Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review
Abstract
:1. Introduction
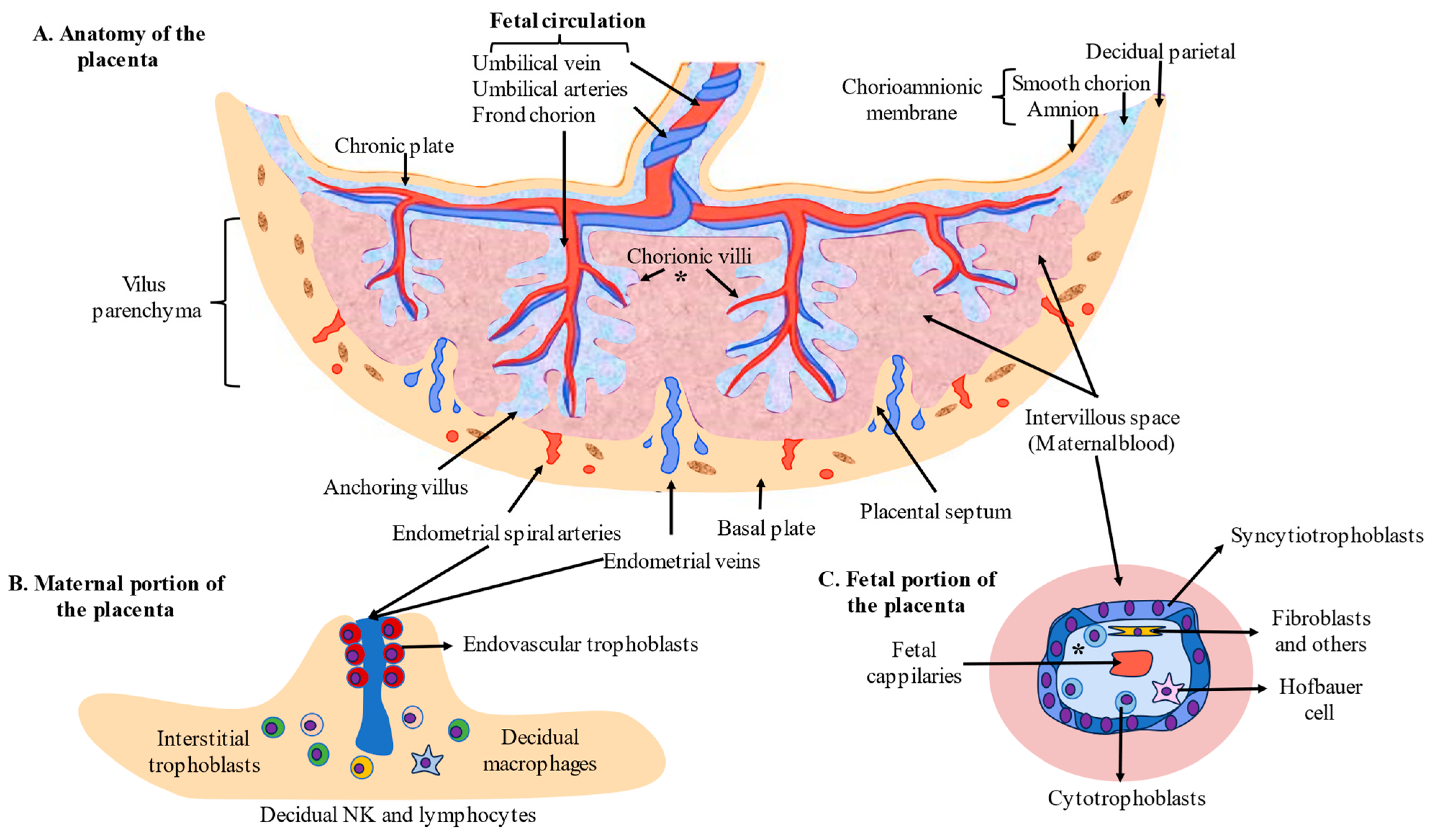
2. Development and Cytoarchitecture of the Placenta
3. Oxidative Stress in the Placenta in Physiological and Pathological Pregnancies
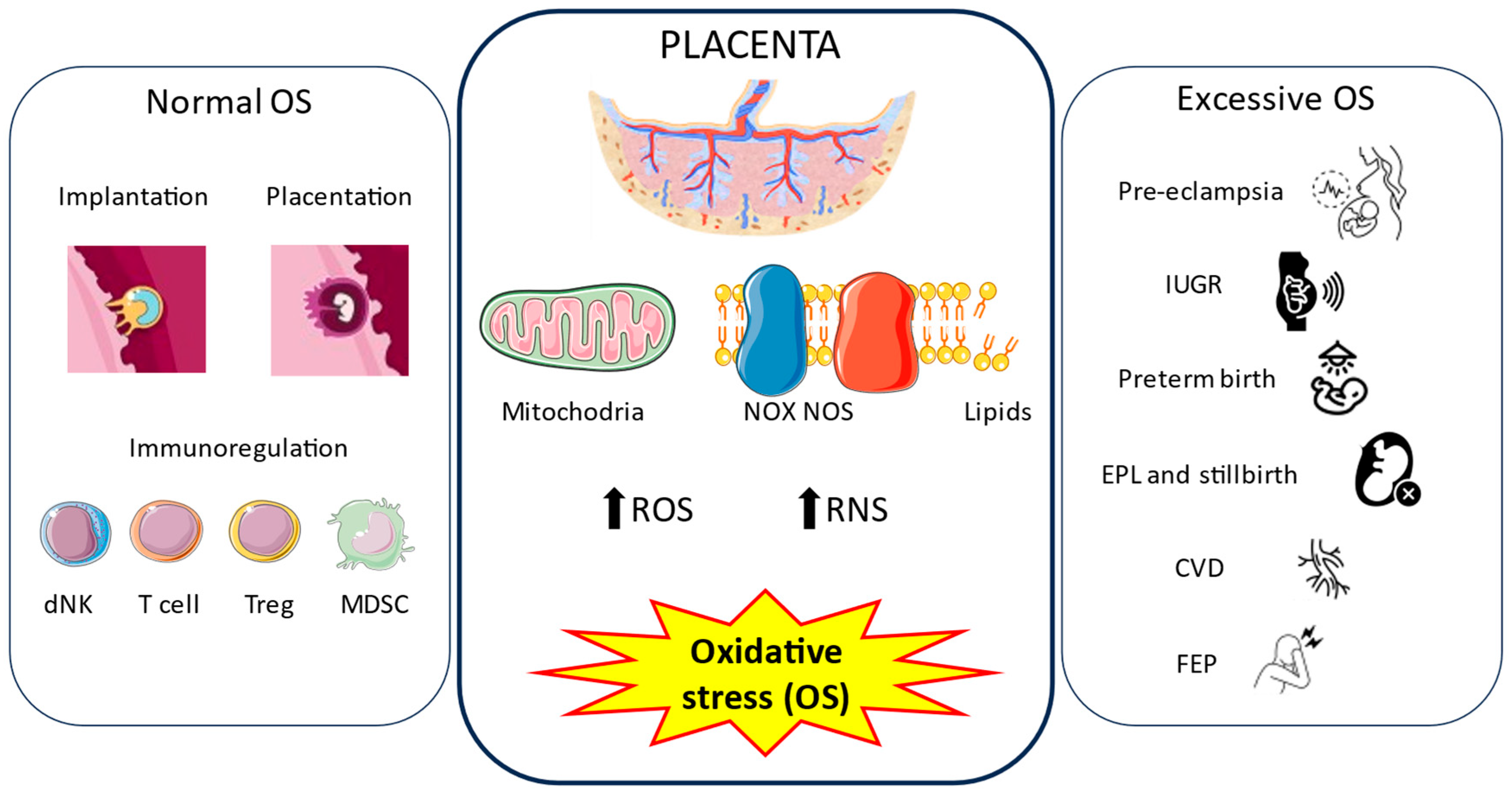
3.1. Physiological Role of Oxidative Stress in the Placenta
3.1.1. Origin and Consequences of OS in the Placenta
3.1.2. Implantation and Placentation
3.1.3. Immunomodulation
3.2. Oxidative Stress in Disease Conditions
3.2.1. Pre-Eclampsia
3.2.2. Intrauterine Growth Restriction
3.2.3. Gestational Diabetes Mellitus
3.2.4. Spontaneous Preterm Birth
3.2.5. Early Pregnancy Loss and Stillbirth
3.2.6. Excessive Gestational Weight Gain
3.2.7. Chronic Venous Disease
3.2.8. First-Episode Psychosis
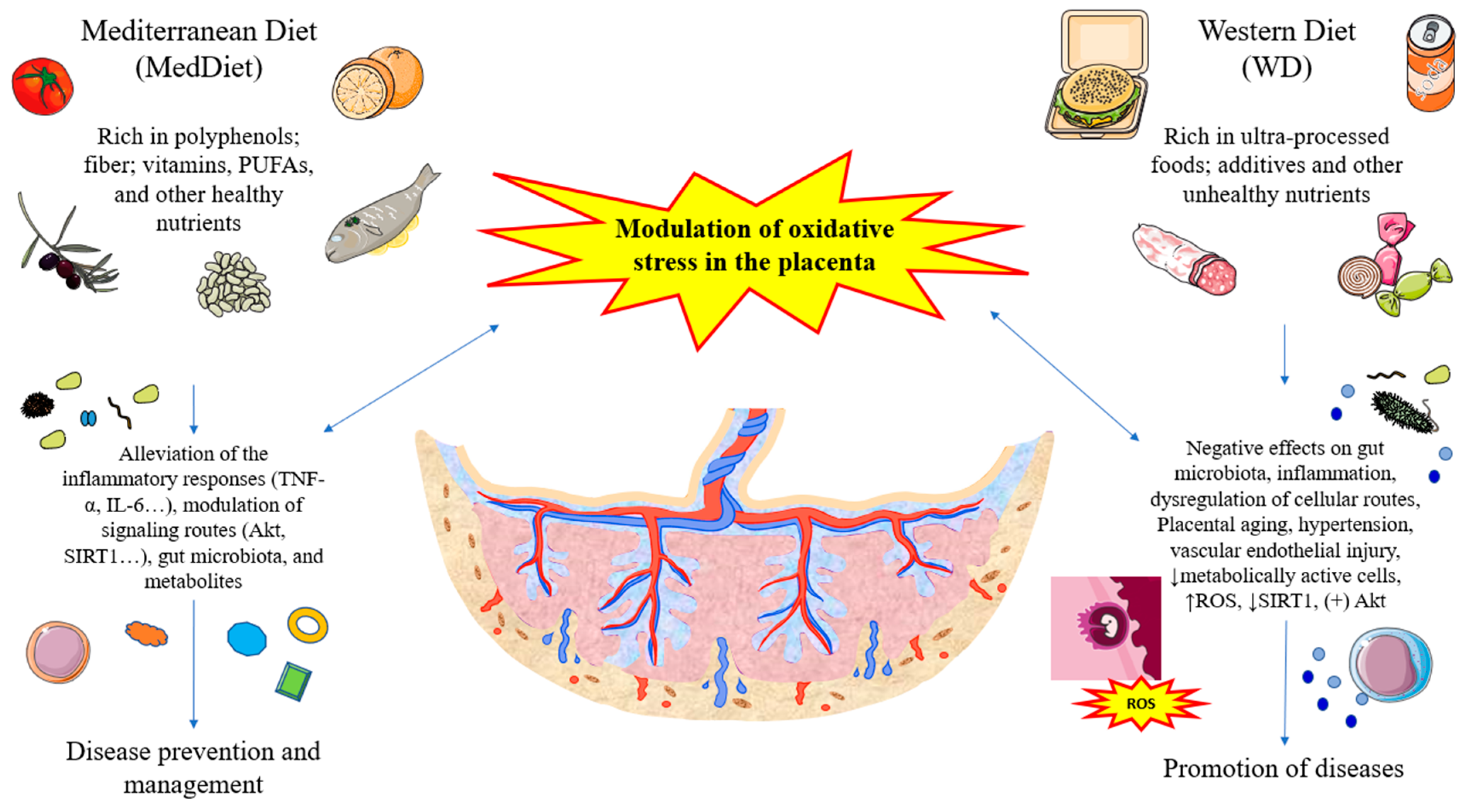
4. Maternal Diet, Oxidative Stress, and the Placenta Connection and Translational Opportunities
4.1. Role of Mediterranean Diet and Related Nutrients and Oxidative Stress in the Placenta
4.1.1. Fiber and Polyphenols
4.1.2. Vitamins
4.1.3. Polyunsaturated Fatty Acids
4.1.4. Choline
4.2. Role of Westernized and Unhealthy Dietary Patterns in the Placenta
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preiser, J.C. Oxidative Stress. JPEN J. Parenter. Enteral Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Araźna, M.; Demkc, U. Biochemistry of Oxidative Stress. Adv. Exp. Med. Biol. 2016, 878, 9–19. [Google Scholar]
- Ji, L.L.; Yeo, D. Oxidative Stress: An Evolving Definition. Fac. Rev. 2021, 10, 13. [Google Scholar] [CrossRef]
- Madkour, L.H. Cellular Signaling Pathways with Reactive Oxygen Species (ROS). In Reactive Oxygen Species (ROS), Nanoparticles, and Endoplasmic Reticulum (ER) Stress-Induced Cell Death Mechanism; Academic Press: Cambridge, MA, USA, 2020; pp. 37–79. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128224816000037?via%3Dihub (accessed on 19 September 2023).
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive Nitrogen Species in Cellular Signaling. Exp. Biol. Med. 2015, 240, 711. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016, 27, 89. [Google Scholar] [CrossRef]
- Troiano, N.H. Physiologic and Hemodynamic Changes during Pregnancy. AACN Adv. Crit. Care 2018, 29, 273–283. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative Stress: Normal Pregnancy versus Preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative Stress in Pregnancy and Reproduction. Obstet. Med. 2016, 9, 113. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. BioMed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351. [Google Scholar] [CrossRef]
- Staud, F.; Karahoda, R. Trophoblast: The Central Unit of Fetal Growth, Protection and Programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Renaud, S.J.; Jeyarajah, M.J. How Trophoblasts Fuse: An in-Depth Look into Placental Syncytiotrophoblast Formation. Cell. Mol. Life Sci. 2022, 79, 433. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S. Chapter 4, Cell Types of the Placenta. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53245/ (accessed on 19 September 2023).
- Turco, M.Y.; Moffett, A. Development of the Human Placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Huppertz, B. Human Placentation. Encycl. Reprod. 2018, 2, 431–439. [Google Scholar] [CrossRef]
- Kapila, V.; Chaudhry, K. Physiology, Placenta; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Barker, D.; Osmond, C.; Grant, S.; Thornburg, K.L.; Cooper, C.; Ring, S.; Davey-Smith, G. Maternal Cotyledons at Birth Predict Blood Pressure in Childhood. Placenta 2013, 34, 672–675. [Google Scholar] [CrossRef]
- Huppertz, B. The Anatomy of the Normal Placenta. J. Clin. Pathol. 2008, 61, 1296–1302. [Google Scholar] [CrossRef]
- Sanguansermsri, D.; Pongcharoen, S. Pregnancy Immunology: Decidual Im-Mune Cells. Asian Pac. J. Allergy Immunol. 2008, 26, 171–181. [Google Scholar] [PubMed]
- Kaufmann, P.; Huppertz, B.; Frank, H.G. The Fibrinoids of the Human Placenta: Origin, Composition and Functional Relevance. Ann. Anat. 1996, 178, 485–501. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef]
- Lagouge, M.; Larsson, N.G. The Role of Mitochondrial DNA Mutations and Free Radicals in Disease and Ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef]
- Mistry, H.D.; Williams, P.J. The Importance of Antioxidant Micronutrients in Pregnancy. Oxid. Med. Cell. Longev. 2011, 2011, 841749. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Fortunato, S.J.; Yu, J.; Milne, G.L.; Sanchez, S.; Drobek, C.O.; Lappas, M.; Taylor, R.N. Cigarette Smoke Induces Oxidative Stress and Apoptosis in Normal Term Fetal Membranes. Placenta 2011, 32, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Qiao, Y.; Maiti, K.; Smith, R. Involvement of Oxidative Stress in Placental Dysfunction, the Pathophysiology of Fetal Death and Pregnancy Disorders. Reproduction 2023, 166, R25–R38. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The Role of NFκB in Healthy and Preeclamptic Placenta: Trophoblasts in the Spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.P.; Crocker, I.P.; Mor, G. Placental Apoptosis in Health and Disease. Am. J. Reprod. Immunol. 2010, 64, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and Placental Development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Hypoxia and Reproductive Health: Oxygen and Development of the Human Placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, Oxidative Stress and Inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Herrera, E.A.; Krause, B.; Ebensperger, G.; Reyes, R.V.; Casanello, P.; Parra-Cordero, M.; Llanos, A.J. The Placental Pursuit for an Adequate Oxidant Balance between the Mother and the Fetus. Front. Pharmacol. 2014, 5, 93513. [Google Scholar] [CrossRef]
- Agarwal, A.; Rosas, I.M.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef]
- Hansen, J.M.; Jones, D.P.; Harris, C. The Redox Theory of Development. Antioxid. Redox Signal. 2020, 32, 715. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Pereira, R.D.; De Long, N.E.; Wang, R.C.; Yazdi, F.T.; Holloway, A.C.; Raha, S. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. BioMed Res. Int. 2015, 2015, 814543. [Google Scholar] [CrossRef]
- Wu, F.; Tian, F.J.; Lin, Y.; Xu, W.M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- Harlin, H.; Hanson, M.; Johansson, C.C.; Sakurai, D.; Poschke, I.; Norell, H.; Malmberg, K.-J.; Kiessling, R. The CD16- CD56(Bright) NK Cell Subset Is Resistant to Reactive Oxygen Species Produced by Activated Granulocytes and Has Higher Antioxidative Capacity than the CD16+ CD56(Dim) Subset. J. Immunol. 2007, 179, 4513–4519. [Google Scholar] [CrossRef] [PubMed]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune Responses at the Maternal-Fetal Interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef] [PubMed]
- Peraldi, M.-N.; Berrou, J.; Dulphy, N.; Seidowsky, A.; Haas, P.; Boissel, N.; Metivier, F.; Randoux, C.; Kossari, N.; Guérin, A.; et al. Oxidative Stress Mediates a Reduced Expression of the Activating Receptor NKG2D in NK Cells from End-Stage Renal Disease Patients. J. Immunol. 2009, 182, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Yang, Y.; Du, Z.; Fan, Y.; Zhao, Y.; Yuan, S. Oxidative Stress on Vessels at the Maternal-Fetal Interface for Female Reproductive System Disorders: Update. Front. Endocrinol. 2023, 14, 1118121. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.E.; Goulwara, S.S.; Whitley, G.S.; Cartwright, J.E. Oxygen Modulates Human Decidual Natural Killer Cell Surface Receptor Expression and Interactions with Trophoblasts. Biol. Reprod. 2014, 91, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Yarosz, E.L.; Chang, C.H. The Role of Reactive Oxygen Species in Regulating T Cell-Mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 2021, 12, 652687. [Google Scholar] [CrossRef]
- Bao, S.H.; Wang, X.P.; De Lin, Q.; Wang, W.J.; Yin, G.J.; Qiu, L.H. Decidual CD4+CD25+CD127dim/- Regulatory T Cells in Patients with Unexplained Recurrent Spontaneous Miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 94–98. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front. Immunol. 2018, 9, 2499. [Google Scholar] [CrossRef]
- Bingisser, R.M.; Tilbrook, P.A.; Holt, P.G.; Kees, U.R. Macrophage-Derived Nitric Oxide Regulates T Cell Activation via Reversible Disruption of the Jak3/STAT5 Signaling Pathway. J. Immunol. 1998, 160, 5729–5734. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of Pre-Eclampsia and the Other Hypertensive Disorders of Pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L. Introduction to ISSHP New Classification of Preeclampsia. Pregnancy Hypertens. 2013, 3, 58–59. [Google Scholar] [CrossRef]
- Raymond, D.; Peterson, E. A Critical Review of Early-Onset and Late-Onset Preeclampsia. Obstet. Gynecol. Surv. 2011, 66, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Jaleel, A.; Tamimi, W.; Al Kadri, H.M.F. Role of Oxidative Stress in the Pathogenesis of Preeclampsia. Arch. Gynecol. Obstet. 2010, 282, 469–474. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of Oxidative Stress in the Dysfunction of the Placental Endothelial Nitric Oxide Synthase in Preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Tashie, W.; Fondjo, L.A.; Owiredu, W.K.B.A.; Ephraim, R.K.D.; Asare, L.; Adu-Gyamfi, E.A.; Seidu, L. Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Larqué, E.; Ruiz-Palacios, M.; Koletzko, B. Placental Regulation of Fetal Nutrient Supply. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 292–297. [Google Scholar] [CrossRef]
- Jansson, T.; Aye, I.L.M.H.; Goberdhan, D.C.I. The Emerging Role of MTORC1 Signaling in Placental Nutrient-Sensing. Placenta 2012, 33 (Suppl. 2), e23–e29. [Google Scholar] [CrossRef]
- Jurado, S.; Saraiva, K.; Marceliano, C.; Souza, V.; Vieira, I. Maternal and Fetal Complications Due to Decreased Nitric Oxide Synthesis during Gestation. Complicat. Pregnancy 2019. [Google Scholar] [CrossRef]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-Medina, C.E.; Lopez, M. Endothelial Dysfunction and Preeclampsia: Role of Oxidative Stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [PubMed]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.L.M.; Davidge, S.T. Prenatal Hypoxia and Placental Oxidative Stress: Linkages to Developmental Origins of Cardiovascular Disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R395–R399. [Google Scholar] [CrossRef] [PubMed]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major Risk Factors for Stillbirth in High-Income Countries: A Systematic Review and Meta-Analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placental Oxidative DNA Damage and Its Repair in Preeclamptic Women with Fetal Growth Restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef]
- Kimura, C.; Watanabe, K.; Iwasaki, A.; Mori, T.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. The Severity of Hypoxic Changes and Oxidative DNA Damage in the Placenta of Early-Onset Preeclamptic Women and Fetal Growth Restriction. J. Matern. Fetal. Neonatal Med. 2013, 26, 491–496. [Google Scholar] [CrossRef]
- Davy, P.; Nagata, M.; Bullard, P.; Fogelson, N.S.; Allsopp, R. Fetal Growth Restriction Is Associated with Accelerated Telomere Shortening and Increased Expression of Cell Senescence Markers in the Placenta. Placenta 2009, 30, 539–542. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Wang, J.Q.; Liu, J.P. Molecular Regulation of Telomerase Activity in Aging. Protein Cell 2011, 2, 726–738. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Gestational Diabetes Mellitus. Saudi Med. J. 2015, 36, 399–406. [Google Scholar] [CrossRef]
- Yaping, X.; Chunhong, L.; Huifen, Z.; Fengfeng, H.; Huibin, H.; Meijing, Z. Risk Factors Associated with Gestational Diabetes Mellitus: A Retrospective Case-Control Study. Int. J. Diabetes Dev. Ctries. 2022, 42, 91–100. [Google Scholar] [CrossRef]
- Lin, P.C.; Hung, C.H.; Chan, T.F.; Lin, K.C.; Hsu, Y.Y.; Tzeng, Y.L. The Risk Factors for Gestational Diabetes Mellitus: A Retrospective Study. Midwifery 2016, 42, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Paradela, A.; Asunción Sánchez-Gil, M.; Rodriguez-Martin, S.; De León-Luis, J.A.; Pereda-Cerquella, C.; Bujan, J.; Guijarro, L.G.; et al. Unfolding the Role of Placental-Derived Extracellular Vesicles in Pregnancy: From Homeostasis to Pathophysiology. Front. Cell Dev. Biol. 2022, 10, 1060850. [Google Scholar] [CrossRef] [PubMed]
- Schliefsteiner, C.; Hirschmugl, B.; Kopp, S.; Curcic, S.; Bernhart, E.M.; Marsche, G.; Lang, U.; Desoye, G.; Wadsack, C. Maternal Gestational Diabetes Mellitus Increases Placental and Foetal Lipoprotein-Associated Phospholipase A2 Which Might Exert Protective Functions against Oxidative Stress. Sci. Rep. 2017, 7, 12628. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Vervaart, P.P.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Altered Placental Oxidative Stress Status in Gestational Diabetes Mellitus. Placenta 2004, 25, 78–84. [Google Scholar] [CrossRef]
- Lopez-Tinoco, C.; Visiedo, F.; Roca-Rodriguez, M.; Rosendo, C.; Mateos, R.; Segundo, C.; Aguilar-Diosdado, M. Oxidative Stress in the Gestational Diabetes Mellitus Mother and Placenta. Endocr. Abstr. 2016, 41, GP76. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; De Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F.A. The Worldwide Incidence of Preterm Birth: A Systematic Review of Maternal Mortality and Morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Gravett, M.G.; Iams, J.; Papageorghiou, A.T.; Waller, S.A.; Kramer, M.; Culhane, J.; Barros, F.; Conde-Agudelo, A.; Bhutta, Z.A.; et al. The Preterm Birth Syndrome: Issues to Consider in Creating a Classification System. Am. J. Obstet. Gynecol. 2012, 206, 113–118. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Yu, J.; Basanta-Henry, P.; Brou, L.; Berga, S.L.; Fortunato, S.J.; Taylor, R.N. Short Fetal Leukocyte Telomere Length and Preterm Prelabor Rupture of the Membranes. PLoS ONE 2012, 7, e0031136. [Google Scholar] [CrossRef] [PubMed]
- Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; Vermeulen, N.; et al. ESHRE Guideline: Recurrent Pregnancy Loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Lucas, E.S.; Dyer, N.P.; Murakami, K.; Hou Lee, Y.; Chan, Y.W.; Grimaldi, G.; Muter, J.; Brighton, P.J.; Moore, J.D.; Patel, G.; et al. Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells 2016, 34, 346–356. [Google Scholar] [CrossRef]
- Zidi-Jrah, I.; Hajlaoui, A.; Mougou-Zerelli, S.; Kammoun, M.; Meniaoui, I.; Sallem, A.; Brahem, S.; Fekih, M.; Bibi, M.; Saad, A.; et al. Relationship between Sperm Aneuploidy, Sperm DNA Integrity, Chromatin Packaging, Traditional Semen Parameters, and Recurrent Pregnancy Loss. Fertil. Steril. 2016, 105, 58–64. [Google Scholar] [CrossRef]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The Role of Oxidative Stress in Patients with Recurrent Pregnancy Loss: A Review. Reprod. Health 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A.; Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. Free Radic. Med. Biol. 2019, 1, 11–22. [Google Scholar] [CrossRef]
- Sutan, R.; Campbell, D.; Prescott, G.J.; Smith, W.C.S. The Risk Factors for Unexplained Antepartum Stillbirths in Scotland, 1994 to 2003. J. Perinatol. 2010, 30, 311–318. [Google Scholar] [CrossRef]
- Stacey, T.; Thompson, J.M.D.; Mitchell, E.A.; Ekeroma, A.J.; Zuccollo, J.M.; McCowan, L.M.E. The Auckland Stillbirth Study, a Case-Control Study Exploring Modifiable Risk Factors for Third Trimester Stillbirth: Methods and Rationale. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 3–8. [Google Scholar] [CrossRef]
- Pinar, H.; Goldenberg, R.L.; Koch, M.A.; Heim-Hall, J.; Hawkins, H.K.; Shehata, B.; Abramowsky, C.; Parker, C.B.; Dudley, D.J.; Silver, R.M.; et al. Placental Findings in Singleton Stillbirths. Obstet. Gynecol. 2014, 123, 325–336. [Google Scholar] [CrossRef]
- Ferrari, F.; Facchinetti, F.; Saade, G.; Menon, R. Placental Telomere Shortening in Stillbirth: A Sign of Premature Senescence? J. Matern. Fetal. Neonatal Med. 2016, 29, 1283–1288. [Google Scholar] [CrossRef]
- Smith, R.; Maiti, K.; Aitken, R.J. Unexplained Antepartum Stillbirth: A Consequence of Placental Aging? Placenta 2013, 34, 310–313. [Google Scholar] [CrossRef]
- McDowell, M.; Cain, M.A.; Brumley, J. Excessive Gestational Weight Gain. J. Midwifery Womens Health 2019, 64, 46–54. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, X.; Yi, H.; Tang, S.; You, H. Determinants of Excessive Gestational Weight Gain: A Systematic Review and Meta-Analysis. Arch. Public Health 2022, 80, 1–12. [Google Scholar] [CrossRef]
- Gujski, M.; Szukiewicz, D.; Chołuj, M.; Sawicki, W.; Bojar, I. Fetal and Placental Weight in Pre-Gestational Maternal Obesity (PGMO) vs. Excessive Gestational Weight Gain (EGWG)—A Preliminary Approach to the Perinatal Outcomes in Diet-Controlled Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 530. [Google Scholar] [CrossRef]
- Solis Paredes, J.M.; Perichart Perera, O.; Montoya Estrada, A.; Reyes Muñoz, E.; Espino, Y.; Sosa, S.; Ortega Castillo, V.; Medina Bastidas, D.; Tolentino Dolores, M.; Sanchez Martinez, M.; et al. Gestational Weight Gain Influences the Adipokine-Oxidative Stress Association during Pregnancy. Obes. Facts 2021, 14, 604. [Google Scholar] [CrossRef]
- Santos-Rosendo, C.; Mateos, R.M.; Vázquez-Fonseca, L.; Ábalos-Martínez, J.; Olmedo-Iglesias, H.; Melero-Jiménez, V.; Fajardo-Expósito, M.A.; Bugatto, F.; Visiedo, F. Influence of Gestational Weight Gain on Placental Oxidative Stress in Women with Normal Pre-Pregnancy Body Mass Index. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martinez, O.; Rodriguez-Martín, S.; Funes Moñux, R.M.; Saz, J.V.; Bravo, C.; De Leon-Luis, J.A.; Ruiz-Minaya, M.; Pekarek, L.; Saez, M.A.; et al. Irregular Expression of Cellular Stress Response Markers in the Placenta of Women with Chronic Venous Disease. Antioxidants 2022, 11, 2277. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Rodriguez-Martín, S.; Funes Moñux, R.M.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. Evidence of Increased Oxidative Stress in the Placental Tissue of Women Who Suffered an Episode of Psychosis during Pregnancy. Antioxidants 2023, 12, 179. [Google Scholar] [CrossRef]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-Associated Venous Insufficiency Course with Placental and Systemic Oxidative Stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef]
- Ortega, M.A.; Pekarek, T.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, L.; Rodriguez-Martín, S.; Funes Moñux, R.M.; Bravo, C.; De León-Luis, J.A.; Lahera, G.; et al. A Review: Integrative Perspectives on the Features and Clinical Management of Psychotic Episodes in Pregnancy. J. Clin. Med. 2023, 12, 656. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Gelaye, B.; Fricchione, G.L.; Avillach, P.; Karlson, E.W.; Williams, M.A. Adverse Obstetric and Neonatal Outcomes Complicated by Psychosis among Pregnant Women in the United States. BMC Pregnancy Childbirth 2018, 18, 120. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Rodríguez-Martín, S.; Funes Moñux, R.M.; Pekarek, L.; Bravo, C.; De Leon-Luis, J.A.; Saez, M.A.; Guijarro, L.G.; et al. Women with Psychotic Episodes during Pregnancy Show Increased Markers of Placental Damage with Tenney-Parker Changes. Histol. Histopathol. 2023. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Funes Moñux, R.M.; Rodriguez-Martín, S.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules 2023, 13, 120. [Google Scholar] [CrossRef]
- Ortega, M.A.; García-Montero, C.; Fraile-Martinez, Ó.; De Leon-Oliva, D.; Boaru, D.L.; Bravo, C.; De Leon-Luis, J.A.; Saez, M.A.; Asúnsolo, A.; Romero-Gerechter, I.; et al. Assessment of Tissue Expression of the Oxytocin-Vasopressin Pathway in the Placenta of Women with a First-Episode Psychosis during Pregnancy. Int. J. Mol. Sci. 2023, 24, 10254. [Google Scholar] [CrossRef]
- Mate, A.; Reyes-Goya, C.; Santana-Garrido, Á.; Vázquez, C.M. Lifestyle, Maternal Nutrition and Healthy Pregnancy. Curr. Vasc. Pharmacol. 2021, 19, 132–140. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The Importance of Nutrition in Pregnancy and Lactation: Lifelong Consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Francis, E.C.; Dabelea, D.; Boyle, K.E.; Jansson, T.; Perng, W. Maternal Diet Quality Is Associated with Placental Proteins in the Placental Insulin/Growth Factor, Environmental Stress, Inflammation, and MTOR Signaling Pathways: The Healthy Start ECHO Cohort. J. Nutr. 2022, 152, 816–825. [Google Scholar] [CrossRef]
- Rasool, A.; Alvarado-Flores, F.; O’Tierney-Ginn, P. Placental Impact of Dietary Supplements: More Than Micronutrients. Clin. Ther. 2021, 43, 226–245. [Google Scholar] [CrossRef]
- Myatt, L.; Thornburg, K.L. Effects of Prenatal Nutrition and the Role of the Placenta in Health and Disease. Methods Mol. Biol. 2018, 1735, 19–46. [Google Scholar] [CrossRef]
- Kinshella, M.L.W.; Omar, S.; Scherbinsky, K.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Moore, S.E.; Elango, R. Effects of Maternal Nutritional Supplements and Dietary Interventions on Placental Complications: An Umbrella Review, Meta-Analysis and Evidence Map. Nutrients 2021, 13, 472. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean Diet: Epidemiological and Molecular Aspects. Mol. Aspects Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef]
- Reijnders, I.F.; Mulders, A.G.M.G.J.; Van Der Windt, M.; Steegers, E.A.P.; Steegers-Theunissen, R.D.S.P.M. The Impact of Periconceptional Maternal Lifestyle on Clinical Features and Biomarkers of Placental Development and Function: A Systematic Review. Hum. Reprod. Update 2019, 25, 72–94. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Acosta-Manzano, P.; Migueles, J.H.; Varela-López, A.; Baena-García, L.; Quiles, J.L.; Aparicio, V.A. Influence of an Exercise Intervention plus an Optimal Mediterranean Diet Adherence during Pregnancy on the Telomere Length of the Placenta. The GESTAFIT Project. Placenta 2023, 136, 42–45. [Google Scholar] [CrossRef]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; de la Serrano Delgado, V.C.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and Its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive Substances of Plant Origin. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–35. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary Reference Intake (DRI) Value for Dietary Polyphenols: Are We Heading in the Right Direction? Br. J. Nutr. 2008, 99 (Suppl. 3), S55–S58. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is There Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Garciá-Rincón, I.; Rufián-Henares, J.A.; Pastoriza, S. A Useful and Simple Tool to Evaluate and Compare the Intake of Total Dietary Polyphenols in Different Populations. Public Health Nutr. 2021, 24, 3818. [Google Scholar] [CrossRef]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Martel, F.; Monteiro, R.; Calhau, C. Effect of Polyphenols on the Intestinal and Placental Transport of Some Bioactive Compounds. Nutr. Res. Rev. 2010, 23, 47–64. [Google Scholar] [CrossRef]
- Vanhees, K.; Godschalk, R.W.; Sanders, A.; Van Waalwijk van Doorn-Khosrovani, S.B.; Van Schooten, F.J. Maternal Quercetin Intake during Pregnancy Results in an Adapted Iron Homeostasis at Adulthood. Toxicology 2011, 290, 350–358. [Google Scholar] [CrossRef]
- Anachuna, K.K.; Moke, G.E.; Iyare, C.; Katchy, N.; Ben-Azu, B.; Adeniyi, B.; Nwogueze, B.C.; Iyare, E. Prenatal and Early Postnatal Food Restrictions Cause Changes in Brain Oxidative Status and Orexigenic/Anorexigenic Hormones in the Offspring of Rats: Prevention by Quercetin and Kaempferol. Curr. Res. Pharmacol. Drug Discov. 2020, 1, 39–52. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Batmomolin, A.; Khotimah, H.; Ahsan, A.; Wiyasa, I.W.A.; Santoso, S. Effects of Quercetin and Kaempferol (Main Compound of Moringa Oleifera Leaves) Improve IUGR through Decreased Hypoxia. Res. J. Pharm. Technol. 2020, 13, 5831–5836. [Google Scholar] [CrossRef]
- Li, Q.; Yin, L.; Si, Y.; Zhang, C.; Meng, Y.; Yang, W. The Bioflavonoid Quercetin Improves Pathophysiology in a Rat Model of Preeclampsia. Biomed. Pharmacother. 2020, 127, 110122. [Google Scholar] [CrossRef]
- Mahabady, M.K.; Shamsi, M.M.; Ranjbar, R.; Tabandeh, M.R.; Khazaeel, K. Quercetin Improved Histological Structure and Upregulated Adiponectin and Adiponectin Receptors in the Placenta of Rats with Gestational Diabetes Mellitus. Placenta 2021, 106, 49–57. [Google Scholar] [CrossRef]
- Ebegboni, V.J.; Balahmar, R.M.; Dickenson, J.M.; Sivasubramaniam, S.D. The Effects of Flavonoids on Human First Trimester Trophoblast Spheroidal Stem Cell Self-Renewal, Invasion and JNK/P38 MAPK Activation: Understanding the Cytoprotective Effects of These Phytonutrients against Oxidative Stress. Biochem. Pharmacol. 2019, 164, 289–298. [Google Scholar] [CrossRef]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef]
- Kostić, S.; Vilotić, A.; Pirković, A.; Dekanski, D.; Borozan, S.; Nacka-Aleksić, M.; Vrzić-Petronijević, S.; Jovanović Krivokuća, M. Caffeic Acid Protects Human Trophoblast HTR-8/SVneo Cells from H2O2-Induced Oxidative Stress and Genotoxicity. Food Chem. Toxicol. 2022, 163, 112993. [Google Scholar] [CrossRef]
- Wang, P.; Huang, C.X.; Gao, J.J.; Shi, Y.; Li, H.; Yan, H.; Yan, S.J.; Zhang, Z. Resveratrol Induces SIRT1-Dependent Autophagy to Prevent H2O2-Induced Oxidative Stress and Apoptosis in HTR8/SVneo Cells. Placenta 2020, 91, 11–18. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; An, P.; Dang, H.; Liu, Y.; Liu, R. Effects of Resveratrol on Autophagy and the Expression of Inflammasomes in a Placental Trophoblast Oxidative Stress Model. Life Sci. 2020, 256, 117890. [Google Scholar] [CrossRef]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Hull, H.R.; Herman, A.; Gibbs, H.; Gajewski, B.; Krase, K.; Carlson, S.E.; Sullivan, D.K.; Goetz, J. The Effect of High Dietary Fiber Intake on Gestational Weight Gain, Fat Accrual, and Postpartum Weight Retention: A Randomized Clinical Trial. BMC Pregnancy Childbirth 2020, 20, 319. [Google Scholar] [CrossRef]
- Pretorius, R.A.; Palmer, D.J. High-Fiber Diet during Pregnancy Characterized by More Fruit and Vegetable Consumption. Nutrients 2020, 13, 35. [Google Scholar] [CrossRef]
- Hajhoseini, L. Importance of Optimal Fiber Consumption during Pregnancy Dear Editor. Int. J. Women’s Health Reprod. Sci 2013, 1, 76–79. [Google Scholar] [CrossRef]
- Huang, S.; Wu, D.; Hao, X.; Nie, J.; Huang, Z.; Ma, S.; Chen, Y.; Chen, S.; Wu, J.; Sun, J.; et al. Dietary Fiber Supplementation during the Last 50 Days of Gestation Improves the Farrowing Performance of Gilts by Modulating Insulin Sensitivity, Gut Microbiota, and Placental Function. J. Anim. Sci. 2023, 101, skad021. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Zhang, L.; Mao, Z.; Lin, Y.; Xu, S.; Fang, Z.; Che, L.; Feng, B.; Li, J.; et al. Dietary Fiber Supplementation in Gestating Sow Diet Improved Fetal Growth and Placental Development and Function Through Serotonin Signaling Pathway. Front. Vet. Sci. 2022, 9, 831703. [Google Scholar] [CrossRef]
- Lin, Y.; Han, X.F.; Fang, Z.F.; Che, L.Q.; Wu, D.; Wu, X.Q.; Wu, C.M. The Beneficial Effect of Fiber Supplementation in High- or Low-Fat Diets on Fetal Development and Antioxidant Defense Capacity in the Rat. Eur. J. Nutr. 2012, 51, 19–27. [Google Scholar] [CrossRef]
- Peng, X.; Huang, Y.; Wang, G.; He, Y.; Hu, L.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Li, J.; et al. Maternal Long-Term Intake of Inulin Improves Fetal Development through Gut Microbiota and Related Metabolites in a Rat Model. J. Agric. Food Chem. 2022, 70, 1840–1851. [Google Scholar] [CrossRef]
- Lin, Y.; Han, X.F.; Fang, Z.F.; Che, L.Q.; Nelson, J.; Yan, T.H.; Wu, D. Beneficial Effects of Dietary Fibre Supplementation of a High-Fat Diet on Fetal Development in Rats. Br. J. Nutr. 2011, 106, 510–518. [Google Scholar] [CrossRef]
- Vasdeki, D.; Tsamos, G.; Koufakis, T.; Goulis, D.G.; Asimakopoulos, B.; Michou, V.; Patriarcheas, V.; Kotsa, K. “You Are My Sunshine, My Only Sunshine”: Maternal Vitamin D Status and Supplementation in Pregnancy and Their Effect on Neonatal and Childhood Outcomes. Hormones 2023, 1–16. [Google Scholar] [CrossRef]
- Oh, C.; Keats, E.C.; Bhutta, Z.A. Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 491. [Google Scholar] [CrossRef]
- Sauder, K.A.; Harte, R.N.; Ringham, B.M.; Guenther, P.M.; Bailey, R.L.; Alshawabkeh, A.; Cordero, J.F.; Dunlop, A.L.; Ferranti, E.P.; Elliott, A.J.; et al. Disparities in Risks of Inadequate and Excessive Intake of Micronutrients during Pregnancy. J. Nutr. 2021, 151, 3555–3569. [Google Scholar] [CrossRef]
- Özdemir, A.A.; Gündemir, Y.E.; Küçük, M.; Sarıcı, D.Y.; Elgörmüş, Y.; Çağ, Y.; Bilek, G. Vitamin D Deficiency in Pregnant Women and Their Infants. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 44. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Zheng, B.; Zheng, Z.; Zhang, H.; Zheng, J.; Sun, C.; Chen, H.; Yang, J.; Wang, Z.; et al. Maternal 25-Hydroxyvitamin D Deficiency Promoted Metabolic Syndrome and Downregulated Nrf2/CBR1 Pathway in Offspring. Front. Pharmacol. 2020, 11, 97. [Google Scholar] [CrossRef]
- Varshney, S.; Adela, R.; Kachhawa, G.; Dada, R.; Kulshreshtha, V.; Kumari, R.; Agarwal, R.; Khadgawat, R. Disrupted Placental Vitamin D Metabolism and Calcium Signaling in Gestational Diabetes and Pre-Eclampsia Patients. Endocrine 2023, 80, 191–200. [Google Scholar] [CrossRef]
- Sundrani, D.; Chavan-Gautam, P.; Pisal, H.; Mehendale, S.; Joshi, S. Matrix Metalloproteinases-2, -3 and Tissue Inhibitors of Metalloproteinases-1, -2 in Placentas from Preterm Pregnancies and Their Association with One-Carbon Metabolites. Reproduction 2013, 145, 401–410. [Google Scholar] [CrossRef]
- Chen, H.A.N.; Qian, N.; Yan, L.; Jiang, H. Role of Serum Vitamin A and E in Pregnancy. Exp. Ther. Med. 2018, 16, 5185. [Google Scholar] [CrossRef]
- Gázquez, A.; Sánchez-Campillo, M.; Arnao, M.B.; Barranco, A.; Rueda, R.; Jensen, S.K.; Chan, J.P.; Kuchan, M.J.; Larqué, E. Natural Vitamin E Supplementation during Pregnancy in Rats Increases RRR-α-Tocopherol Stereoisomer Proportion and Enhances Fetal Antioxidant Capacity, Compared to Synthetic Vitamin E Administration. Ann. Nutr. Metab. 2023, 79, 60–69. [Google Scholar] [CrossRef]
- Johnston, P.C.; McCance, D.R.; Holmes, V.A.; Young, I.S.; McGinty, A. Placental Antioxidant Enzyme Status and Lipid Peroxidation in Pregnant Women with Type 1 Diabetes: The Effect of Vitamin C and E Supplementation. J. Diabetes Complicat. 2016, 30, 109–114. [Google Scholar] [CrossRef]
- Bala, R.; Verma, R.; Budhwar, S.; Prakash, N.; Sachan, S. Fetal Hyperhomocysteinemia Is Associated with Placental Inflammation and Early Breakdown of Maternal-Fetal Tolerance in Pre-Term Birth. Am. J. Reprod. Immunol. 2022, 88, e13589. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, Y.; Zhao, X.; Zhang, L.; Li, J.; Zhang, Y.; Wang, P.; Liang, H. Dietary Folic Acid Supplementation Attenuates Maternal High-Fat Diet-Induced Fetal Intrauterine Growth Retarded via Ameliorating Placental Inflammation and Oxidative Stress in Rats. Nutrients 2023, 15, 3263. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Torky, H.; Fouad, M.A.; GadAllah, S.H.; Waked, N.M.; Gayed, A.S.; Salem, A.K. Role of Antioxidants in Gestational Diabetes Mellitus and Relation to Fetal Outcome: A Randomized Controlled Trial. J. Matern. Fetal Neonatal Med. 2016, 29, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cao, C.; Li, J.; Meng, Q.; Cheng, B.; Shi, B.; Shan, A. Lycopene Modulates Placental Health and Fetal Development Under High-Fat Diet During Pregnancy of Rats. Mol. Nutr. Food Res. 2021, 65, 2001148. [Google Scholar] [CrossRef]
- Pietrantoni, E.; Del Chierico, F.; Rigon, G.; Vernocchi, P.; Salvatori, G.; Manco, M.; Signore, F.; Putignani, L. Docosahexaenoic Acid Supplementation during Pregnancy: A Potential Tool to Prevent Membrane Rupture and Preterm Labor. Int. J. Mol. Sci. 2014, 15, 8024–8036. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dove, E.; Bhatt, D.L. Omega-3 Fatty Acids. In Clinical Lipidology: A Companion to Braunwald’s Heart Disease; Elsevier: Amsterdam, The Netherlands, 2024; pp. 169–183.e3. [Google Scholar] [CrossRef]
- Tahaei, H.; Gignac, F.; Pinar, A.; Fernandez-Barrés, S.; Romaguera, D.; Vioque, J.; Santa-Marina, L.; Subiza-Pérez, M.; Llop, S.; Soler-Blasco, R.; et al. Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Ulti-Centre Population-Based Birth Cohort Study in Spain. Nutrients 2022, 14, 518. [Google Scholar] [CrossRef]
- Ma, R.; Wang, S.; Xue, M.; Zhang, H.; He, Z.; Jueraitetibaike, K.; Ge, X.; Chen, L.; Yao, B. Effects of N-3 PUFA Supplementation on Oocyte in Vitro Maturation in Mice with Polycystic Ovary Syndrome. J. Ovarian Res. 2023, 16, 1–10. [Google Scholar] [CrossRef]
- Yamagata, K. Fatty Acids Act on Vascular Endothelial Cells and Influence the Development of Cardiovascular Disease. Prostaglandins Other Lipid Mediat. 2023, 165, 106704. [Google Scholar] [CrossRef]
- Zailani, H.; Satyanarayanan, S.K.; Liao, W.C.; Liao, H.F.; Huang, S.Y.; Gałecki, P.; Su, K.P.; Chang, J.P.C. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. J. Clin. Med. 2023, 12, 2653. [Google Scholar] [CrossRef]
- Zou, H.Y.; Zhang, H.J.; Zhao, Y.C.; Li, X.Y.; Wang, Y.M.; Zhang, T.T.; Xue, C.H. N-3 PUFA Deficiency Aggravates Streptozotocin-Induced Pancreatic Injury in Mice but Dietary Supplementation with DHA/EPA Protects the Pancreas via Suppressing Inflammation, Oxidative Stress and Apoptosis. Mar. Drugs 2023, 21, 39. [Google Scholar] [CrossRef]
- Kasture, V.; Sundrani, D.; Dalvi, S.; Swamy, M.; Kale, A.; Joshi, S. Maternal Omega-3 Fatty Acids and Vitamin E Improve Placental Angiogenesis in Late-Onset but Not Early-Onset Preeclampsia. Mol. Cell. Biochem. 2019, 461, 159–170. [Google Scholar] [CrossRef]
- Kasture, V.; Kale, A.; Randhir, K.; Sundrani, D.; Joshi, S. Effect of Maternal Omega-3 Fatty Acids and Vitamin E Supplementation on Placental Apoptotic Markers in Rat Model of Early and Late Onset Preeclampsia. Life Sci. 2019, 239, 117038. [Google Scholar] [CrossRef]
- Mezouar, D.; Merzouk, H.; Merzouk, A.S.; Merzouk, S.A.; Belarbi, B.; Narce, M. In Vitro Effects of Vitamins C and E, n-3 and n-6 PUFA and n-9 MUFA on Placental Cell Function and Redox Status in Type 1 Diabetic Pregnant Women. Placenta 2016, 42, 114–121. [Google Scholar] [CrossRef]
- Melody, S.M.; Vincent, R.; Mori, T.A.; Mas, E.; Barden, A.E.; Waddell, B.J.; Keelan, J.A. Effects of Omega-3 and Omega-6 Fatty Acids on Human Placental Cytokine Production. Placenta 2015, 36, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Boulis, T.S.; Rochelson, B.; Novick, O.; Xue, X.; Chatterjee, P.K.; Gupta, M.; Solanki, M.H.; Akerman, M.; Metz, C.N. Omega-3 Polyunsaturated Fatty Acids Enhance Cytokine Production and Oxidative Stress in a Mouse Model of Preterm Labor. J. Perinat. Med. 2014, 42, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Mori, T.A.; Keelan, J.A.; Waddell, B.J. Maternal Dietary Omega-3 Fatty Acid Supplementation Reduces Placental Oxidative Stress and Increases Fetal and Placental Growth in the Rat. Biol. Reprod. 2013, 88, 1–8. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal Omega-3 Fatty Acid Intake Increases Placental Labyrinthine Antioxidant Capacity but Does Not Protect against Fetal Growth Restriction Induced by Placental Ischaemia-Reperfusion Injury. Reproduction 2013, 146, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.P.; Joshi, A.A.; Joshi, S.R. Maternal Micronutrients, Omega-3 Fatty Acids, and Placental PPARγ Expression. Appl. Physiol. Nutr. Metab. 2014, 39, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Šušnjara, P.; Kolobarić, N.; Matić, A.; Mihaljević, Z.; Stupin, A.; Marczi, S.; Drenjančević, I. Consumption of Hen Eggs Enriched with N-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E and Lutein Incites Anti-Inflammatory Conditions in Young, Healthy Participants—A Randomized Study. Front. Biosci. 2022, 27, 332. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Critical Role During Fetal Development and Dietary Requirements in Adults. Annu. Rev. Nutr. 2006, 26, 229. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef]
- Kwan, S.T.C.; King, J.H.; Grenier, J.K.; Yan, J.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation during Normal Murine Pregnancy Alters the Placental Epigenome: Results of an Exploratory Study. Nutrients 2018, 10, 417. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal Choline Intake Alters the Epigenetic State of Fetal Cortisol-Regulating Genes in Humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bar, H.Y.; Yan, J.; Jones, S.; Brannon, P.M.; West, A.A.; Perry, C.A.; Ganti, A.; Pressman, E.; Devapatla, S.; et al. A Higher Maternal Choline Intake among Third-Trimester Pregnant Women Lowers Placental and Circulating Concentrations of the Antiangiogenic Factor Fms-like Tyrosine Kinase-1 (SFLT1). FASEB J. 2013, 27, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Klatt, K.C.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Alters Fetal Growth Patterns in a Mouse Model of Placental Insufficiency. Nutrients 2017, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Qiao, J.; Wu, Y.; Ren, Y. The Impact of a High Fat Diet on Bones: Potential Mechanisms. Food Funct. 2021, 12, 963–975. [Google Scholar] [CrossRef]
- Guldstrand, M.C.; Simberg, C.L. High-Fat Diets: Healthy or Unhealthy? Clin. Sci. 2007, 113, 397–399. [Google Scholar] [CrossRef]
- Liu, A.G.; Ford, N.A.; Hu, F.B.; Zelman, K.M.; Mozaffarian, D.; Kris-Etherton, P.M. A Healthy Approach to Dietary Fats: Understanding the Science and Taking Action to Reduce Consumer Confusion. Nutr. J. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Knudsen, V.K.; Orozova-Bekkevold, I.M.; Mikkelsen, T.B.; Wolff, S.; Olsen, S.F. Major Dietary Patterns in Pregnancy and Fetal Growth. Eur. J. Clin. Nutr. 2008, 62, 463–470. [Google Scholar] [CrossRef]
- Paula, W.O.; Patriota, E.S.O.; Gonçalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3242. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yu, H.R.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Sheen, J.M.; Lin, Y.J.; Chang, K.A.; Chen, C.C.; Tsai, C.C.; et al. Maternal Obesity Related to High Fat Diet Induces Placenta Remodeling and Gut Microbiome Shaping That Are Responsible for Fetal Liver Lipid Dysmetabolism. Front. Nutr. 2021, 8, 736944. [Google Scholar] [CrossRef]
- Napso, T.; Lean, S.C.; Lu, M.; Mort, E.J.; Desforges, M.; Moghimi, A.; Bartels, B.; El-Bacha, T.; Fowden, A.L.; Camm, E.J.; et al. Diet-Induced Maternal Obesity Impacts Feto-Placental Growth and Induces Sex-Specific Alterations in Placental Morphology, Mitochondrial Bioenergetics, Dynamics, Lipid Metabolism and Oxidative Stress in Mice. Acta Physiol. 2022, 234, e13795. [Google Scholar] [CrossRef]
- Upadhyay, A.; Anjum, B.; Godbole, N.M.; Rajak, S.; Shukla, P.; Tiwari, S.; Sinha, R.A.; Godbole, M.M. Time-Restricted Feeding Reduces High-Fat Diet Associated Placental Inflammation and Limits Adverse Effects on Fetal Organ Development. Biochem. Biophys. Res. Commun. 2019, 514, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bondarczuk, N.H.; Schmidt, N.P.; Breyer, G.M.; de Moura, A.C.; Molz, P.; Barshack, A.G.; de Souza da Motta, S.; Guedes, R.P.; Giovenardi, M. A High-Fat Diet Changes Placental Morphology but Does Not Change Biochemical Parameters, Placental Oxidative Stress or Cytokine Levels. Placenta 2023, 135, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Sun, R.; Chen, Y.C.; Kang, L.; Wang, C.T.; Chiu, C.F.; Wu, H.T. Aspartame Consumption during Pregnancy Impairs Placenta Growth in Mice through Sweet Taste Receptor-Reactive Oxygen Species-Dependent Pathway. J. Nutr. Biochem. 2023, 113, 109228. [Google Scholar] [CrossRef]
- Rodrigues, H.; Silva, C.; Martel, F. The Effects of Aspartame on the HTR8/SVneo Extravillous Trophoblast Cell Line. Reprod. Biol. 2022, 22, 100678. [Google Scholar] [CrossRef]
- Yin, L.; Xu, L.; Chen, B.; Zheng, X.; Chu, J.; Niu, Y.; Ma, T. SRT1720 Plays a Role in Oxidative Stress and the Senescence of Human Trophoblast HTR8/SVneo Cells Induced by D-Galactose through the SIRT1/FOXO3a/ROS Signalling Pathway. Reprod. Toxicol. 2022, 111, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arvizu, M.; Bjerregaard, A.A.; Madsen, M.T.B.; Granström, C.; Halldorsson, T.I.; Olsen, S.F.; Gaskins, A.J.; Rich-Edwards, J.W.; Rosner, B.A.; Chavarro, J.E. Sodium Intake during Pregnancy, but Not Other Diet Recommendations Aimed at Preventing Cardiovascular Disease, Is Positively Related to Risk of Hypertensive Disorders of Pregnancy. J. Nutr. 2020, 150, 159–166. [Google Scholar] [CrossRef]
- Bank, T.C.; Grasch, J.L.; Chung, J.; Mercer, B.M.; McNeil, R.B.; Parry, S.; Saade, G.; Shanks, A.; Silver, R.M.; Simhan, H.; et al. Sodium Intake and the Development of Hypertensive Disorders of Pregnancy. Am. J. Obstet. Gynecol. MFM 2023, 5, 101166. [Google Scholar] [CrossRef]
- Mente, A.; O’donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Vickers, M.H.; Harrison, C.J.; Segovia, S.A.; Gray, C. High Fat and/or High Salt Intake during Pregnancy Alters Maternal Meta-inflammation and Offspring Growth and Metabolic Profiles. Physiol. Rep. 2014, 2, e12110. [Google Scholar] [CrossRef]
- Eisele, N.; Klossner, R.; Escher, G.; Rudloff, S.; Larionov, A.; Theilig, F.; Mohaupt, M.G.; Mistry, H.D.; Gennari-Moser, C. Physiological and Molecular Responses to Altered Sodium Intake in Rat Pregnancy. J. Am. Heart Assoc. 2018, 7, e008363. [Google Scholar] [CrossRef]
- Beauséjour, A.; Bibeau, K.; Lavoie, J.C.; St-Louis, J.; Brochu, M. Placental Oxidative Stress in a Rat Model of Preeclampsia. Placenta 2007, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Leandro, S.M.; Furukawa, L.N.S.; Shimizu, M.H.M.; Casarini, D.E.; Seguro, A.C.; Patriarca, G.; Coelho, M.S.; Dolnikoff, M.S.; Heimann, J.C. Low Birth Weight in Response to Salt Restriction during Pregnancy Is Not Due to Alterations in Uterine-Placental Blood Flow or the Placental and Peripheral Renin-Angiotensin System. Physiol. Behav. 2008, 95, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Imai, Y. The Impact of Salt Intake during and after Pregnancy. Hypertens. Res. 2018, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Montero, C.; Fraile-Martinez, O.; De Leon-Oliva, D.; Boaru, D.L.; Garcia-Puente, L.M.; De León-Luis, J.A.; Bravo, C.; Diaz-Pedrero, R.; Lopez-Gonzalez, L.; Álvarez-Mon, M.; et al. Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review. Antioxidants 2023, 12, 1918. https://doi.org/10.3390/antiox12111918
García-Montero C, Fraile-Martinez O, De Leon-Oliva D, Boaru DL, Garcia-Puente LM, De León-Luis JA, Bravo C, Diaz-Pedrero R, Lopez-Gonzalez L, Álvarez-Mon M, et al. Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review. Antioxidants. 2023; 12(11):1918. https://doi.org/10.3390/antiox12111918
Chicago/Turabian StyleGarcía-Montero, Cielo, Oscar Fraile-Martinez, Diego De Leon-Oliva, Diego Liviu Boaru, Luis M. Garcia-Puente, Juan A. De León-Luis, Coral Bravo, Raul Diaz-Pedrero, Laura Lopez-Gonzalez, Melchor Álvarez-Mon, and et al. 2023. "Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review" Antioxidants 12, no. 11: 1918. https://doi.org/10.3390/antiox12111918
APA StyleGarcía-Montero, C., Fraile-Martinez, O., De Leon-Oliva, D., Boaru, D. L., Garcia-Puente, L. M., De León-Luis, J. A., Bravo, C., Diaz-Pedrero, R., Lopez-Gonzalez, L., Álvarez-Mon, M., García-Honduvilla, N., Saez, M. A., & Ortega, M. A. (2023). Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review. Antioxidants, 12(11), 1918. https://doi.org/10.3390/antiox12111918











