Multi-Oxidant Environment as a Suicidal Inhibitor of Myeloperoxidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protein Reconstitution
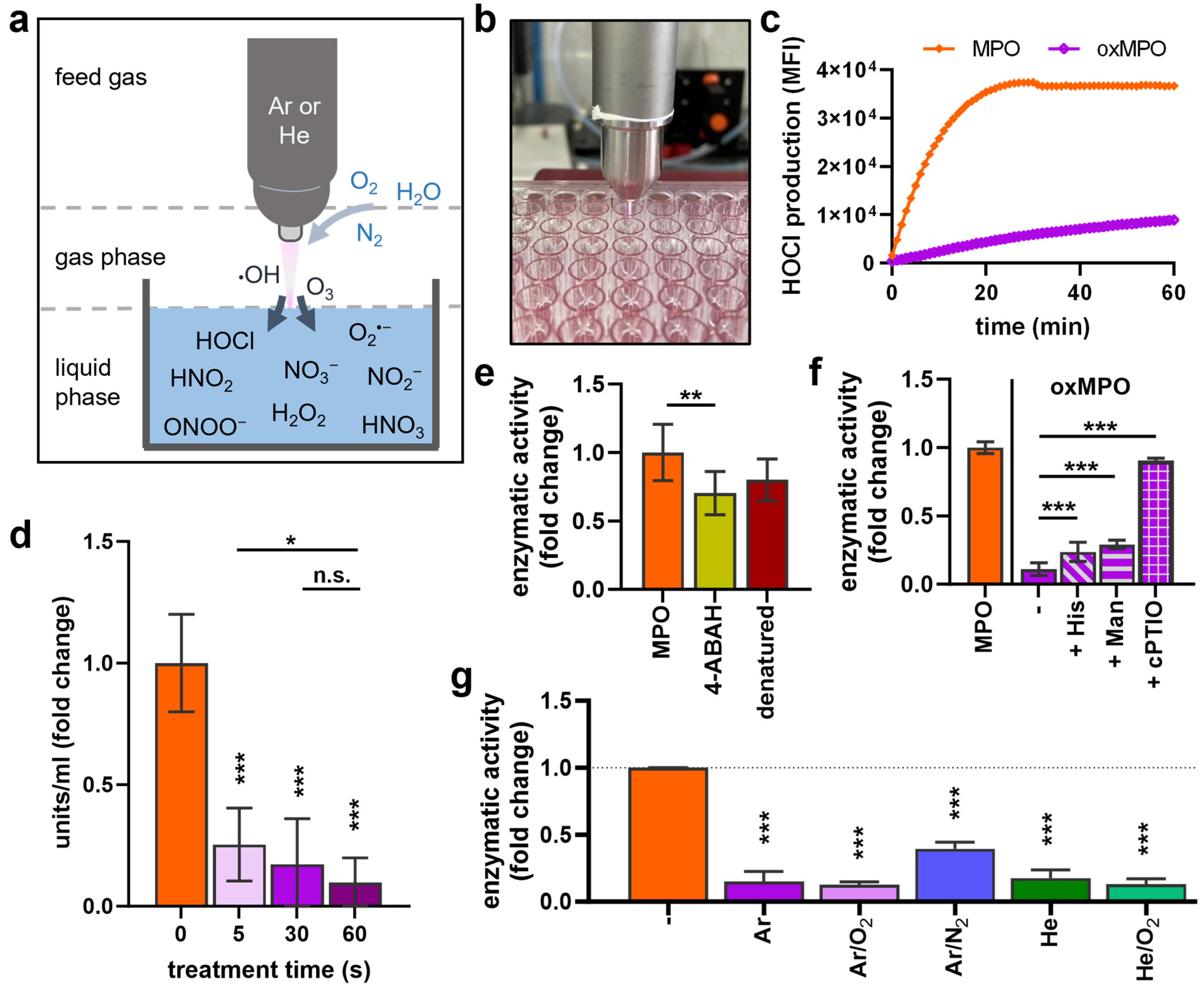
2.2. Plasma Device and Treatment
2.3. MPO Activity Assays
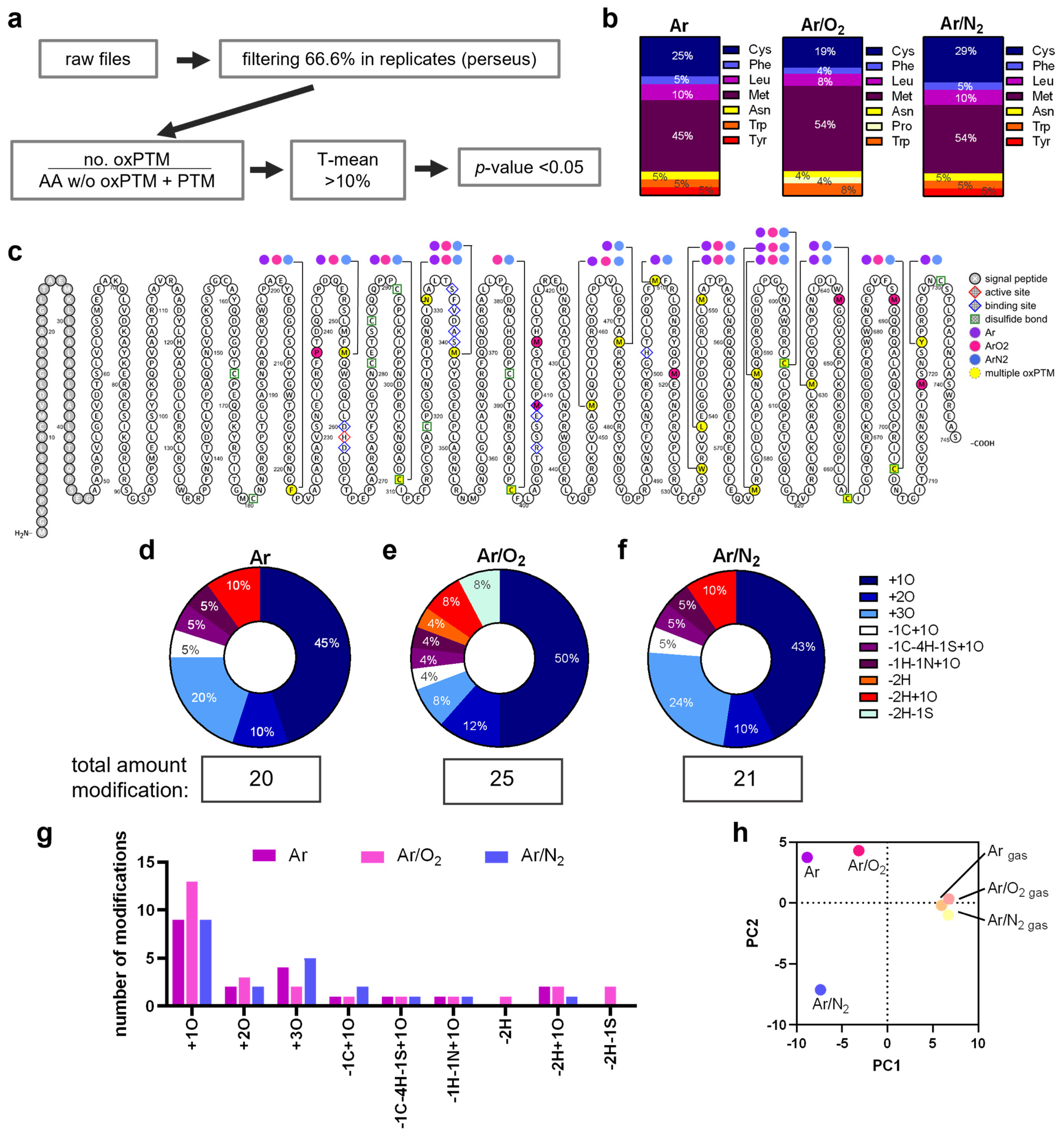
2.4. High-Resolution LC-MS2 Measurements of MPO
2.5. LC-MS/MS Data Analysis
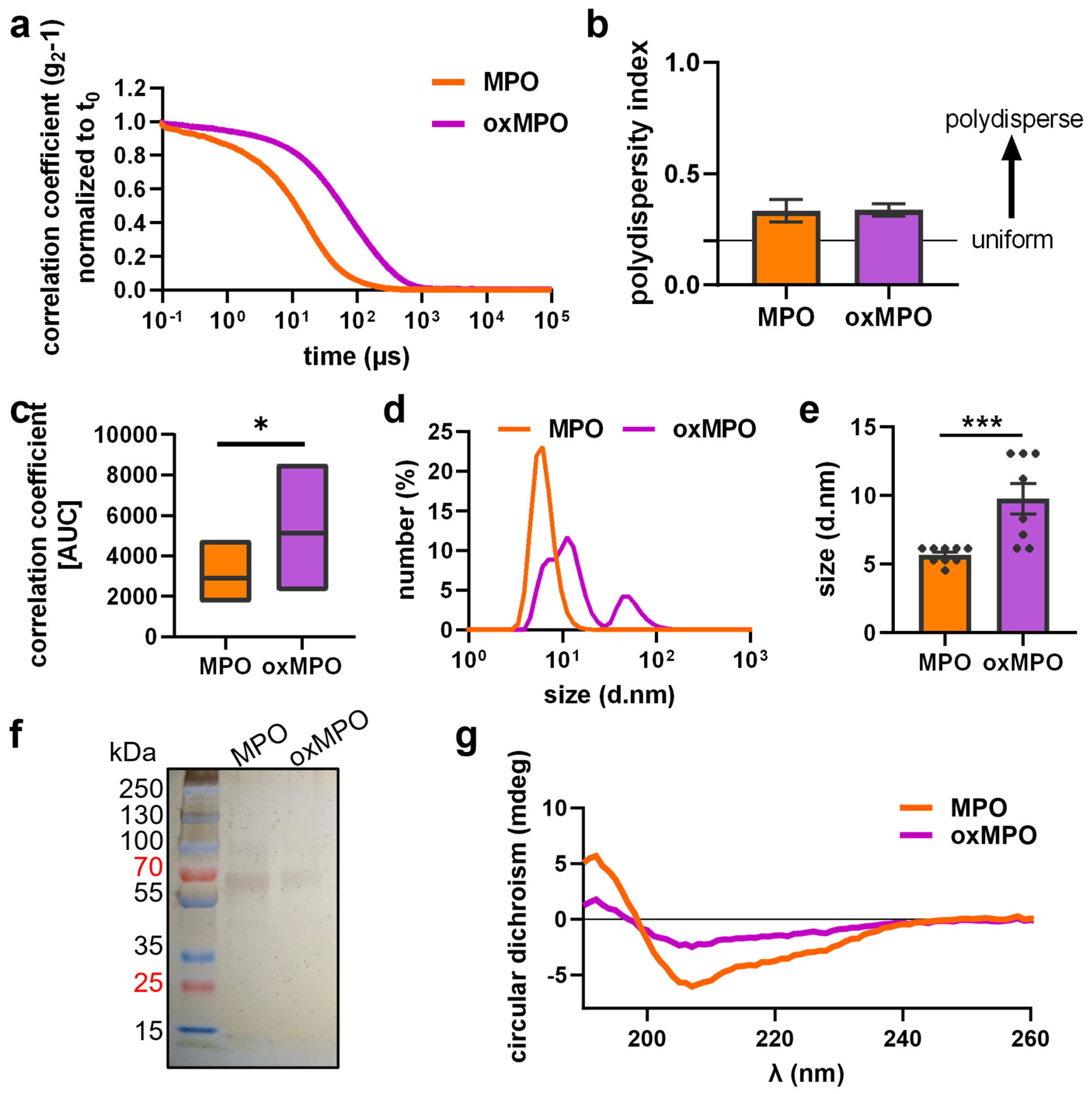
2.6. Photon Correlation Spectroscopy
2.7. Circular Dichroism (CD) Spectroscopy
2.8. SDS Page and Silver Staining
2.9. Statistical Analysis

3. Results
3.1. Plasma Treatment Decreased MPO Activity
3.2. Plasma Treatment Modified the Protein Structure of MPO
3.3. Plasma Treatment Provoked oxPTMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Nadel, J.; Tumanov, S.; Kong, S.M.Y.; Chen, W.; Giannotti, N.; Sivasubramaniam, V.; Rashid, I.; Ugander, M.; Jabbour, A.; Stocker, R. Intraplaque Myeloperoxidase Activity as Biomarker of Unstable Atheroma and Adverse Clinical Outcomes in Human Atherosclerosis. JACC Adv. 2023, 2, 100310. [Google Scholar] [CrossRef]
- Michaelsson, E.; Lund, L.H.; Hage, C.; Shah, S.J.; Voors, A.A.; Saraste, A.; Redfors, B.; Grove, E.L.; Barasa, A.; Richards, A.M.; et al. Myeloperoxidase Inhibition Reverses Biomarker Profiles Associated with Clinical Outcomes in HFpEF. JACC Heart Fail. 2023, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, A.; Borichevsky, G.M.; Edwards, T.S.; Hirschfeld, E.; Mules, T.C.; Frampton, C.M.A.; Day, A.S.; Hampton, M.B.; Kettle, A.J.; Gearry, R.B. Faecal Myeloperoxidase as a Biomarker of Endoscopic Activity in Inflammatory Bowel Disease. J. Crohns Colitis 2022, 16, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.C.D.; Murray, H.C.; Hill, M.; van Leeuwen, E.; Highet, B.; Magon, N.J.; Osanlouy, M.; Mathiesen, S.N.; Mockett, B.; Singh-Bains, M.K.; et al. Neutrophil-vascular interactions drive myeloperoxidase accumulation in the brain in Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 38. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Thomson, L.; Tenopoulou, M.; Lightfoot, R.; Tsika, E.; Parastatidis, I.; Martinez, M.; Greco, T.M.; Doulias, P.T.; Wu, Y.; Tang, W.H.; et al. Immunoglobulins against tyrosine-nitrated epitopes in coronary artery disease. Circulation 2012, 126, 2392–2401. [Google Scholar] [CrossRef]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef]
- Paumann-Page, M.; Furtmuller, P.G.; Hofbauer, S.; Paton, L.N.; Obinger, C.; Kettle, A.J. Inactivation of human myeloperoxidase by hydrogen peroxide. Arch. Biochem. Biophys. 2013, 539, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kajer, T.B.; Fairfull-Smith, K.E.; Yamasaki, T.; Yamada, K.; Fu, S.; Bottle, S.E.; Hawkins, C.L.; Davies, M.J. Inhibition of myeloperoxidase- and neutrophil-mediated oxidant production by tetraethyl and tetramethyl nitroxides. Free Radic. Biol. Med. 2014, 70, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Maiocchi, S.; Ku, J.; Hawtrey, T.; De Silvestro, I.; Malle, E.; Rees, M.; Thomas, S.R.; Morris, J.C. Polyamine-Conjugated Nitroxides Are Efficacious Inhibitors of Oxidative Reactions Catalyzed by Endothelial-Localized Myeloperoxidase. Chem. Res. Toxicol. 2021, 34, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Steiling, H.; Munz, B.; Werner, S.; Brauchle, M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999, 247, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma-Air-Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Winter, J.; Iseni, S.; Dunnbier, M.; Weltmann, K.D.; Reuter, S. Reactive species output of a plasma jet with a shielding gas device-combination of FTIR absorption spectroscopy and gas phase modelling. J. Phys. D Appl. Phys. 2014, 47, 145201. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, M.; Sun, D.-W.; Tiwari, B.K. Correlation of plasma generated long-lived reactive species in aqueous and gas phases with different feeding gases. Plasma Sources Sci. Technol. 2023, 32, 045015. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.D.; Wende, K. Basic Research in Plasma Medicine—A Throughput Approach from Liquids to Cells. J. Vis. Exp. 2017, 129, e56331. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. Rsc. Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Clemen, R.; Arlt, K.; Miebach, L.; von Woedtke, T.; Bekeschus, S. Oxidized Proteins Differentially Affect Maturation and Activation of Human Monocyte-Derived Cells. Cells 2022, 11, 3659. [Google Scholar] [CrossRef]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.W.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice. Adv. Sci. 2021, 8, 2003395. [Google Scholar] [CrossRef]
- Nasri, Z.; Memari, S.; Wenske, S.; Clemen, R.; Martens, U.; Delcea, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Singlet Oxygen-Induced Phospholipase A2 Inhibition: A Major Role for Interfacial Tryptophan Dioxidation. Chemistry 2021, 27, 14702–14710. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Tresp, H.; Hammer, M.U.; Weltmann, K.D. Controlling the Ambient Air Affected Reactive Species Composition in the Effluent of an Argon Plasma Jet. IEEE Trans. Plasma Sci. 2012, 40, 2788–2794. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Cecchini, A.L.; Bekeschus, S. Conductive Gas Plasma Treatment Augments Tumor Toxicity of Ringer’s Lactate Solutions in a Model of Peritoneal Carcinomatosis. Antioxidants 2022, 11, 1439. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Wilkinson, F.; Helman, W.P.; Ross, A.B. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet-State of Molecular-Oxygen in Solution—an Expanded and Revised Compilation. J. Phys. Chem. Ref. Data 1995, 24, 663–1021. [Google Scholar] [CrossRef]
- Wenske, S.; Lackmann, J.W.; Busch, L.M.; Bekeschus, S.; von Woedtke, T.; Wende, K. Reactive species driven oxidative modifications of peptides-Tracing physical plasma liquid chemistry. J. Appl. Phys. 2021, 129, 193305. [Google Scholar] [CrossRef]
- Aratani, Y.; Koyama, H.; Nyui, S.; Suzuki, K.; Kura, F.; Maeda, N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun. 1999, 67, 1828–1836. [Google Scholar] [CrossRef]
- Ahmad, G.; Chami, B.; Liu, Y.; Schroder, A.L.; San Gabriel, P.T.; Gao, A.; Fong, G.; Wang, X.; Witting, P.K. The Synthetic Myeloperoxidase Inhibitor AZD3241 Ameliorates Dextran Sodium Sulfate Stimulated Experimental Colitis. Front. Pharmacol. 2020, 11, 556020. [Google Scholar] [CrossRef] [PubMed]
- Kaindlstorfer, C.; Sommer, P.; Georgievska, B.; Mather, R.J.; Kugler, A.R.; Poewe, W.; Wenning, G.K.; Stefanova, N. Failure of Neuroprotection Despite Microglial Suppression by Delayed-Start Myeloperoxidase Inhibition in a Model of Advanced Multiple System Atrophy: Clinical Implications. Neurotox. Res. 2015, 28, 185–194. [Google Scholar] [CrossRef]
- Groitl, B.; Jakob, U. Thiol-based redox switches. Biochim. Biophys. Acta 2014, 1844, 1335–1343. [Google Scholar] [CrossRef]
- Wenske, S.; Lackmann, J.W.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15, 061008. [Google Scholar] [CrossRef] [PubMed]
- Lackmann, J.W.; Baldus, S.; Steinborn, E.; Edengeiser, E.; Kogelheide, F.; Langklotz, S.; Schneider, S.; Leichert, L.I.O.; Benedikt, J.; Awakowicz, P.; et al. A dielectric barrier discharge terminally inactivates RNase A by oxidizing sulfur-containing amino acids and breaking structural disulfide bonds. J. Phys. D Appl. Phys. 2015, 48, 494003. [Google Scholar] [CrossRef]
- Krewing, M.; Stepanek, J.J.; Cremers, C.; Lackmann, J.W.; Schubert, B.; Muller, A.; Awakowicz, P.; Leichert, L.I.O.; Jakob, U.; Bandow, J.E. The molecular chaperone Hsp33 is activated by atmospheric-pressure plasma protecting proteins from aggregation. J. R. Soc. Interface 2019, 16, 20180966. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Bruno, G.; Jablonowski, H.; Kogelheide, F.; Offerhaus, B.; Held, J.; Schulz-von der Gathen, V.; Stapelmann, K.; von Woedtke, T.; Wende, K. Nitrosylation vs. oxidation—How to modulate cold physical plasmas for biological applications. PLoS ONE 2019, 14, e0216606. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Wenske, S.; Lackmann, J.W.; Lalk, M.; von Woedtke, T.; Wende, K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules 2020, 10, 1687. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Jablonowski, H.; Schmidt-Bleker, A.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Non-touching plasma-liquid interaction—Where is aqueous nitric oxide generated? Phys. Chem. Chem. Phys. 2018, 20, 25387–25398. [Google Scholar] [CrossRef]
- Tresp, H.; Hammer, M.U.; Weltmann, K.-D.; Reuter, S. Effects of Atmosphere Composition and Liquid Type on Plasma-Generated Reactive Species in Biologically Relevant Solutions. Plasma Med. 2013, 3, 45–55. [Google Scholar] [CrossRef]
- Fiedler, T.J.; Davey, C.A.; Fenna, R.E. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 A resolution. J. Biol. Chem. 2000, 275, 11964–11971. [Google Scholar] [CrossRef] [PubMed]
- Colas, C.; Ortiz de Montellano, P.R. Autocatalytic radical reactions in physiological prosthetic heme modification. Chem. Rev. 2003, 103, 2305–2332. [Google Scholar] [CrossRef]
- Karimi, M.; Crossett, B.; Cordwell, S.J.; Pattison, D.I.; Davies, M.J. Characterization of disulfide (cystine) oxidation by HOCl in a model peptide: Evidence for oxygen addition, disulfide bond cleavage and adduct formation with thiols. Free Radic. Biol. Med. 2020, 154, 62–74. [Google Scholar] [CrossRef]
- Kramer, A.C.; Torreggiani, A.; Davies, M.J. Effect of Oxidation and Protein Unfolding on Cross-Linking of beta-Lactoglobulin and alpha-Lactalbumin. J. Agric. Food Chem. 2017, 65, 10258–10269. [Google Scholar] [CrossRef]
- Grishkovskaya, I.; Paumann-Page, M.; Tscheliessnig, R.; Stampler, J.; Hofbauer, S.; Soudi, M.; Sevcnikar, B.; Oostenbrink, C.; Furtmuller, P.G.; Djinovic-Carugo, K.; et al. Structure of human promyeloperoxidase (proMPO) and the role of the propeptide in processing and maturation. J. Biol. Chem. 2017, 292, 8244–8261. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, Y.; Lillig, C.H. Cysteinyl and methionyl redox switches: Structural prerequisites and consequences. Redox Biol. 2023, 65, 102832. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Sokolov, A.V.; Kostevich, V.A.; Vasilyev, V.B.; Panasenko, O.M. Enzymatic and Bactericidal Activity of Monomeric and Dimeric Forms of Myeloperoxidase. Biochem. Suppl. Ser. B Biomed. Chem. 2018, 12, 258–265. [Google Scholar] [CrossRef]
- Heinecke, J.W.; Li, W.; Francis, G.A.; Goldstein, J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J. Clin. Investig. 1993, 91, 2866–2872. [Google Scholar] [CrossRef]
- Lund, M.N.; Luxford, C.; Skibsted, L.H.; Davies, M.J. Oxidation of myosin by haem proteins generates myosin radicals and protein cross-links. Biochem. J. 2008, 410, 565–574. [Google Scholar] [CrossRef]
- Andrekopoulos, C.; Zhang, H.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Bicarbonate enhances alpha-synuclein oligomerization and nitration: Intermediacy of carbonate radical anion and nitrogen dioxide radical. Biochem. J. 2004, 378, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kapp, E.A.; Lothian, A.; Roberts, A.M.; Vasil’ev, Y.V.; Boughton, B.A.; Barnham, K.J.; Kok, W.M.; Hutton, C.A.; Masters, C.L.; et al. Characterization and Identification of Dityrosine Cross-Linked Peptides Using Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Al-Hilaly, Y.K.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.; Morris, K.L.; Sada, A.A.; Thorpe, J.R.; et al. A central role for dityrosine crosslinking of Amyloid-beta in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 83. [Google Scholar] [CrossRef]
- Zeng, L.; Mathew, A.V.; Byun, J.; Atkins, K.B.; Brosius, F.C., 3rd; Pennathur, S. Myeloperoxidase-derived oxidants damage artery wall proteins in an animal model of chronic kidney disease-accelerated atherosclerosis. J. Biol. Chem. 2018, 293, 7238–7249. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Eiserich, J.P.; Brennan, M.L.; Jackson, R.M.; Alexander, C.B.; Freeman, B.A. Spatial mapping of pulmonary and vascular nitrotyrosine reveals the pivotal role of myeloperoxidase as a catalyst for tyrosine nitration in inflammatory diseases. Free Radic. Biol. Med. 2002, 33, 1010. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Ye, Y.Z.; Anderson, P.G.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler 1994, 375, 81–88. [Google Scholar] [CrossRef]
- Vlasak, J.; Bussat, M.C.; Wang, S.; Wagner-Rousset, E.; Schaefer, M.; Klinguer-Hamour, C.; Kirchmeier, M.; Corvaia, N.; Ionescu, R.; Beck, A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal. Biochem. 2009, 392, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zubarev, R.A. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis 2010, 31, 1764–1772. [Google Scholar] [CrossRef]
- Doyle, H.A.; Gee, R.J.; Mamula, M.J. Altered immunogenicity of isoaspartate containing proteins. Autoimmunity 2007, 40, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mamula, M.J.; Gee, R.J.; Elliott, J.I.; Sette, A.; Southwood, S.; Jones, P.J.; Blier, P.R. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 1999, 274, 22321–22327. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemen, R.; Minkus, L.; Singer, D.; Schulan, P.; von Woedtke, T.; Wende, K.; Bekeschus, S. Multi-Oxidant Environment as a Suicidal Inhibitor of Myeloperoxidase. Antioxidants 2023, 12, 1936. https://doi.org/10.3390/antiox12111936
Clemen R, Minkus L, Singer D, Schulan P, von Woedtke T, Wende K, Bekeschus S. Multi-Oxidant Environment as a Suicidal Inhibitor of Myeloperoxidase. Antioxidants. 2023; 12(11):1936. https://doi.org/10.3390/antiox12111936
Chicago/Turabian StyleClemen, Ramona, Lara Minkus, Debora Singer, Paul Schulan, Thomas von Woedtke, Kristian Wende, and Sander Bekeschus. 2023. "Multi-Oxidant Environment as a Suicidal Inhibitor of Myeloperoxidase" Antioxidants 12, no. 11: 1936. https://doi.org/10.3390/antiox12111936
APA StyleClemen, R., Minkus, L., Singer, D., Schulan, P., von Woedtke, T., Wende, K., & Bekeschus, S. (2023). Multi-Oxidant Environment as a Suicidal Inhibitor of Myeloperoxidase. Antioxidants, 12(11), 1936. https://doi.org/10.3390/antiox12111936





