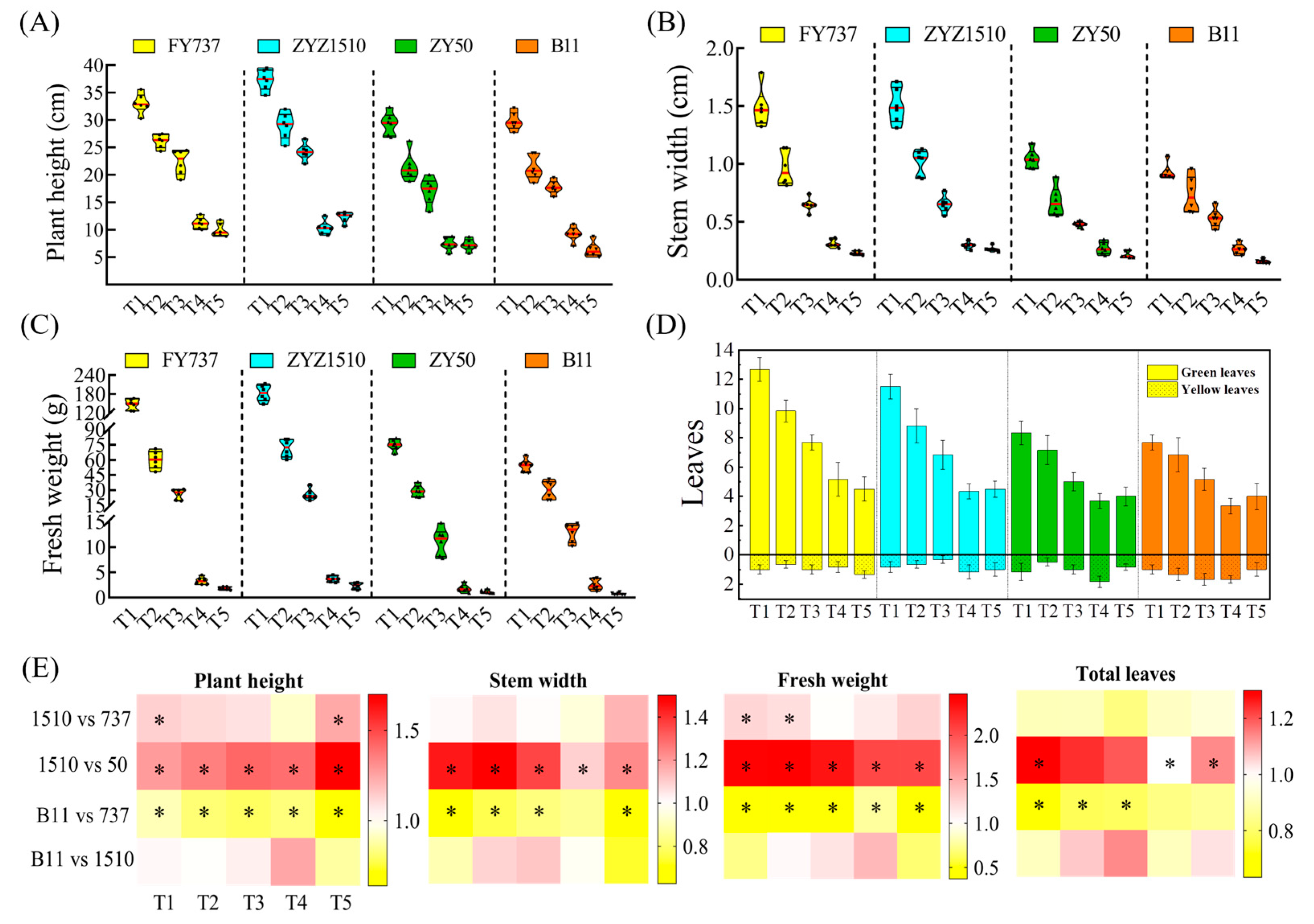
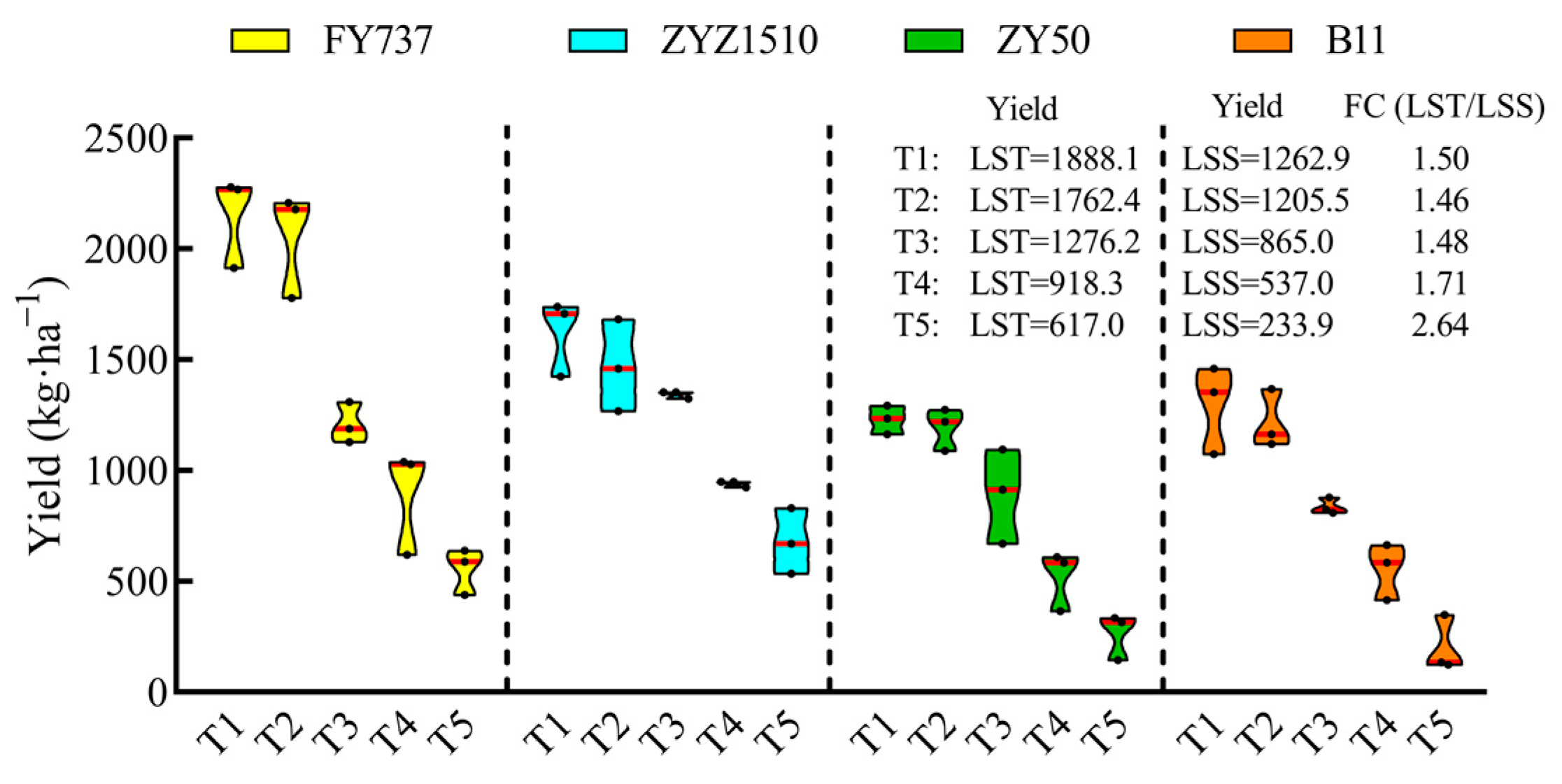
3.1. The Growth and Yield Were Significantly Repressed with the Delay in Seeding Date, While ZYZ1510 and FY737 Were Better According to UAV Imaging and Agronomic Parameters
3.2. Obviously Lower ROS Levels and Stress Degrees Were Detected in LST Cultivars Compared with LSS
3.3. The Stronger Activities of Antioxidase May Account for the Better Growth of LST Cultivars under Late Seeding Conditions, though Their Contents Were Decreased with the Delay in Seeding
3.4. The Increased Accumulation of Osmoregulation Substances Contributed to a Higher Late Seeding Tolerance
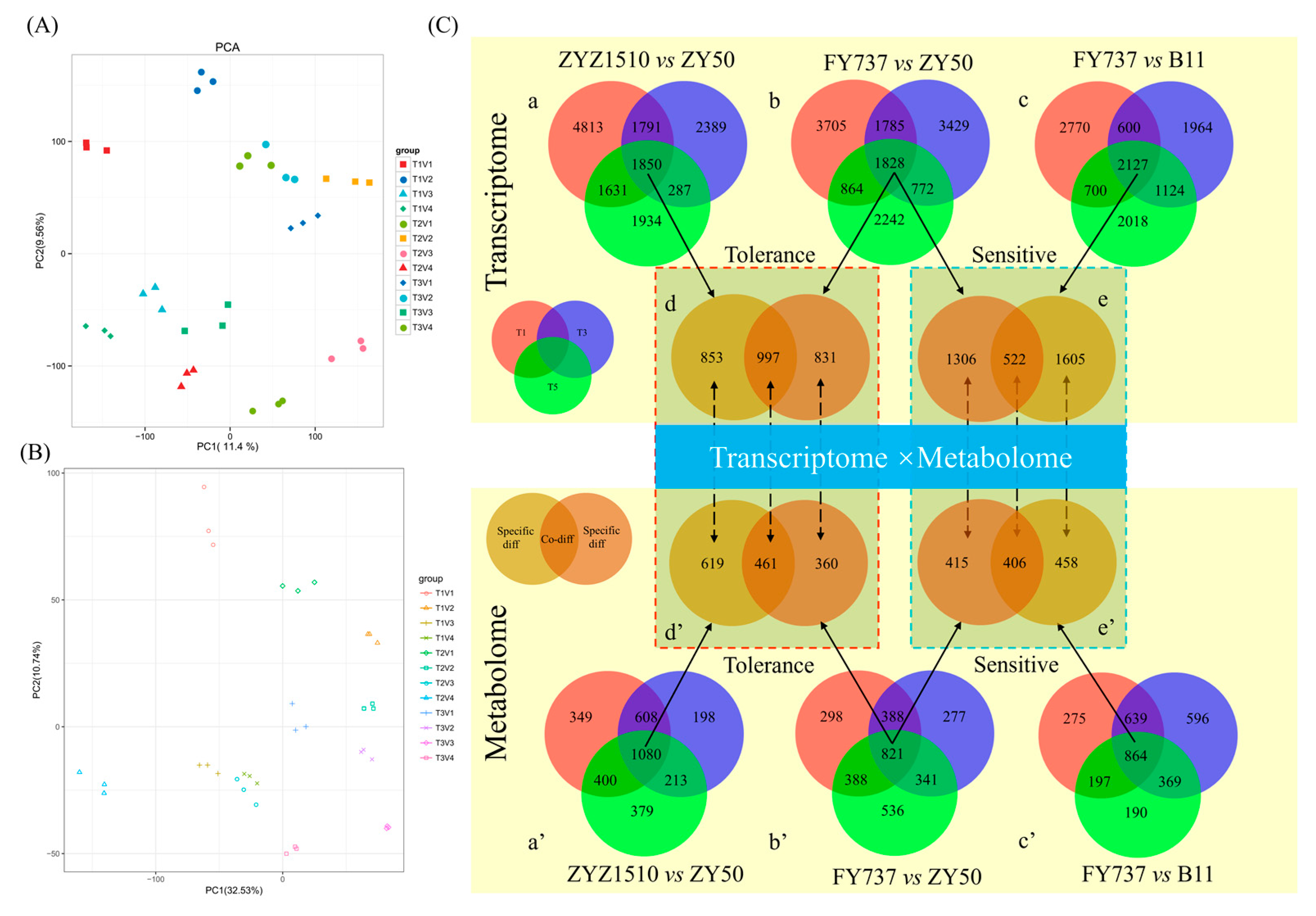
3.5. Identification and WGCNA Analysis of Candidate Late-Seeding-Tolerant/-Sensitive DEGs and DAMs
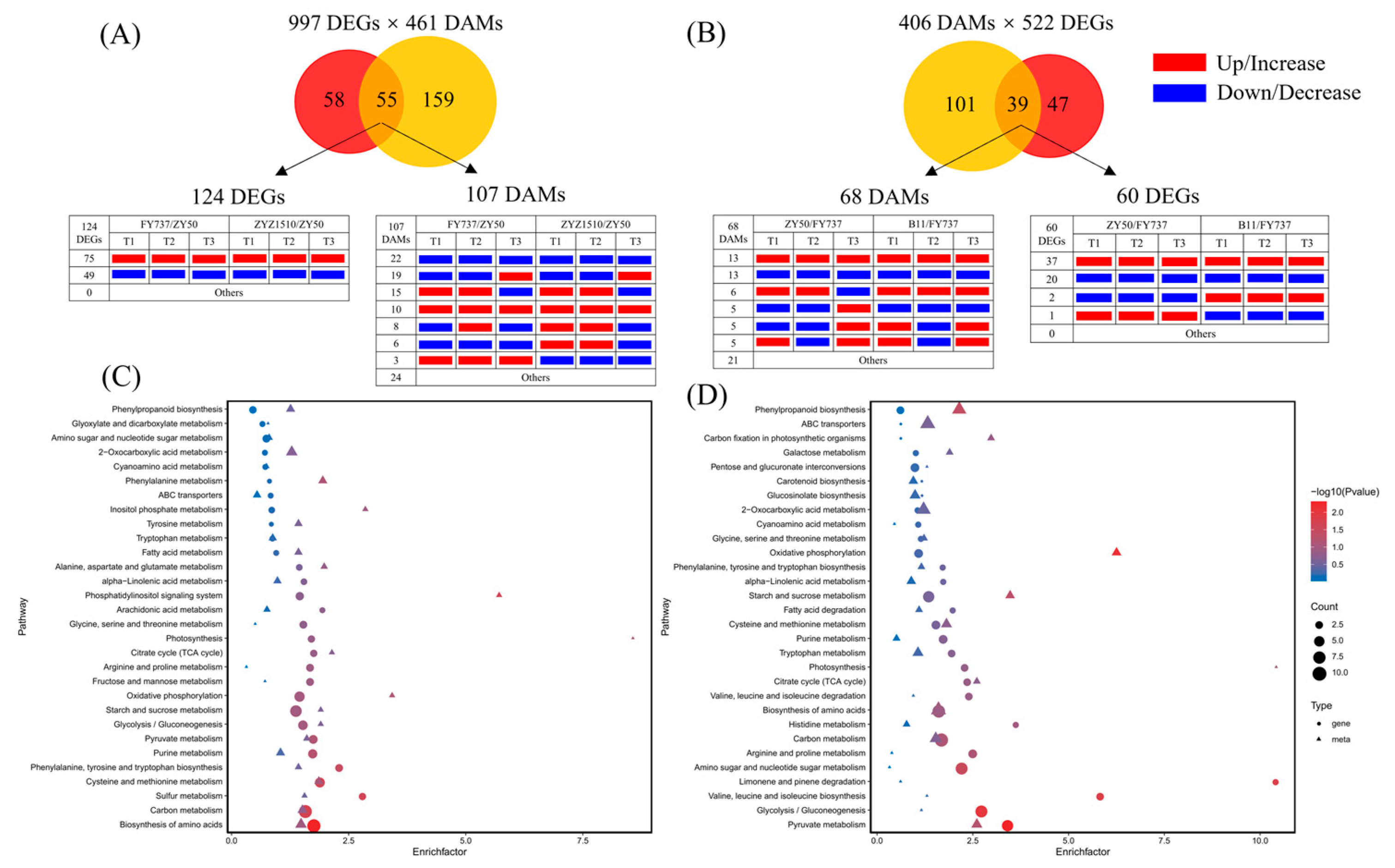
3.6. Candidate Genes, Metabolites and Pathways Involved in Late Seeding Response Were Further Identified via Pathway Interaction Analysis
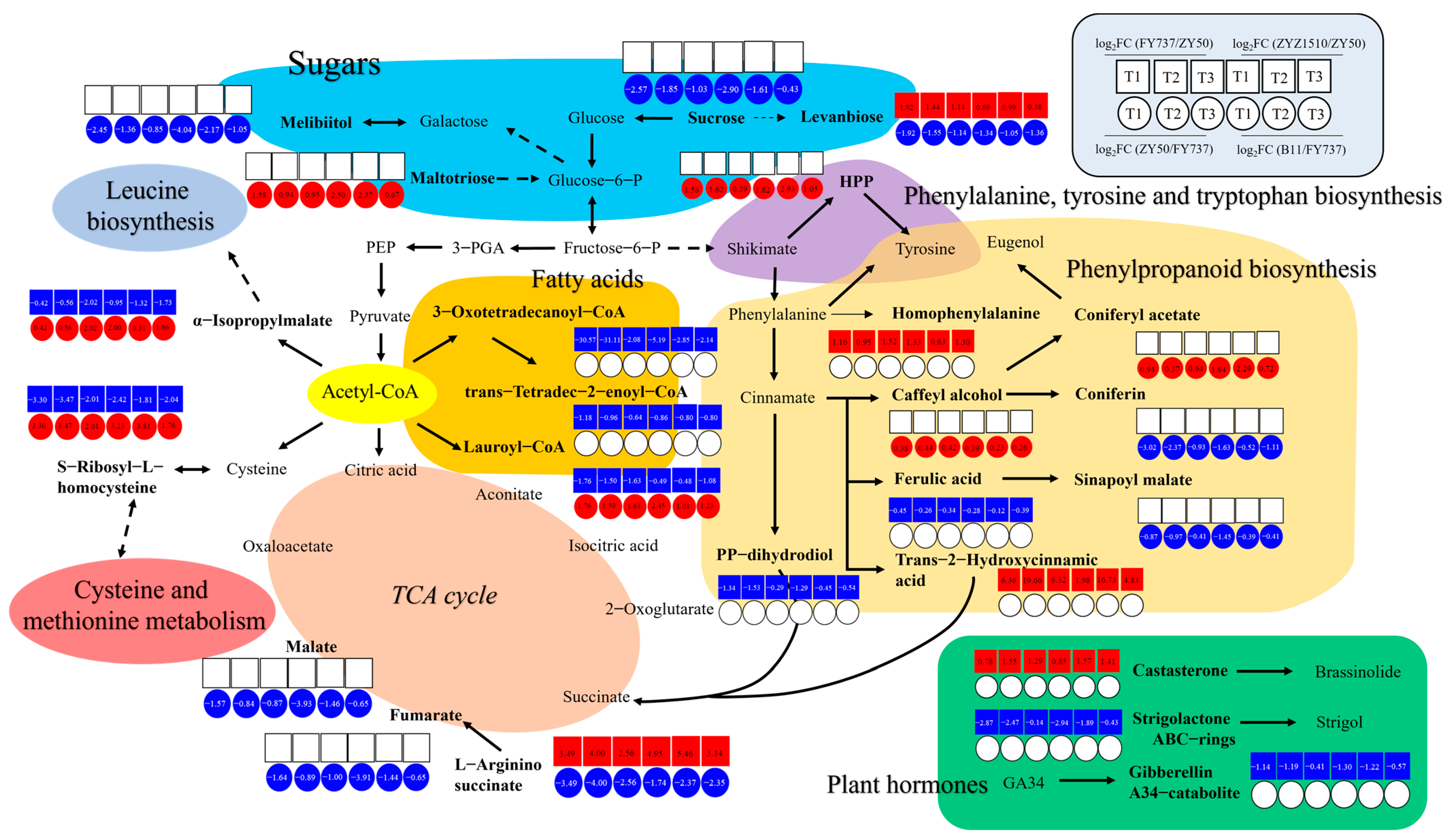
3.7. TCA and Phenylpropanoid Biosynthesis Dominated the Regulation Network Underlying the Regulation of Late Seeding Tolerance/Sensitivity
3.8. Complicated Gene Regulation Network of Late Seeding Response under Late Seeding Conditions
4. Discussion
4.1. Rapeseed Production Was Largely Restricted by Climate Change under the Rice–Rape Rotation System, While LST Cultivars Showed Better Yield Resilience than LSS Cultivars
4.2. Antioxidase and Osmoregulation Substances Played Important Roles in the Adaption Process of Rapeseed to Late Seeding
4.3. Interaction Analysis of Multi-Omics Contributed to the Mechanism Identification of Late Seeding Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Joshua, E.; Delphine, D.; Alex, C.R.; Christoph, M.; Almu, A.; Kenneth, J.B.; Christian, F.; Michael, G.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Zabel, F.; Mueller, C.; Elliott, J.; Minoli, S.; Jagermeyr, J.; Schneider, J.M.; Franke, J.A.; Moyer, E.; Dury, M.; Francois, L.; et al. Large potential for crop production adaptation depends on available future varieties. Glob. Chang. Biol. 2021, 27, 3870–3882. [Google Scholar] [CrossRef]
- Restivo, I.; Basilicata, M.G.; Giardina, I.C.; Massaro, A.; Pepe, G.; Salviati, E.; Pecoraro, C.; Carbone, D.; Cascioferro, S.; Parrino, B.; et al. A Combination of Polymethoxyflavones from Citrus sinensis and Prenylflavonoids from Humulus lupulus Counteracts IL-1β-Induced Differentiated Caco-2 Cells Dysfunction via a Modulation of NF-κB/Nrf2 Activation. Antioxidants 2023, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Ewert, F.; Martre, P.; Roetter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Hua, S.J.; Lin, B.G.; Hussain, N.; Zhang, Y.F.; Yu, H.S.; Ren, Y.; Ding, H.D.; Zhang, D.Q. Delayed planting affects seed yield, biomass production, and carbohydrate allocation in canola (Brassica napus L.). Int. J. Agric. Biol. 2014, 14, 671–680. [Google Scholar]
- Xian, M.Z.; Yang, P.; Hu, L.Y.; Xu, Z.H. Comprehensive evaluation of low temperature tolerance in rapeseed during germination and emergence periods. Crops 2015, 5, 116–122. [Google Scholar]
- Bigot, S.; Buges, J.; Gilly, L.; Jacques, C.; Le Boulch, P.; Berger, M.; Delcros, P.; Domergue, J.B.; Koehl, A.; Ley-Ngardigal, B.; et al. Pivotal roles of environmental sensing and signaling mechanisms in plant responses to climate change. Glob. Chang. Biol. 2018, 24, 5573–5589. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, Y.H.; Sun, L.X.; Xu, X.L.; Fan, D.L.; Zhong, H.L.; Liang, Z.R.; Ficsher, G. Changes in production potentials of rapeseed in the Yangtze River Basin of China under climate change: A multi-model ensemble approach. J. Geogr. Sci. 2018, 28, 1700–1714. [Google Scholar] [CrossRef]
- Luo, T.; Sheng, Z.W.; Zhang, C.N.; Li, Q.; Liu, X.Y.; Qu, Z.J.; Xu, Z.H. Seed Characteristics affect low-temperature stress tolerance performance of rapeseed (Brassica napus L.) during seed germination and seedling emergence stages. Agronomy 2022, 12, 1969. [Google Scholar] [CrossRef]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.K.; Liu, L.J.; Shi, J.Q.; Zhao, Y.G.; Qin, L.; Chen, C.; Wang, H.Z. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Li, X.; Zuo, Q.S.; Chang, H.B.; Bai, G.P.; Kuai, J.; Zhou, G.S. Higher density planting benefits mechanical harvesting of rapeseed in the Yangtze River Basin of China. Field Crop Res. 2018, 218, 97–105. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.F.; Hussain, N.; Ma, S.L.; Ye, G.R.; Zhang, D.Q.; Hua, S.J. Seedling age and quality upon transplanting affect seed yield of canola (Brassica napus L.). Can. J. Plant Sci. 2014, 94, 1461–1469. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Sprague, S.J.; Fettell, N.A.; Pengilley, G.C. Re-evaluating sowing time of spring canola (Brassica napus L.) in south-eastern Australia-how early is too early? Crop Pasture Sci. 2016, 67, 381–396. [Google Scholar] [CrossRef]
- Gorzin, M.; Ghaderi-Far, F.; Sadeghipour, H.R.; Zeinali, E. Induced thermo-dormancy in rapeseed (Brassica napus L.) cultivars by sub- and supra-optimal temperatures. J. Plant Growth Regul. 2020, 40, 2164–2177. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, Y.; Xu, H.; Qiu, H.; Sun, L.; Zhong, H.; Liu, J. The potential contribution of growing rapeseed in winter fallow fields across Yangtze River Basin to energy and food security in China. Resour. Conserv. Recycl. 2021, 164, 105159. [Google Scholar] [CrossRef]
- Zabel, F.; Delzeit, R.; Schneider, J.M.; Seppelt, R.; Mauser, W.; Vaclaavik, T. Global impacts of future cropland expansion and intensification on agricultural markets and biodiversity. Nat. Commun. 2019, 10, 2844. [Google Scholar] [CrossRef]
- Zhang, C.N.; Luo, T.; Liu, J.H.; Xian, M.Z.; Yuan, J.Z.; Hu, L.Y.; Xu, Z.H. Evaluation of the low-temperature tolerance of rapeseed genotypes at the germination and seedling emergence stages. Crop Sci. 2019, 59, 1709–1717. [Google Scholar] [CrossRef]
- Qin, M.; Li, H.; Guo, Z.; Zhu, Y.; Wang, R.; Zhang, M.; Zhang, Q.; Xu, Y.; Song, J.; Huang, Z. Phenotypic damage and transcriptomic responses of flower buds in rapeseed (Brassica napus L.) under low-temperature stress. Ind. Crop Prod. 2023, 198, 116669. [Google Scholar] [CrossRef]
- Mehmood, S.S.; Lu, G.Y.; Luo, D.; Hussain, M.A.; Raza, A.; Zafar, Z.; Zhang, X.K.; Cheng, Y.; Zou, X.L.; Lv, Y. Integrated analysis of transcriptomics and proteomics provides insights into the molecular regulation of cold response in Brassica napus. Environ. Exp. Bot. 2021, 187, 104480. [Google Scholar] [CrossRef]
- Liu, L.; Pu, Y.Y.; Niu, Z.X.; Wu, J.Y.; Fang, Y.; Xu, J.; Xu, F.; Yue, J.L.; Ma, L.; Li, X.C.; et al. Transcriptomic insights into root development and overwintering transcriptional memory of Brassica rapa L. Grown in the field. Front. Plant Sci. 2022, 13, 900708. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Luo, T.; Khan, M.N.; Hu, L.Y.; Xu, Z.H. Identifying differentially expressed genes associated with tolerance against low temperature stress in Brassica napus through transcriptome analysis. Int. J. Agric. Biol. 2017, 19, 273–281. [Google Scholar] [CrossRef]
- Yue, J.B.; Feng, H.K.; Jin, X.L.; Yuan, H.H.; Li, Z.H.; Zhou, C.Q.; Yang, G.L.; Tian, Q.J. A comparison of crop parameters estimation using images from UAV-mounted snapshot hyperspectral sensor and high-definition digital camera. Remote Sens. 2018, 10, 1138. [Google Scholar] [CrossRef]
- Hao, P.F.; Qiu, C.W.; Ding, G.H.; Vincze, E.; Zhang, G.P.; Zhang, Y.S.; Wu, F.B. Agriculture organic wastes fermentation CO2 enrichment in greenhouse and the fermentation residues can improve growth, yield and fruit quality in tomato. J. Clean. Prod. 2020, 275, 123885. [Google Scholar] [CrossRef]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Gupta, V.K.; Upadhyay, R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME). Front. Plant Sci. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Hao, P.F.; Lin, B.G.; Ren, Y.; Hu, H.; Xue, B.W.; Huang, L.; Hua, S.J. Auxin-regulated timing of transition from vegetative to reproductive growth in rapeseed (Brassica napus L.) under different nitrogen application rates. Front. Plant Sci. 2022, 13, 927662. [Google Scholar] [CrossRef]
- Liu, Y.J.; Ye, S.H.; Yuan, G.G.; Ma, X.W.; Heng, S.P.; Yi, B.; Ma, C.Z.; Shen, J.X.; Tu, J.X.; Fu, T.D.; et al. Gene silencing of BnaA09.ZEP and BnaC09.ZEP confers orange color in Brassica napus flowers. Plant J. 2020, 104, 932–949. [Google Scholar] [CrossRef]
- Zhang, G.; He, P.J.; Tan, H.S.; Budhu, A.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Yfantis, H.G.; Lee, D.H.; Maitra, A.; et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effect in human pancreatic cancer. Clin. Cancer Res. 2013, 19, 4983–4993. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Yue, J.H.; Dong, Y.; Liu, S.H.; Jia, Y.A.; Li, C.X.; Wang, Z.Y.; Gong, S.F. Integrated proteomic and metabolomic analyses provide insights into acquisition of embryogenic ability in agapanthus praecox. Front. Plant Sci. 2022, 13, 858065. [Google Scholar] [CrossRef]
- Liu, X.H.; Wei, R.; Tian, M.Y.; Liu, J.C.; Ruan, Y.; Sun, C.X.; Liu, C.L. Combined transcriptome and metabolome profiling provide insights into cold responses in Rapeseed (Brassica napus L.) genotypes with contrasting cold-stress sensitivity. Int. J. Mol. Sci. 2022, 23, 13546. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernard, S.M.; Markelz, R.J.C.; Ort, D.R.; Placella, S.A.; Rogers, A.; Smith, M.D.; Sudderth, E.A.; Weaton, D.J. Gene expression profiling: Opening the black box of plant ecosystem responses to global change. Glob. Chang. Biol. 2009, 15, 1201–1213. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A. Climate change CO2, and defense: The metabolic, redox and signaling perspectives. Trends Plant Sci. 2017, 22, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef]
- Wu, W.Z.; Yang, H.B.; Xing, P.; Dong, Y.; Shen, J.; Wu, G.F.; Zheng, S.; Da, L.; He, J.T.; Wu, Y.J. Comparative transcriptome analysis revealed the freezing tolerance signaling events in winter rapeseed (Brassica rapa L.). Front. Genet. 2022, 13, 871825. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.Y.; Wang, Z.R.; Baskin, C.C.; Baskin, J.M.; Ye, R.H.; Sun, H.L.; Zhang, Y.Y.; Ye, X.H.; Liu, G.F.; Yang, X.J.; et al. Seed germination responses to seasonal temperature and drought stress are species-specific but not related to seed size in a desert steppe: Implications for effect of climate change on community structure. Ecol. Evol. 2019, 9, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Luo, W.; Zhao, Y.; Xu, Y.Y.; Song, S.H.; Chong, K. Comparative metabolomic analysis reveals a reactive oxygen species dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol. 2016, 211, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.F.; Wang, W.R.; Jiang, M.Y.; Yang, L.Y.; Zhou, X.R. QTL mapping for low temperature germination in rapeseed. Sci. Rep. 2021, 11, 23382. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yao, F.; Xue, T.T.; Wang, Z.L.; Wang, Y.; Cao, X.; Hui, M.; Wu, D.; Li, Y.H.; Wang, H. Sprayed biodegradable liquid film improved the freezing tolerance of cv. Cabernet Sauvignon by up-regulating soluble protein and carbohydrate levels and alleviating oxidative damage. Front. Plant Sci. 2022, 13, 1021483. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Li, Y.Y.; Zhong, H.; Li, X.L. Role of osmotic regulation and cryoprotectant substances in the freezing tolerance of alfalfa in cold, dry conditions. Legume Res. 2022, 45, 952–959. [Google Scholar] [CrossRef]
- Lu, J.Y.; Nawaz, M.A.; Wei, N.N.; Cheng, F.; Bie, Z.L. Suboptimal temperature acclimation enhances chilling tolerance by improving photosynthetic adaptability and osmoregulation ability in watermelon. Hortic. Plant J. 2020, 6, 49–60. [Google Scholar] [CrossRef]
- Ohbayashi, I.; Huang, S.B.; Fukaki, H.; Song, X.M.; Sun, S.; Moria, M.T.; Tasaka, M.; Millar, A.H.; Furutani, M. Mitochondrial pyruvate dehydrogenase contributes to auxin-regulated organ development. Plant Physiol. 2019, 180, 896–909. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef]
- Xu, Q.; Yin, X.R.; Zeng, J.K.; Ge, H.; Song, M.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Activator-and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J. Exp. Bot. 2014, 65, 4349–4359. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, T.A.; Kim, J.Y.; Wedde, A.E.; Levin, L.A.; Schmitt, K.C.; Schuurink, R.C.; Clark, D.G. PhMYB4 fine-tunes the floral volatile signature of Petunia × hybrida through PhC4H. J. Exp. Bot. 2011, 62, 1133–1143. [Google Scholar] [CrossRef]
- Matros, A.; Amme, S.; Kettig, B.; Buck-Sorlin, G.H.; Sonnewald, U.; Mock, H.P. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 2006, 29, 126–137. [Google Scholar] [CrossRef]
- Lee, D.G.; Ahsan, N.; Lee, S.H.; Lee, J.J.; Bahk, J.D.; Kang, K.Y.; Lee, B.H. Chilling stress-induced proteomic changes in rice roots. J. Plant Physiol. 2009, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Q.; Dai, Z.Z.; Zhang, Y.T.; Iqbal, S.; Lu, S.P.; Guo, L.; Yao, X. Proteome-wide identification of S-sulfenylated cysteines reveals metabolic response to freezing stress after cold acclimation in Brassica napus. Front. Plant Sci. 2022, 13, 1014295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, S.S.; Zhao, S.M.; Chen, D.Y.; Tian, H.Y.; Li, J.; Zhang, L.R.; Li, S.G.; Liu, L.; Shi, C.A. Global crotonylatome and GWAS revealed a TaSRT1-TaPGK model regulating wheat cold tolerance through mediating pyruvate. Sci. Adv. 2023, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Vásquez, J.; Raynal, M.; Delseny, M. A rapeseed cold-inducible transcript encodes a phosphoenolpyruvate carboxykinase. Plant Physiol. 1995, 109, 611–618. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, P.; Lin, B.; Ren, Y.; Hu, H.; Lou, W.; Yi, K.; Xue, B.; Huang, L.; Li, X.; Hua, S. How Antioxidants, Osmoregulation, Genes and Metabolites Regulate the Late Seeding Tolerance of Rapeseeds (Brassica napus L.) during Wintering. Antioxidants 2023, 12, 1915. https://doi.org/10.3390/antiox12111915
Hao P, Lin B, Ren Y, Hu H, Lou W, Yi K, Xue B, Huang L, Li X, Hua S. How Antioxidants, Osmoregulation, Genes and Metabolites Regulate the Late Seeding Tolerance of Rapeseeds (Brassica napus L.) during Wintering. Antioxidants. 2023; 12(11):1915. https://doi.org/10.3390/antiox12111915
Chicago/Turabian StyleHao, Pengfei, Baogang Lin, Yun Ren, Hao Hu, Weidong Lou, Kaige Yi, Bowen Xue, Lan Huang, Xi Li, and Shuijin Hua. 2023. "How Antioxidants, Osmoregulation, Genes and Metabolites Regulate the Late Seeding Tolerance of Rapeseeds (Brassica napus L.) during Wintering" Antioxidants 12, no. 11: 1915. https://doi.org/10.3390/antiox12111915
APA StyleHao, P., Lin, B., Ren, Y., Hu, H., Lou, W., Yi, K., Xue, B., Huang, L., Li, X., & Hua, S. (2023). How Antioxidants, Osmoregulation, Genes and Metabolites Regulate the Late Seeding Tolerance of Rapeseeds (Brassica napus L.) during Wintering. Antioxidants, 12(11), 1915. https://doi.org/10.3390/antiox12111915






