Pressurized Hot Water Extraction of Mangosteen Pericarp and Its Associated Molecular Signatures in Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Chemicals
2.2. Extraction of Compounds Using PHWE
2.3. LC/UV and LC/MSMS Profiling of Mangosteen Pericarp Extracts
2.4. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Antioxidant Assay
2.5. ABTS (Azinobis-3-ethylbenzothiazoline-6-sulfonic Acid) Antioxidant Capacity Assay
2.6. Cell Culture and Treatment with MPE
2.7. RNA Extraction and RNA Sequencing
2.8. Transcriptional Profiling and Regulatory Network Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. PHWE and Chemical Standardization of MPE
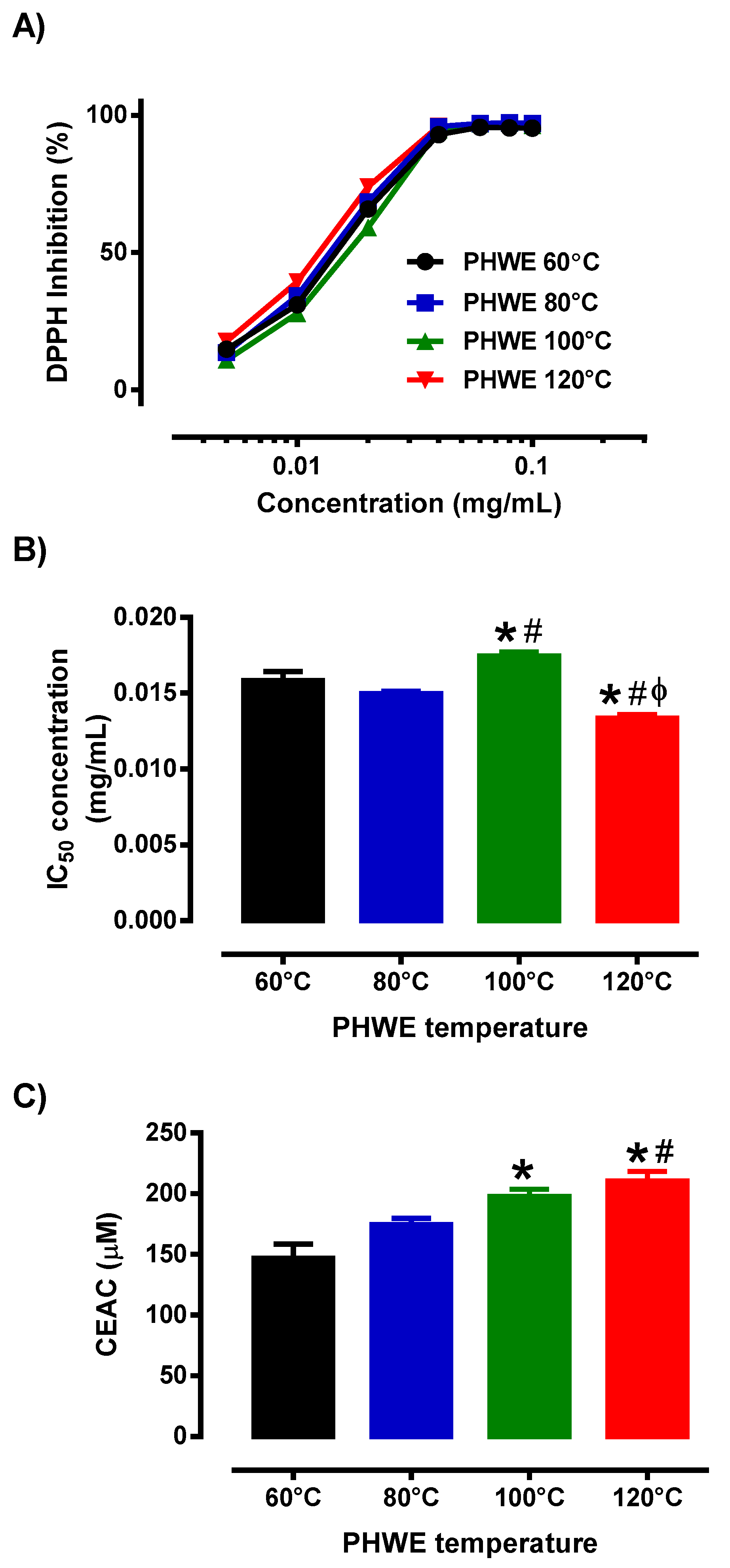
3.2. Antioxidant Activity of MPE
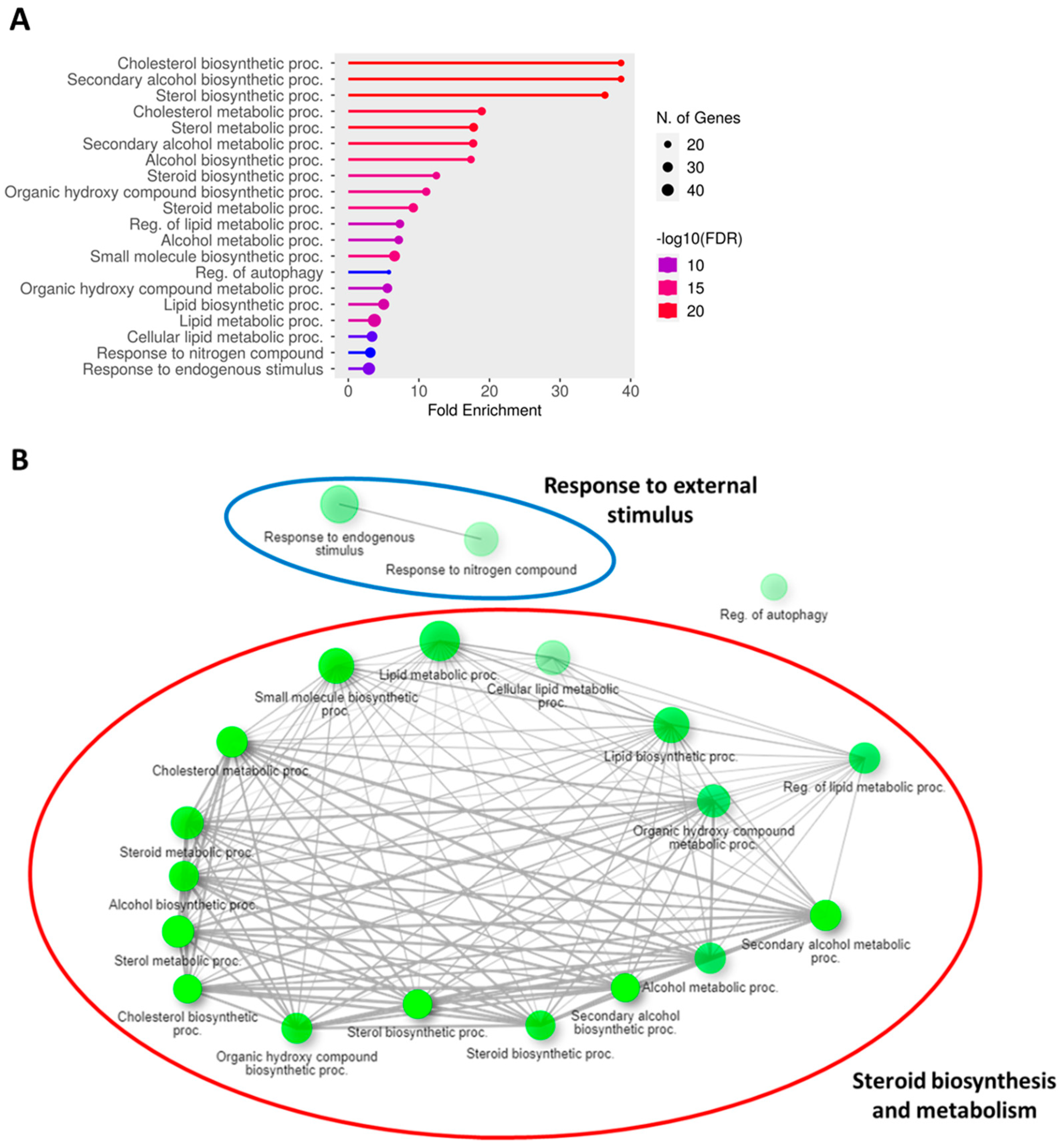
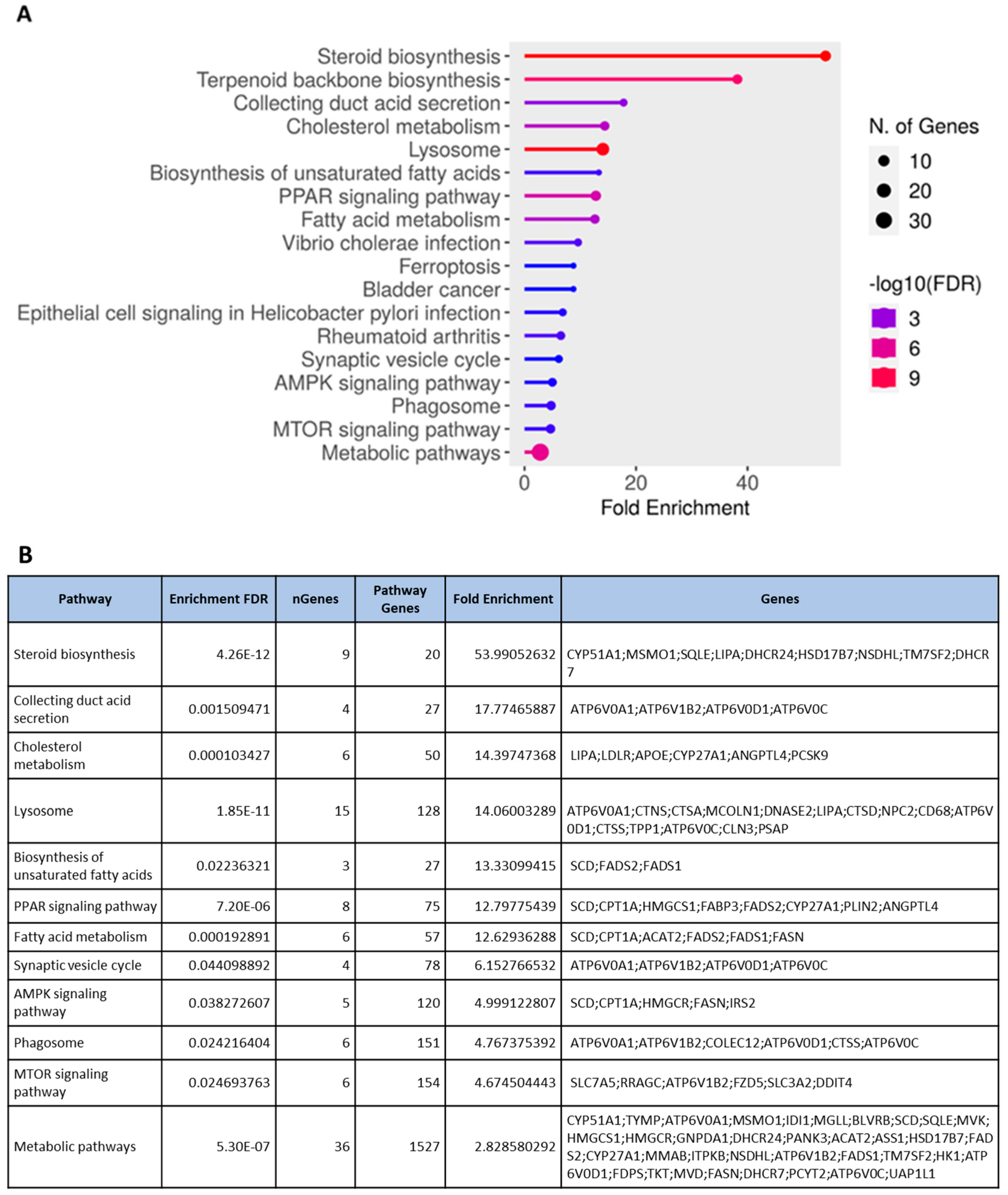
3.3. Transcriptomic Profiling and Molecular Signatures of MPE Treatment in Endothelial Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aizat, W.M.; Ahmad-Hashim, F.H.; Syed Jaafar, S.N. Valorization of mangosteen, “The Queen of Fruits,” and new advances in postharvest and in food and engineering applications: A review. J. Adv. Res. 2019, 20, 61–70. [Google Scholar] [CrossRef]
- John, O.D.; Mushunje, A.T.; Surugau, N.; Guad, R.M. The metabolic and molecular mechanisms of α-mangostin in cardiometabolic disorders (Review). Int. J. Mol. Med. 2022, 50, 120. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar]
- Boutagy, N.E.; Singh, A.K.; Sessa, W.C. Targeting the vasculature in cardiometabolic disease. J. Clin. Investig. 2022, 132, e148556. [Google Scholar] [CrossRef]
- Godson, C.; Guiry, P.; Brennan, E. Lipoxin Mimetics and the Resolution of Inflammation. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 429–448. [Google Scholar] [CrossRef]
- Halade, G.V.; Lee, D.H. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Machmudah, S.; Lestari, S.D.; Widiyastuti; Wahyudiono; Kanda, H.; Winardi, S.; Goto, M. Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J. Supercrit. Fluids 2018, 133, 615–624. [Google Scholar] [CrossRef]
- Muhamad Adyab, N.S.; Rahmat, A.; Abdul Kadir, N.A.A.; Jaafar, H.; Shukri, R.; Ramli, N.S. Mangosteen (Garcinia mangostana) flesh supplementation attenuates biochemical and morphological changes in the liver and kidney of high fat diet-induced obese rats. BMC Complement. Altern. Med. 2019, 19, 344. [Google Scholar] [CrossRef]
- Pan, T.; Chen, R.; Wu, D.; Cai, N.; Shi, X.; Li, B.; Pan, J. Alpha-mangostin suppresses interleukin-1β-induced apoptosis in rat chondrocytes by inhibiting the NF-κB signaling pathway and delays the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2017, 52, 156–162. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Pires, T.C.S.P.; Alves, M.J.; Corrêa, R.C.G.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Barros, L. Insights into the Chemical Composition and In Vitro Bioactive Properties of Mangosteen (Garcinia mangostana L.) Pericarp. Foods 2023, 12, 994. [Google Scholar] [CrossRef]
- Vien, L.C.; Chinnappan, S.; Mogana, R. Antioxidant activity of Garcinia mangostana L and alpha mangostin: A review. Res. J. Pharm. Technol. 2021, 14, 4466–4470. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- EMA. CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities: Residual Solvents; European Medicines Agency: Amsterdam, The Netherlands, 2013.
- FDA. Botanical Drug Development, Guidance for Industry. 2016. Available online: https://www.fda.gov/files/drugs/published/Botanical-Drug-Development--Guidance-for-Industry.pdf (accessed on 6 September 2023).
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Green extraction and characterization of leaves phenolic compounds: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5155–5193. [Google Scholar] [CrossRef]
- Leo, C.H.; Ong, E.S. Recent advances in the combination of organic solvent-free extraction, chemical standardization, antioxidant assay, and cell culture metabolomics for functional food and its by-product. Crit. Rev. Food Sci. Nutr. 2023, in press. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Semi-Continuous Subcritical Water Extraction of Flavonoids from Citrus unshiu Peel: Their Antioxidant and Enzyme Inhibitory Activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Torres-Mayanga, P.C.; Ávila, P.F.; Tompsett, G.A.; Marostica, M.; Goldbeck, R.; Timko, M.T.; Rostagno, M.; Martinez, J.; et al. Sequential subcritical water process applied to orange peel for the recovery flavanones and sugars. J. Supercrit. Fluids 2020, 160, 104789. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Ong, E.S.; Low, J.; Tan, J.C.W.; Foo, S.Y.; Leo, C.H. Valorization of avocado seeds with antioxidant capacity using pressurized hot water extraction. Sci. Rep. 2022, 12, 13036. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Oh, C.L.Y.; Tan, J.C.W.; Foo, S.Y.; Leo, C.H. Pressurized Hot Water Extraction of Okra Seeds Reveals Antioxidant, Antidiabetic and Vasoprotective Activities. Plants 2021, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.B. Compendium of Terminology in Analytical Chemistry; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-Mangostin-Rich Extracts from Mangosteen Pericarp: Optimization of Green Extraction Protocol and Evaluation of Biological Activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef] [PubMed]
- Yuvanatemiya, V.; Srean, P.; Klangbud, W.K.; Venkatachalam, K.; Wongsa, J.; Parametthanuwat, T.; Charoenphun, N. A Review of the Influence of Various Extraction Techniques and the Biological Effects of the Xanthones from Mangosteen (Garcinia mangostana L.) Pericarps. Molecules 2022, 27, 8775. [Google Scholar] [CrossRef]
- Leo, C.H.; Jelinic, M.; Ng, H.H.; Parry, L.J.; Tare, M. Recent developments in relaxin mimetics as therapeutics for cardiovascular diseases. Curr. Opin. Pharmacol. 2019, 45, 42–48. [Google Scholar] [CrossRef]
- Leo, C.H.; Ou, J.L.M.; Ong, E.S.; Qin, C.X.; Ritchie, R.H.; Parry, L.J.; Ng, H.H. Relaxin Elicits Renoprotective Actions Accompanied by Increasing Bile Acid Levels in Streptozotocin-induced Diabetic Mice. Biomed. Pharmacother. 2023, 162, 114578. [Google Scholar] [CrossRef]
- Ong, E.S.; Pek, C.J.N.; Tan, J.C.W.; Leo, C.H. Antioxidant and Cytoprotective Effect of Quinoa (Chenopodium quinoa Willd.) with Pressurized Hot Water Extraction (PHWE). Antioxidants 2020, 9, 1110. [Google Scholar] [CrossRef]
- Leo, C.H.; Foo, S.Y.; Tan, J.C.W.; Tan, U.X.; Chua, C.K.; Ong, E.S. Green Extraction of Orange Peel Waste Reduces TNFα-Induced Vascular Inflammation and Endothelial Dysfunction. Antioxidants 2022, 11, 1768. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Batut, B.; van den Beek, M.; Doyle, M.A.; Soranzo, N. RNA-Seq Data Analysis in Galaxy. Methods Mol. Biol. 2021, 2248, 367–392. [Google Scholar]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Nawawi, N.I.M.; Ijod, G.; Abas, F.; Ramli, N.S.; Mohd Adzahan, N.; Mohamad Azman, E. Influence of Different Drying Methods on Anthocyanins Composition and Antioxidant Activities of Mangosteen (Garcinia mangostana L.) Pericarps and LC-MS Analysis of the Active Extract. Foods 2023, 12, 2351. [Google Scholar] [CrossRef]
- Ong, E.S. Urine Metabolites and Bioactive compounds from Functional Food, Applications of Liquid Chromatography Mass Spectrometry. Crit. Rev. Anal. Chem. 2023, 23, 5442. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Woodman, O.L.; Chan, E.C.H. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Meeker, W.F.; Boujaoude, M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharmacol. 2005, 46, 302–309. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Jaisupa, N.; Samer, J.; Kosem, N.; Konlata, J.; Rodpai, E.; Pongpan, N. Comparison of the biological activity of two different isolates from mangosteen. J. Pharm. Pharmacol. 2014, 66, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Ngawhirunpat, T.; Opanasopi, P.; Sukma, M.; Sittisombut, C.; Kat, A.; Adachi, I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharm. Biol. 2010, 48, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Suttirak, W.; Manurakchinakorn, S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol. 2014, 51, 3546–3558. [Google Scholar] [CrossRef]
- Jelinic, M.; Jackson, K.L.; O’Sullivan, K.; Singh, J.; Giddy, T.; Deo, M.; Parry, L.J.; Ritchie, R.H.; Woodman, O.L.; Head, G.A.; et al. Endothelium-dependent relaxation is impaired in Schlager hypertensive (BPH/2J) mice by region-specific mechanisms in conductance and resistance arteries. Life Sci. 2023, 320, 121542. [Google Scholar] [CrossRef]
- John, O.D.; Brown, L.; Panchal, S.K. Rind from purple mangosteen (Garcinia mangostana) attenuates diet-induced physiological and metabolic changes in obese rats. Nutrients 2019, 13, 319. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Sukandar, E.; Kurniati, N.F.; Adnyana, I.K. Preventive effect on obesity of mangosteen (Garcinia mangostana L.) pericarp ethanolic extract by reduction of fatty acid synthase level in monosodium glutamate and high-calorie diet-induced male wistar rats. Asian J. Pharm. Clin. Res. 2016, 9, 257–260. [Google Scholar]
- Choi, Y.H.; Bae, J.K.; Chae, H.S.; Kim, Y.M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.W. α-Mangostin regulates hepatic steatosis and obesity through SirT1-AMPK and PPARγ pathways in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef]
- Chae, H.S.; Kim, Y.M.; Bae, J.K.; Sorchhann, S.; Yim, S.; Han, L.; Paik, J.H.; Choi, Y.H.; Chin, Y.W. Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating AMPK. J. Med. Food 2016, 19, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Tousian, H.; Razavi, B.M.; Hosseinzadeh, H. Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. Daru 2020, 28, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.D.; Vijayaragavan, K. Ameliorative prospective of alpha-mangostin, a xanthone derivative from Garcinia mangostana against beta-adrenergic cathecolamine-induced myocardial toxicity and anomalous cardiac TNF-α and COX-2 expressions in rats. Exp. Toxicol. Pathol. 2008, 60, 357–364. [Google Scholar] [CrossRef]
- Lee, A.Y.; Pant, A.; Pojchanun, K.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.-X.; Leo, C.H.; Wong, G.; Chua, C.K.; et al. Three-Dimensional Printing of Food Foams Stabilized by Hydrocolloids for Hydration in Dysphagia. Int. J. Bioprint. 2021, 7, 393. [Google Scholar] [CrossRef]
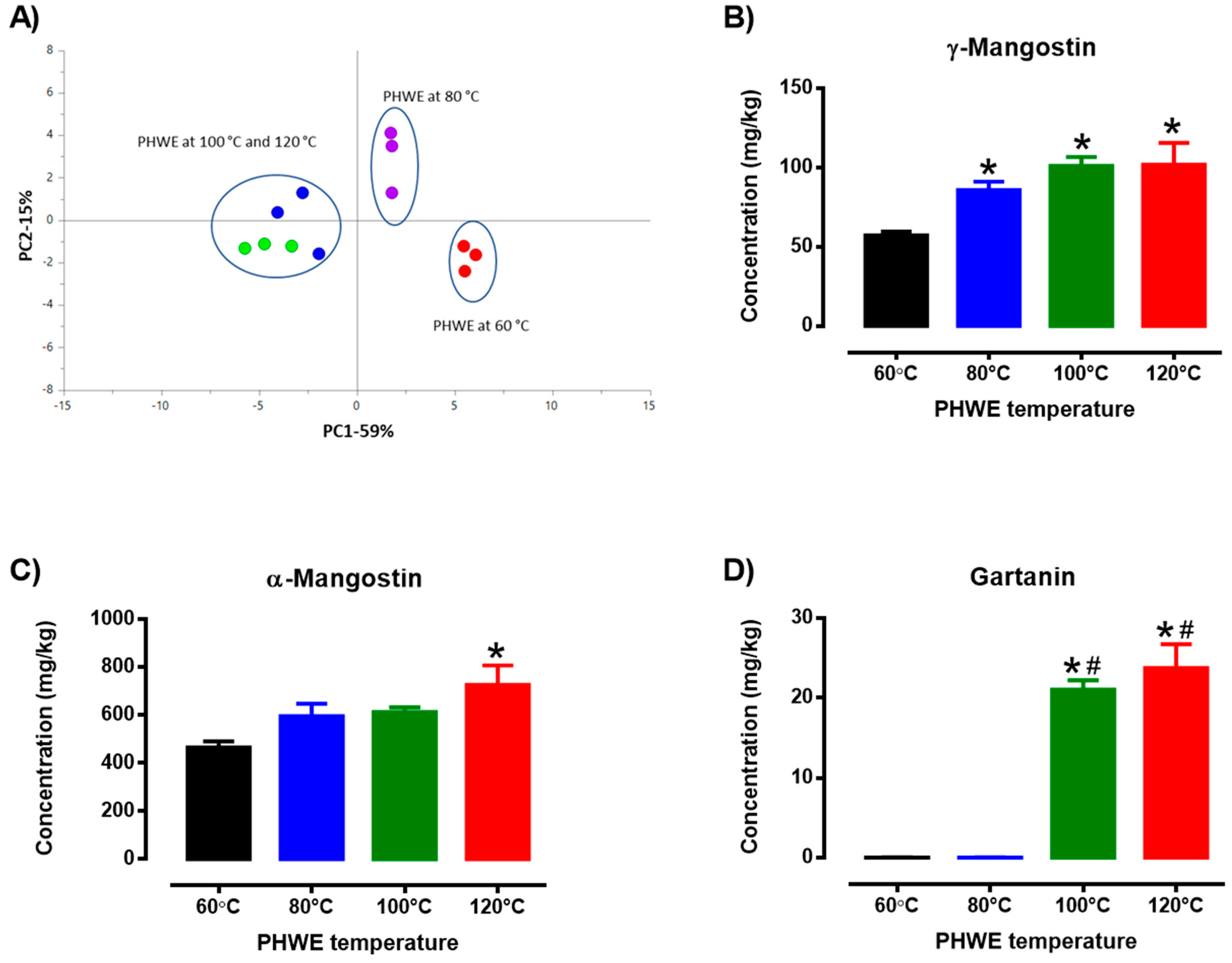

| No | Retention Time (Min) | Compound | Chemical Structure | Mass-to-Charge Ratio (m/z) | Tandem MS (MS/MS) |
|---|---|---|---|---|---|
| 1 | 9.1 | Gartanin | C23H24O6 | 395 | 395, 379, 351, 337, 325, 297, 285, 151, 124 |
| 2 | 9.1 | α-Mangostin | C24H26O6 | 409 | 393, 377, 351, 339, 307, 295, 282, 267, 255, 242 |
| 3 | 8.5 | γ- Mangostin | C23H24O6 | 395 | 395, 351, 339, 309, 297, 283, 271, 255, 242, 177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.S.Y.; Shanmugham, M.; Chin, Y.L.; An, J.; Chua, C.K.; Ong, E.S.; Leo, C.H. Pressurized Hot Water Extraction of Mangosteen Pericarp and Its Associated Molecular Signatures in Endothelial Cells. Antioxidants 2023, 12, 1932. https://doi.org/10.3390/antiox12111932
Tan SSY, Shanmugham M, Chin YL, An J, Chua CK, Ong ES, Leo CH. Pressurized Hot Water Extraction of Mangosteen Pericarp and Its Associated Molecular Signatures in Endothelial Cells. Antioxidants. 2023; 12(11):1932. https://doi.org/10.3390/antiox12111932
Chicago/Turabian StyleTan, Sakeena Si Yu, Meyammai Shanmugham, Yu Ling Chin, Jia An, Chee Kai Chua, Eng Shi Ong, and Chen Huei Leo. 2023. "Pressurized Hot Water Extraction of Mangosteen Pericarp and Its Associated Molecular Signatures in Endothelial Cells" Antioxidants 12, no. 11: 1932. https://doi.org/10.3390/antiox12111932







