Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
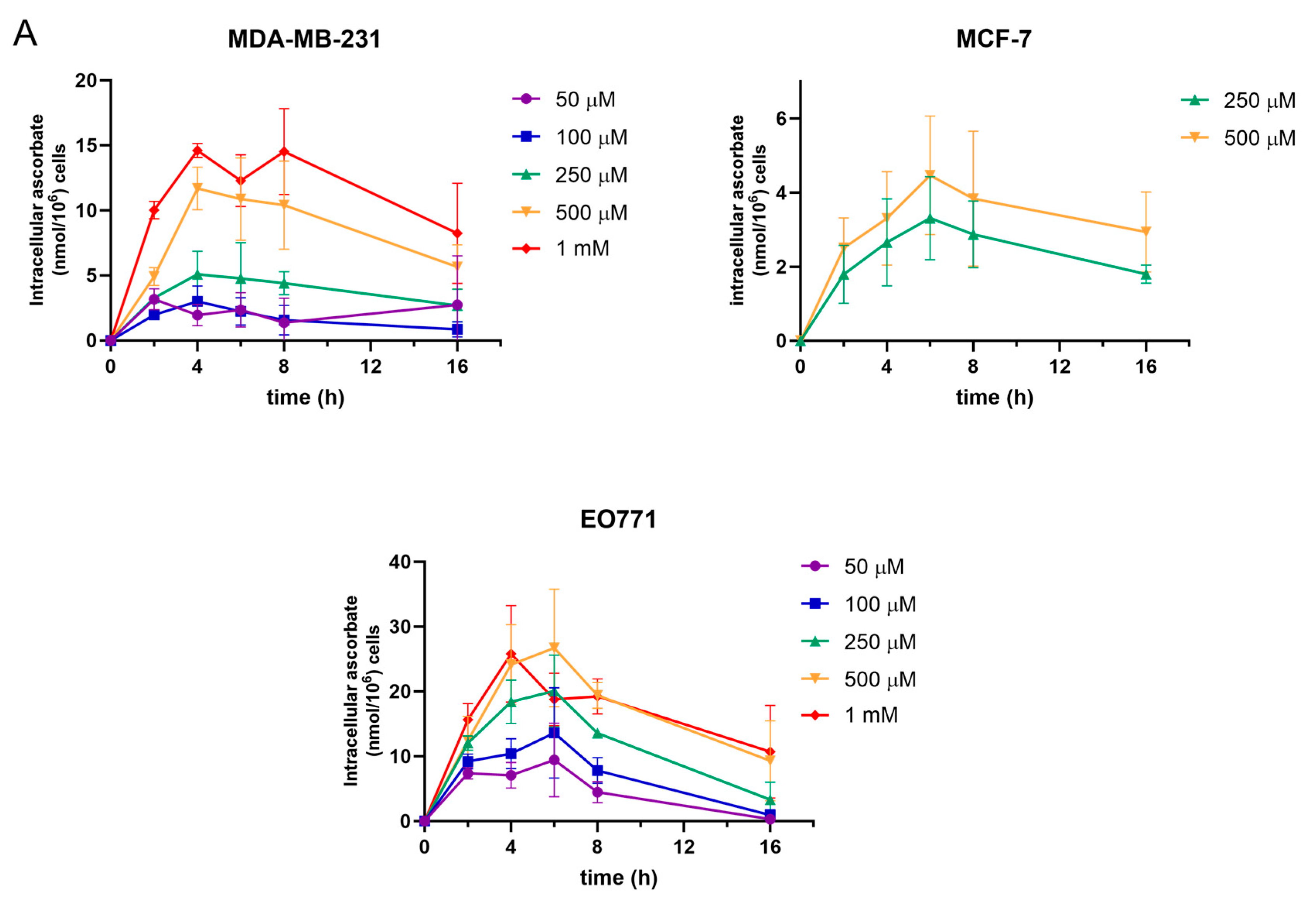
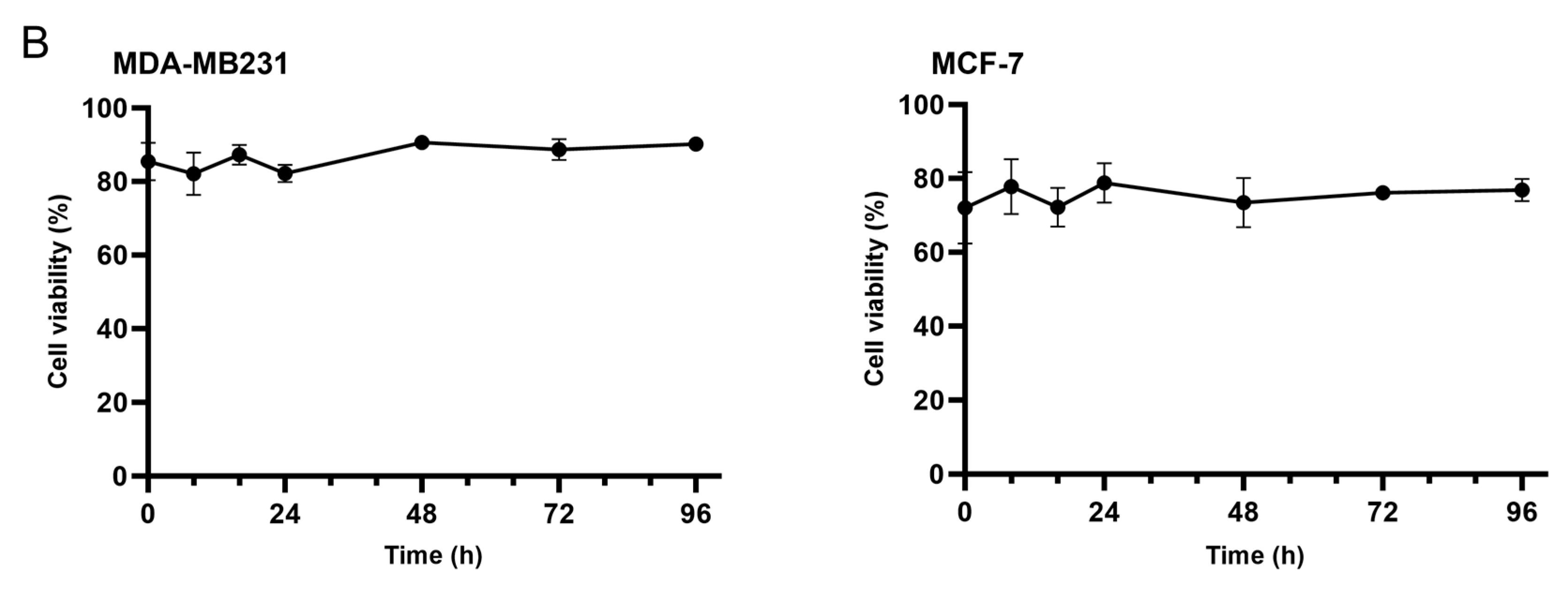
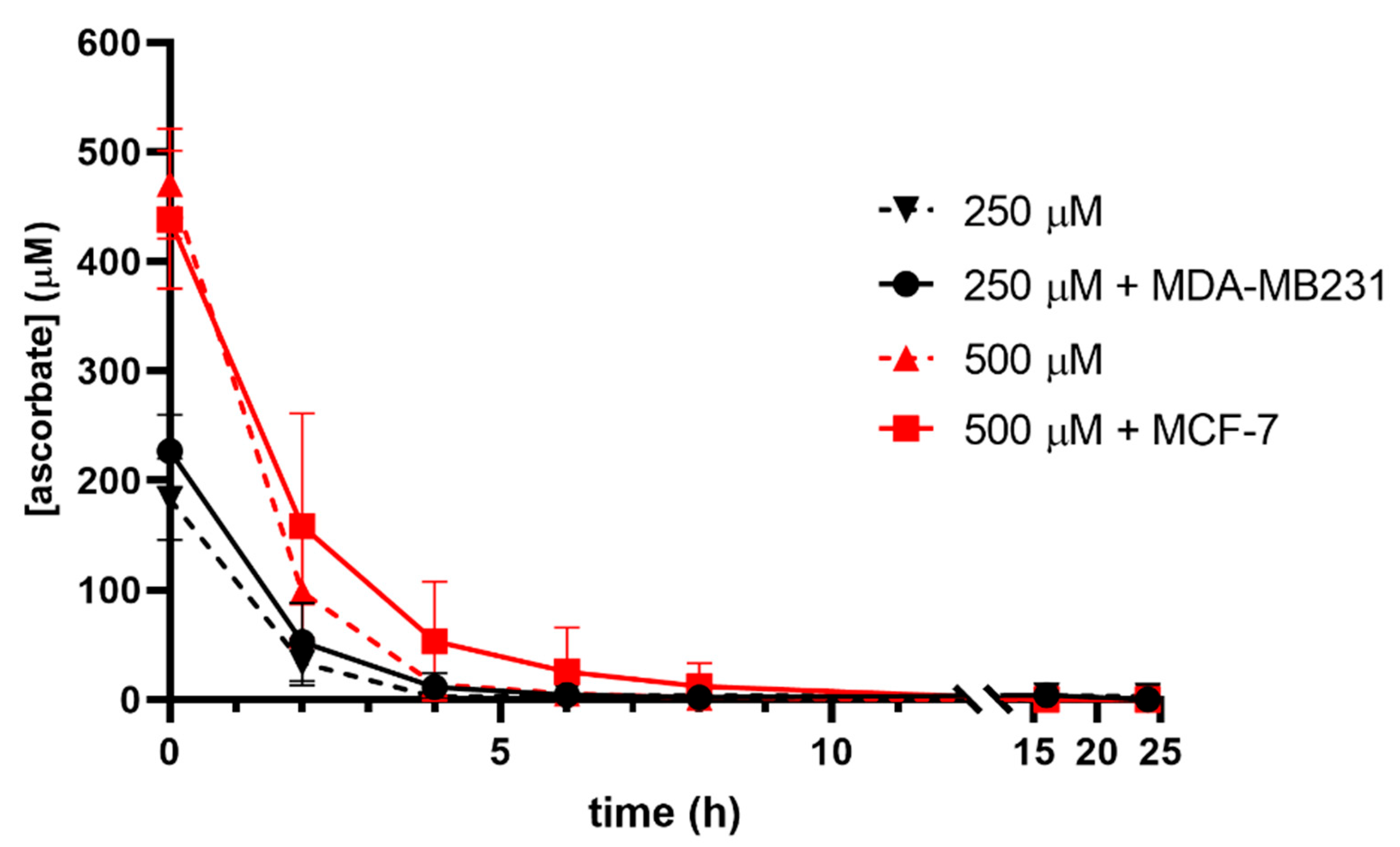
3.1. Ascorbate Uptake Kinetics
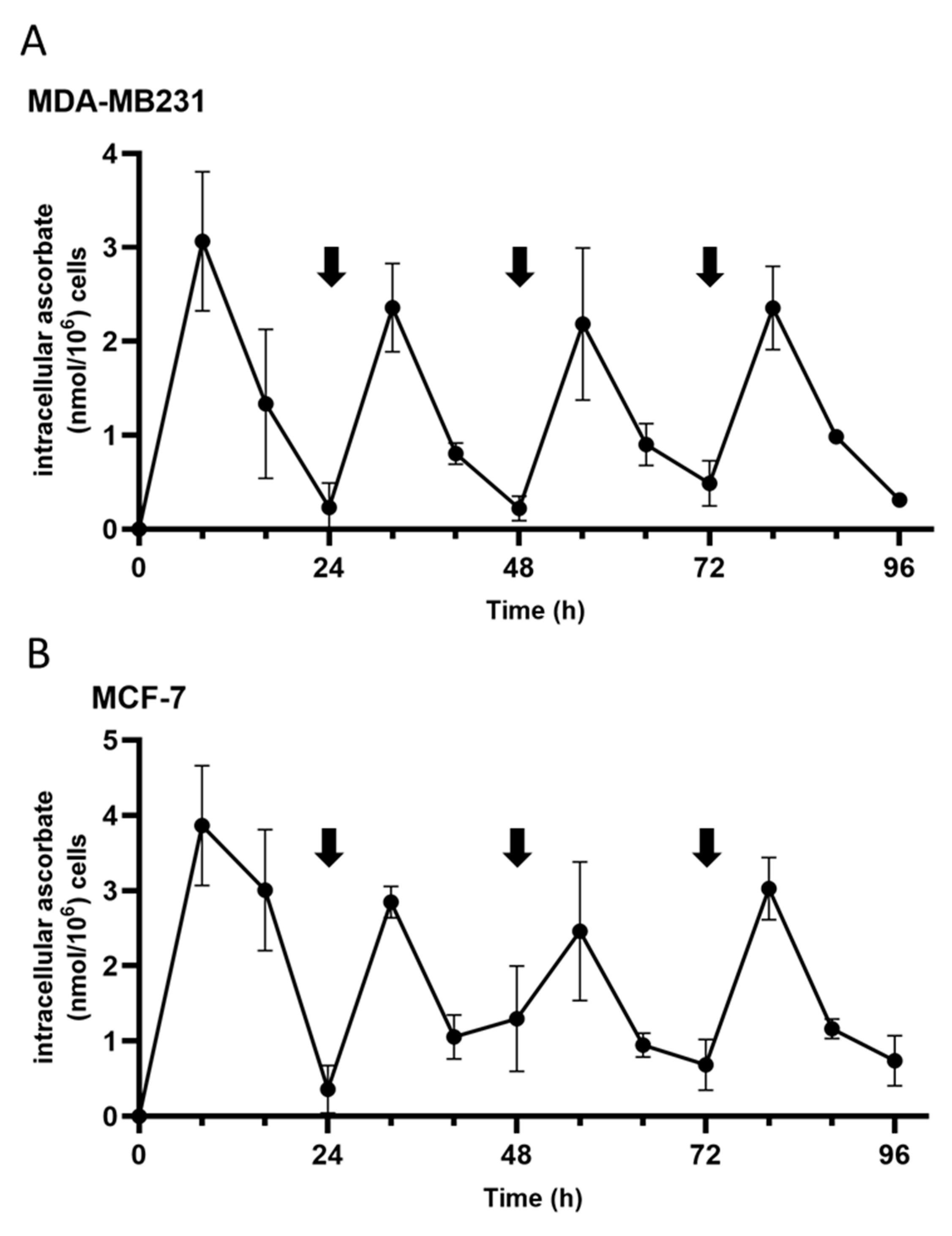
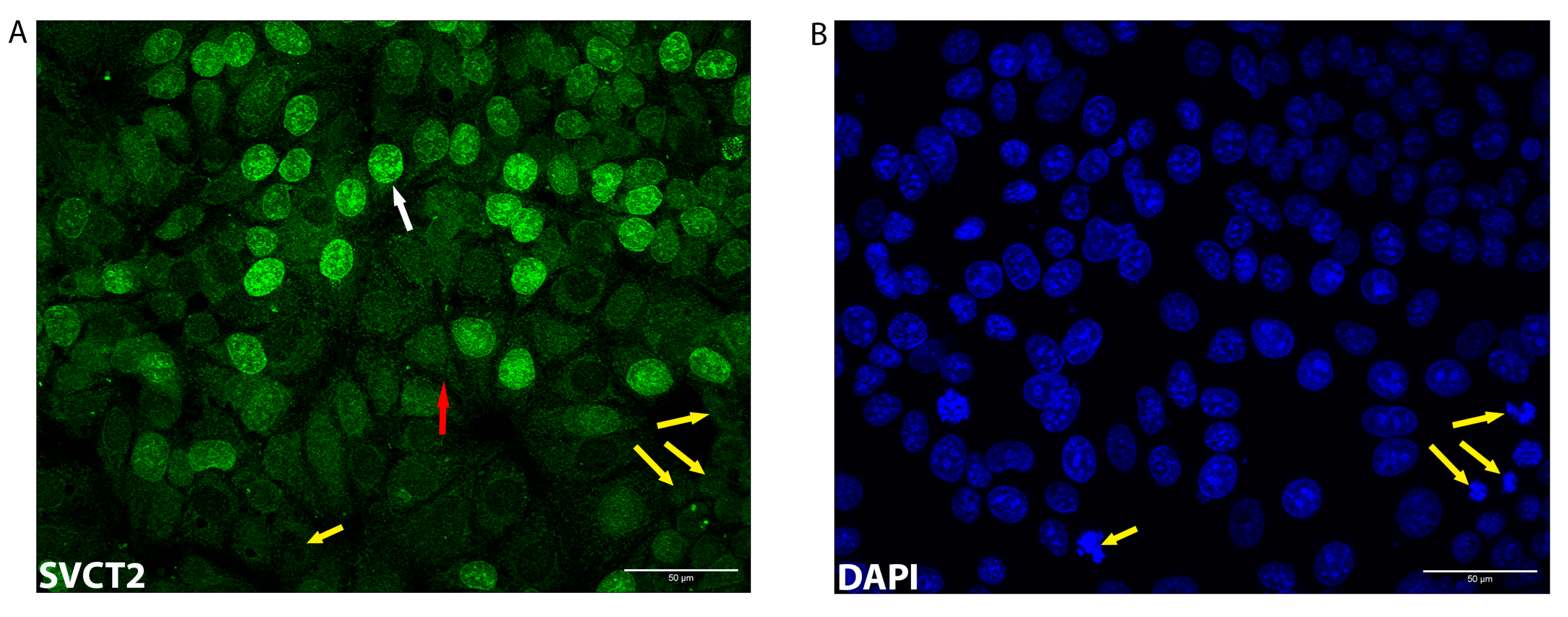
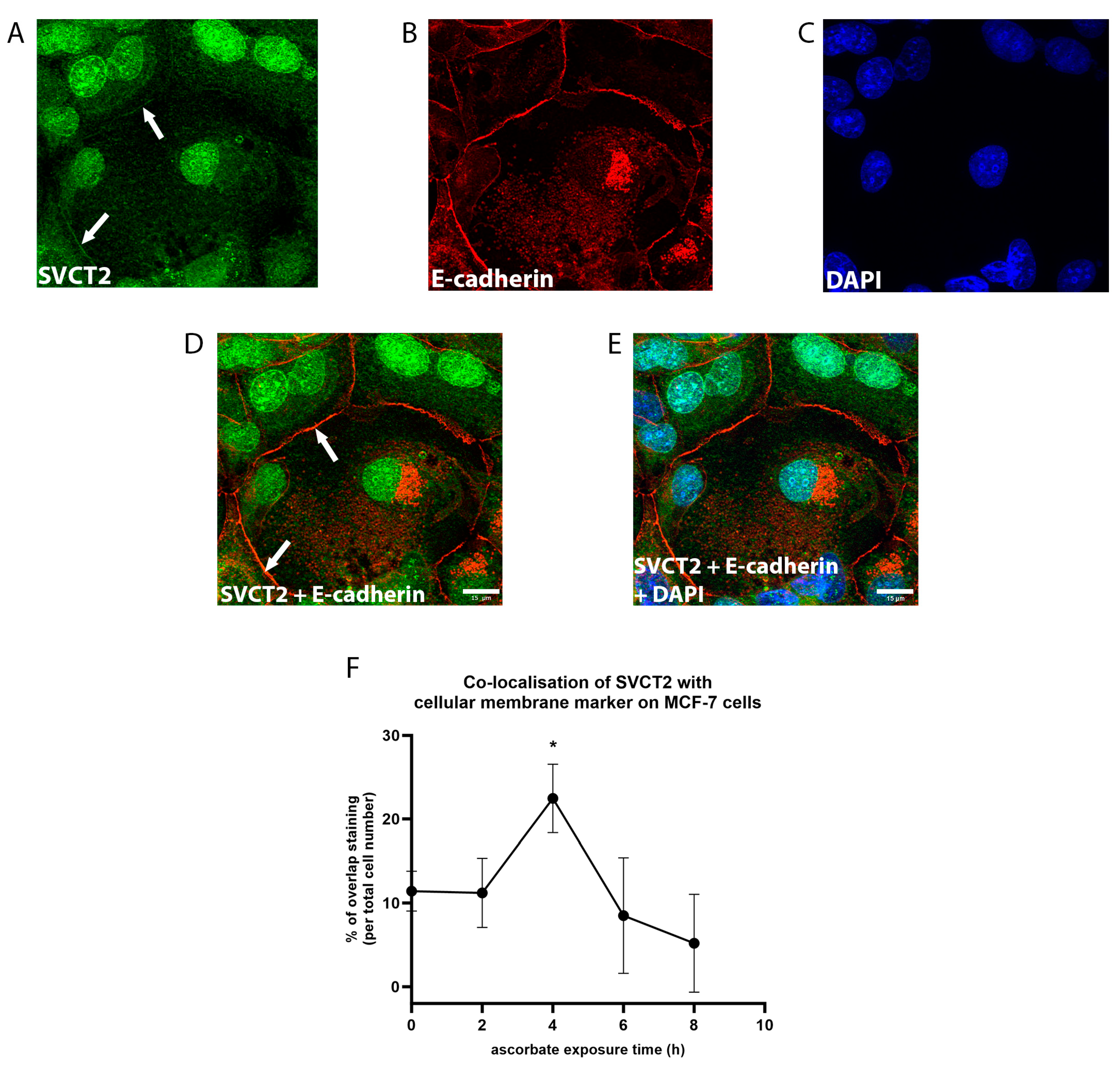
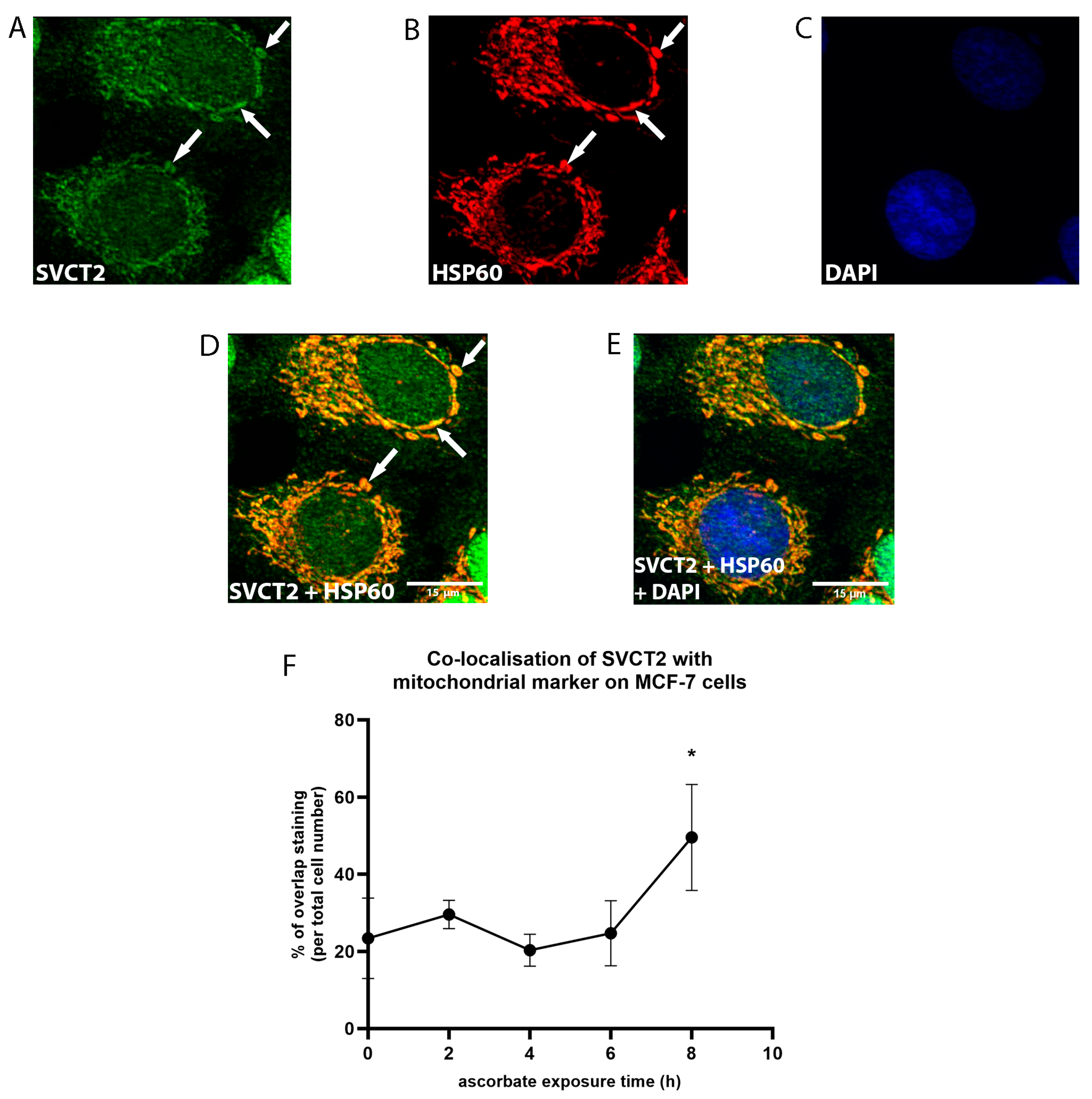
3.2. SVCT2 Localisation and Kinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Lindblad, M.; Tveden-Nyborg, P.; Lykkesfeldt, J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013, 5, 2860–2879. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Fletcher, S.C.; Coleman, M.L. Human 2-oxoglutarate-dependent oxygenases: Nutrient sensors, stress responders, and disease mediators. Biochem. Soc. Trans. 2020, 48, 1843–1858. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Ascorbate as an enzyme cofactor. In Vitamin C New Biochemical and Functional Insights; Chen, Q., Vissers, M.C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 71–98. [Google Scholar]
- Losman, J.A.; Koivunen, P.; Kaelin, W.G., Jr. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 2020, 20, 710–726. [Google Scholar] [CrossRef]
- Halliwel, B.; Gutteridge, J.M.C. Antioxidant defences: Endogenous and diet derived. In Free Radicals in Biology and Medicine; Oxford University Press, Inc.: New York, NY, USA, 2007. [Google Scholar]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Godoy, A.; Ormazabal, V.; Moraga-Cid, G.; Zúñiga, F.A.; Sotomayor, P.; Barra, V.; Vasquez, O.; Montecinos, V.; Mardones, L.; Guzmán, C.; et al. Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2 activated by sodium and absolute dependence of bivalent cations. J. Biol. Chem. 2007, 282, 615–624. [Google Scholar] [CrossRef]
- Wang, Y.; Mackenzie, B.; Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem. Biophys. Res. Commun. 2000, 267, 488–494. [Google Scholar] [CrossRef]
- Boyer, J.C.; Campbell, C.E.; Sigurdson, W.J.; Kuo, S.-M. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem. Biophys. Res. Commun. 2005, 334, 150–156. [Google Scholar] [CrossRef]
- Banhegyi, G.; Szarka, A.; Mandl, J. Role of Ascorbate and Dehydroascorbic Acid in Metabolic Integration of the Cell. In Vitamin C New Biochemical and Functional Insights; Chen, Q., Vissers, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 99–112. [Google Scholar]
- Smith-Diaz, C.C.; Magon, N.J.; McKenzie, J.L.; Hampton, M.B.; Vissers, M.C.M.; Das, A.B. Ascorbate Inhibits Proliferation and Promotes Myeloid Differentiation in TP53-Mutant Leukemia. Front. Oncol. 2021, 11, 709543. [Google Scholar] [CrossRef]
- Munoz-Montesino, C.; Roa, F.J.; Peña, E.; González, M.; Sotomayor, K.; Inostroza, E.; Muñoz, C.A.; González, I.; Maldonado, M.; Soliz, C.; et al. ochondrial ascorbic acid transport is mediated by a low-affinity form of the sodium-coupled ascorbic acid transporter-2. Free Radic. Biol. Med. 2014, 70, 241–254. [Google Scholar] [CrossRef]
- Pena, E.; Roa, F.J.; Inostroza, E.; Sotomayor, K.; González, M.; Gutierrez-Castro, F.A.; Maurin, M.; Sweet, K.; Labrousse, C.; Gatica, M.; et al. Increased expression of mitochondrial sodium-coupled ascorbic acid transporter-2 (mitSVCT2) as a central feature in breast cancer. Free Radic. Biol. Med. 2019, 135, 283–292. [Google Scholar] [CrossRef]
- Roa, F.J.; Peña, E.; Inostroza, E.; Sotomayor, K.; González, M.; Gutierrez-Castro, F.A.; Maurin, M.; Sweet, K.; Labrousse, C.; Gatica, M.; et al. Data on SVCT2 transporter expression and localization in cancer cell lines and tissues. Data Brief 2019, 25, 103972. [Google Scholar] [CrossRef]
- Zhitkovich, A. Nuclear and Cytoplasmic Functions of Vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Simu, S.; Marcovici, I.; Dobrescu, A.; Malita, D.; Dehelean, C.A.; Coricovac, D.; Olaru, F.; Draghici, G.A.; Navolan, D. Insights into the Behavior of Triple-Negative MDA-MB-231 Breast Carcinoma Cells Following the Treatment with 17β-Ethinylestradiol and Levonorgestrel. Molecules 2021, 26, 2776. [Google Scholar] [CrossRef]
- Horwitz, K.B.; Costlow, M.E.; McGuire, W.L. MCF-7: A human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids 1975, 26, 785–795. [Google Scholar] [CrossRef]
- Johnstone, C.N.; Smith, Y.E.; Cao, Y.; Burrows, A.D.; Cross, R.S.N.; Ling, X.; Redvers, R.P.; Doherty, J.P.; Eckhardt, B.L.; Natoli, A.L.; et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis. Models Mech. 2015, 8, 237–251. [Google Scholar] [CrossRef]
- Le Naour, A.; Rossary, A.; Vasson, M.P. EO771, is it a well-characterized cell line for mouse mammary cancer model? Limit and uncertainty. Cancer Med. 2020, 9, 8074–8085. [Google Scholar] [CrossRef]
- Pullar, J.M.; Bayer, S.; Carr, A.C. Appropriate Handling, Processing and Analysis of Blood Samples Is Essential to Avoid Oxidation of Vitamin C to Dehydroascorbic Acid. Antioxidants 2018, 7, 29. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.V.; Ramalingam, J.; Long, L.H.; Halliwell, B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid. Redox Signal. 2001, 3, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Michels, A.J.; Frei, B. Myths, artifacts, and fatal flaws: Identifying limitations and opportunities in vitamin C research. Nutrients 2013, 5, 5161–5192. [Google Scholar] [CrossRef]
- Wilson, M.K.; Baguley, B.C.; Wall, C.; Jameson, M.B.; Findlay, M.P. Review of high-dose intravenous vitamin C as an anticancer agent. Asia-Pac. J. Clin. Oncol. 2014, 10, 22–37. [Google Scholar] [CrossRef]
- Kuiper, C.; Vissers, M.C.M.; Hicks, K.O. Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue. Free Radic. Biol. Med. 2014, 77, 340–352. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Horiguchi, Y.; Furukawa, F.; Fujita, M.; Imamura, S. Ultrastructural localisation of E-cadherin cell adhesion molecule on the cytoplasmic membrane of keratinocytes in vivo and in vitro. J. Histochem. Cytochem. 1994, 42, 1333–1340. [Google Scholar] [CrossRef]
- Kaul, Z.; Yaguchi, T.; Kaul, S.C.; Wadhwa, R. Quantum dot-based protein imaging and functional significance of two mitochondrial chaperones in cellular senescence and carcinogenesis. Ann. N. Y. Acad. Sci. 2006, 1067, 469–473. [Google Scholar] [CrossRef]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C.M. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef]
- Wohlrab, C.; Kuiper, C.; Vissers, M.C.; Phillips, E.; Robinson, B.A.; Dachs, G.U. Ascorbate modulates the hypoxic pathway by increasing intracellular activity of the HIF hydroxylases in renal cell carcinoma cells. Hypoxia 2019, 7, 17–31. [Google Scholar] [CrossRef]
- Campbell, E.J.; Vissers, M.C.M.; Dachs, G.U. Ascorbate availability affects tumor implantation-take rate and increases tumor rejection in Gulo−/− mice. Hypoxia 2016, 4, 41–52. [Google Scholar]
- Travaglini, S.; Gurnari, C.; Antonelli, S.; Silvestrini, G.; Noguera, N.I.; Ottone, T.; Voso, M.T. The Anti-Leukemia Effect of Ascorbic Acid: From the Pro-Oxidant Potential to the Epigenetic Role in Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2022, 10, 930205. [Google Scholar] [CrossRef]
- Wohlrab, C.; Vissers, M.C.; Burgess, E.R.; Nonis, M.; Phillips, E.; Robinson, B.A.; Dachs, G.U. Limited Association Between Ascorbate Concentrations and Vitamin C Transporters in Renal Cell Carcinoma Cells and Clinical Samples. Cell Physiol. Biochem. 2021, 55, 553–568. [Google Scholar]
- Cha, J.; Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013, 42, 55–64. [Google Scholar] [CrossRef]
- Tan, C.W.; Gardiner, B.S.; Hirokawa, Y.; Layton, M.J.; Smith, D.W.; Burgess, A.W. Wnt signalling pathway parameters for mammalian cells. PLoS ONE 2012, 7, e31882. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C. Ascorbic acid efflux and re-uptake in endothelial cells: Maintenance of intracellular ascorbate. Mol. Cell. Biochem. 2009, 325, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.C.; Rivas, C.I.; Fischbarg, J.; Golde, D.W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993, 364, 79–82. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.-C.; Whitesell, R.R.; Cobb, C.E. Ascorbate recycling in human erythrocytes: Role of GSH in reducing dehydroascorbate. Free Radic. Biol. Med. 1996, 20, 543–551. [Google Scholar] [CrossRef]
- Washko, P.W.; Wang, Y.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar] [CrossRef]
- Islam, M.S.; Leissing, T.M.; Chowdhury, R.; Hopkinson, R.J.; Schofield, C.J. 2-Oxoglutarate-Dependent Oxygenases. Annu. Rev. Biochem. 2018, 87, 585–620. [Google Scholar] [CrossRef]
- Cheishvili, D.; Boureau, L.; Szyf, M. DNA demethylation and invasive cancer: Implications for therapeutics. Br. J. Pharmacol. 2015, 172, 2705–2715. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Linowiecka, K.; Foksinski, M.; Brozyna, A.A. Vitamin C Transporters and Their Implications in Carcinogenesis. Nutrients 2020, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Frikke-Schmidt, H.; Lykkesfeldt, J. Keeping the intracellular vitamin C at a physiologically relevant level in endothelial cell culture. Anal. Biochem. 2010, 397, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Myllyla, R.; Majamaa, K.; Gunzler, V.; Hanauske-Abel, H.M.; Kivirikko, K.I. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl-4-hydroxylase and lysyl hydroylase. J. Biol. Chem. 1984, 259, 5403–5405. [Google Scholar] [CrossRef]
- Bode, A.M.; Cunningham, L.; Rose, R.C. Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin. Chem. 1990, 36, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Borsook, H.M.; Davenport, H.W.; Jeffreys, C.E.; Warner, R.C. The oxidation of ascorbic acid and its reduction in vitro and in vivo. J. Biol. Chem. 1937, 117, 237–279. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]
- Luo, M.; Fu, A.; Wu, R.; Wei, N.; Song, K.; Lim, S.; Luo, K.Q. High Expression of G6PD Increases Doxorubicin Resistance in Triple Negative Breast Cancer Cells by Maintaining GSH Level. Int. J. Biol. Sci. 2022, 18, 1120–1133. [Google Scholar] [CrossRef]
- Fujiwara, M.; Nagao, N.; Monden, K.; Misumi, M.; Kageyama, K.; Yamamoto, K.; Miwa, N. Enhanced protection against peroxidation-induced mortality of aortic endothelial cells by ascorbic acid-2-O-phosphate abundantly accumulated in the cell as the dephosphorylated form. Free Radic. Res. 1997, 27, 97–104. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praditi, C.; Bozonet, S.M.; Dachs, G.U.; Vissers, M.C.M. Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2. Antioxidants 2023, 12, 1929. https://doi.org/10.3390/antiox12111929
Praditi C, Bozonet SM, Dachs GU, Vissers MCM. Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2. Antioxidants. 2023; 12(11):1929. https://doi.org/10.3390/antiox12111929
Chicago/Turabian StylePraditi, Citra, Stephanie M. Bozonet, Gabi U. Dachs, and Margreet C. M. Vissers. 2023. "Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2" Antioxidants 12, no. 11: 1929. https://doi.org/10.3390/antiox12111929
APA StylePraditi, C., Bozonet, S. M., Dachs, G. U., & Vissers, M. C. M. (2023). Ascorbate Uptake and Retention by Breast Cancer Cell Lines and the Intracellular Distribution of Sodium-Dependent Vitamin C Transporter 2. Antioxidants, 12(11), 1929. https://doi.org/10.3390/antiox12111929







