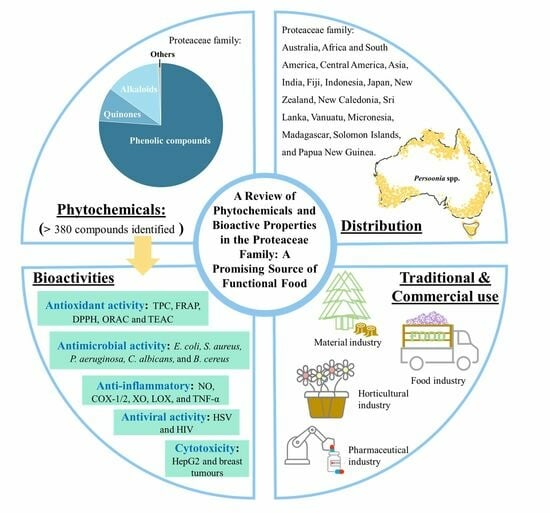
A Review of Phytochemicals and Bioactive Properties in the Proteaceae Family: A Promising Source of Functional Food
Abstract
:1. Introduction
2. Methodology
3. Distribution
4. Traditional and Commercial Use
| Industry | Genus/Specie | Traditional/Commercial Use | References |
|---|---|---|---|
| Food industry | Persoonia, Hicksbeachia, Floydia, Macadamia, Hakea, Brabejum, Finschia, Gevuina, Panopsis, Oreocallis | Seeds, nuts, gum, or fruits have been eaten by Australian Aboriginal people | [7,11] |
| Food industry Pharmaceutical industry | Helicia serrata, H. robusta | Young shoots eaten by Javanese people | [7] |
| Telopea, Lambertia, Grevillea, Banksia, Macadamia | Honey sources | [11,53,54] | |
| Hakea leucoptera | The roots are used for freshwater | [55] | |
| Banksia, Persoonia | Relief of coughs and sore throats | [54,56] | |
| Pharmaceutical industry Horticultural industry | Dilobeia thouarsii, D. cordata | Leaves against S. aureus used for skin infection in Madagascar | [57] |
| Lomatia hirsuta | Leaves used for the treatment of bronchitis and asthma in Chilean traditional medicine | [58] | |
| Faurea saligna | Diarrhea | [7,56,59] | |
| Grevillea, Hakea, Persoonia, Roupala and Xylomelum | Skin infections | ||
| Heliciopsis | Eye infections | ||
| Protea | Skin infections or hyperpigmentation | [60,61] | |
| Helicia robusta | Gastritis or kidney problems | [62,63] | |
| Oreocallis | Liver diseases, bleeding, or inflammation treatments | [7,38] | |
| Helicia, Grevillea | Skin or mouth sores | [56,64] | |
| Grevillea, Hakea, Persoonia, Roupala, Xylomelum, Aulax, Leucadendron, Paranomus, Leucospermum, Mimetes, Heliciopsis, Toronia, Banksia | Skin lightening agent | [7,59,65] | |
| Leucospermum, Persoonia, Hakea, Grevillea, Protea, Serruria, Waratah, Banksia, Telopea, Isopogon, Leucadendron | Colourful horticultural plant | [41,55,66,67] | |
| Material industry | Persoonia | Fishing lines and strings | [11] |
| Grevillea | Cementing compound | [55] | |
| Grevillea, Protea, Darlingia, Buckinghamia, Athertonia and Hakea | Timber | [7,11,42] |
5. Phytochemicals
6. Bioactive Properties
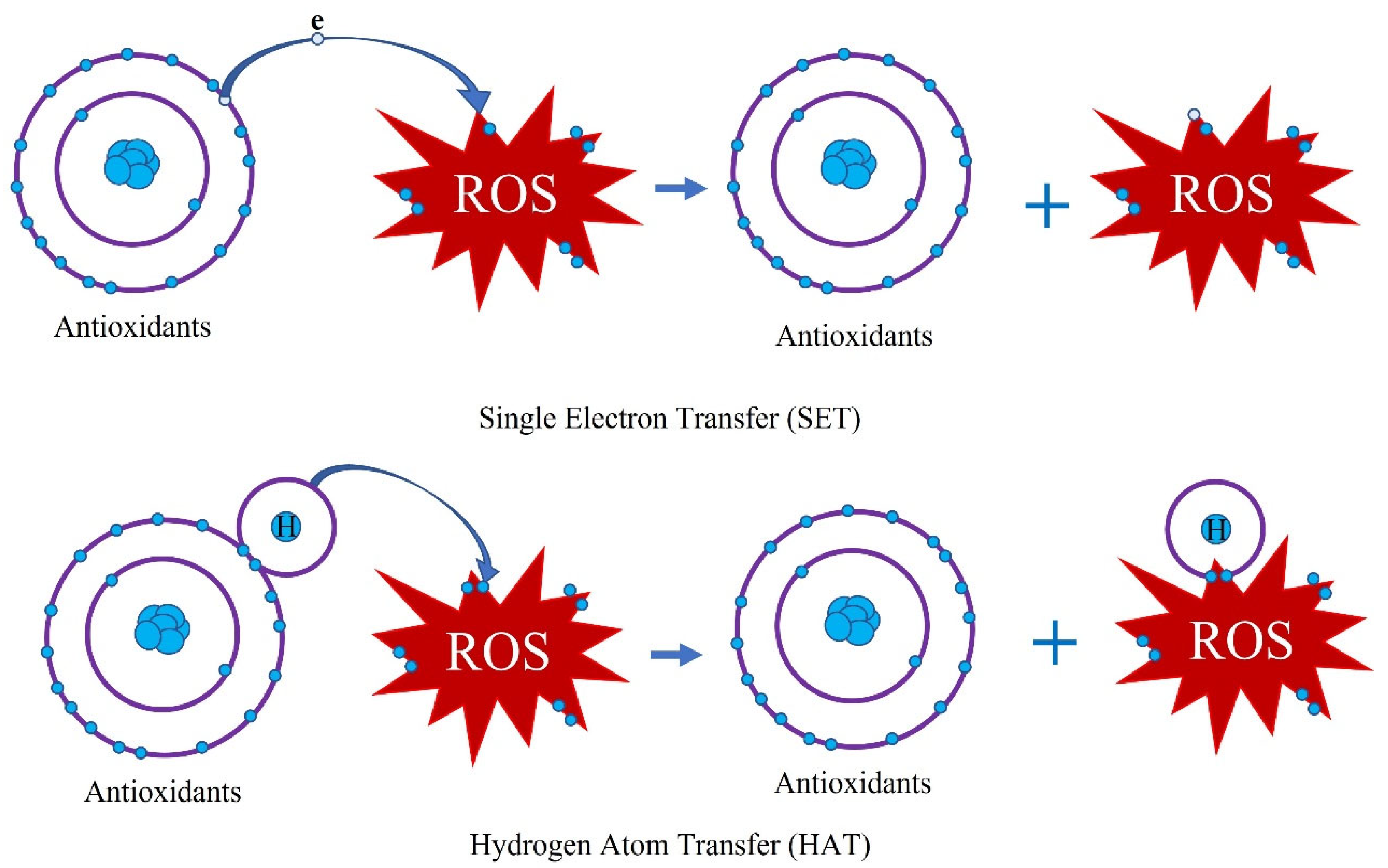
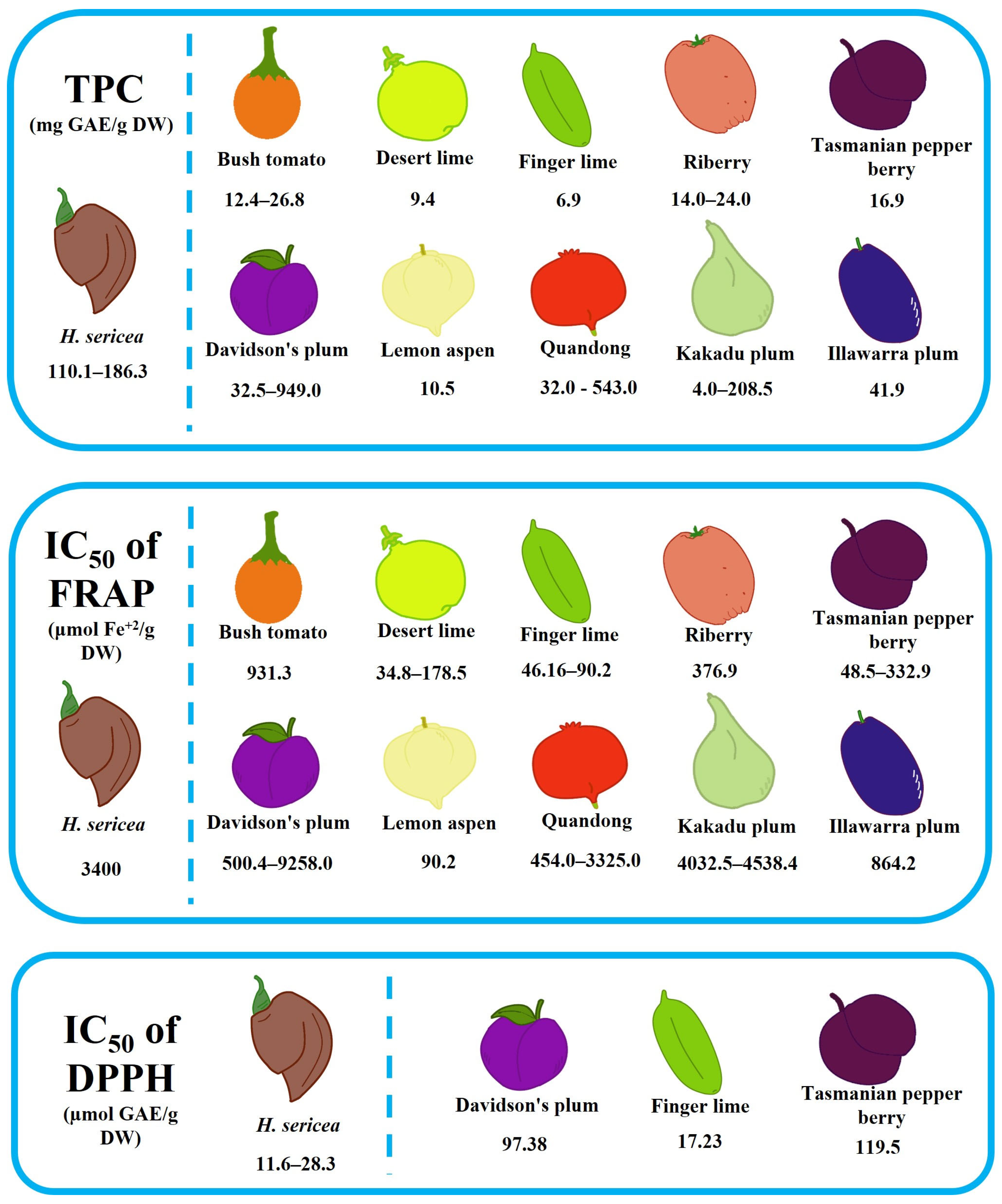
6.1. Antioxidant Activity
| Species | TP | FRAP | DPPH (IC50) | ORAC | TEAC | References |
|---|---|---|---|---|---|---|
| G. avellana (nut) | 1.9–4.6 g GAE/100 g | 51.2–352.8 mM TE/g | 8.9–93.8% inhibition at 100 µg/mL | 273.9–2157.5 μM TE/g | 207.3–1012.8 μM TE/g | [99] |
| Macadamia (nut) | - | - | - | 14.43 ± 2.31 μM TE/g | - | [100] |
| M. integrifolia (nut) | 52.9–108.6 µg GAE/g | 4.7–51.9 µM Fe2+/g | 0–57.0% inhibition (without conc.) | - | 13.3–118.8 mg TE/g | [101] |
| H. terminalis (trunk) | - | - | 156.9 mg/mL | - | - | [98] |
| Faurea. Speciosa (leaf) | 65.4 ± 0.5 mg AAE/g | - | 499.4 ± 5.8 μg/mL | - | - | [102] |
| Protea Susara (aerial part) | - | 4.4 ± 0.1 μM Fe2+/g | 41 ± 2% inhibition at 0.5 mg/mL | - | - | [103] |
| B. menziesii (floral) | 26.1 ± 4.1 mg GAE/100 g | 2.90 ± 0.55 mM Fe2+/kg | 1095 ± 497 µM TE/kg | - | - | [104] |
| B. sessilis (floral) | 31.8 ± 5.5 mg GAE/100 g | 3.12 ± 0.61 mM Fe2+/kg | 1093 ± 263 µM TE/kg | - | - | |
| M. tetraphylla (peel) | 168.22 ± 0.77 mg GAE/g | 1607.82 ± 7.89 μM TE/g | 1128.76 μM TE/g | - | - | [105] |
| O. grandiflora (flower) | - | - | 14.39 ± 1.43 μg/mL | - | - | [38] |
| O. grandiflora (leaf) | - | - | 6.69 ± 1.39 μg/mL | - | - | |
| Roupala paulensis (aerial parts) | 24.27 ± 0.76 g GAE/100 g | - | 37.50 ± 0.46 μg/mL | - | - | [106] |
| Adenanthos sericeus (stem) | - | - | 57.3–82.8 μg/mL | - | - | [107] |
| H. sericea (fruit) | 186.3 mg GAE/g | 3.4 mM Fe2+/g | 11.6 µg/mL | - | - | [108] |
| O. grandiflora (leaf) | 13.97 ± 0.31 GAE mg/100 g | - | 292.37 ± 9.37 µg/mL | - | - | [95] |
| F. saligna (leaf) | - | - | 1.17 ± 0.04 µg/mL | - | - | [109] |
| F. saligna (stem and bark) | - | - | 13 ± 1 µg/mL | - | - | [110] |
| O. grandiflora (flower) | - | - | 955.23 ± 0.25 µg/mL | - | - | [96] |
| Embothrium coccineum (leaf) | - | 0.40–0.53 mM Fe2+/g | 5.27–21.78 mg/mL | 270.61–405.21 μM TE/g | - | [111] |
| H. sericea (stem) | 267.6 ± 5.9 mg GAE/g | - | 9.5 ± 0.1 mg/L | - | - | [112] |
| H. sericea (leaf) | 217.0 ± 2.7 mg GAE/g | - | 13.4 ± 0.4 mg/L | - | - | |
| H. sericea (fruit) | 110.1 ± 2.7 mg GAE/g | - | 28.3 ± 1.8 mg/L | - | - |
6.2. Antimicrobial Activity
| Species | Bacterium Type | MIC | References |
|---|---|---|---|
| H. salicifolia (leaf) | Staphylococcus aureus, S. epidermidis, Enterococcus faecalis Mycobacterium smegmatis, Candida albicans | 15–250 μg/mL | [140] |
| H. salicifolia (bark) | 7.5–250 μg/mL | ||
| H. salicifolia (fruit) | 15–250 μg/mL | ||
| H. sericeae (leaf) | 62–250 μg/mL | ||
| Banksia genus (leaf) | Phytophthora cinnamom | 1–6 mg/mL | [142] |
| Roupala brasiliensis (stem) | C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, Cryptococcus neoformans, Escherichia coli, E. faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, S. aureus | 15.6–>1000 μg/mL | [138] |
| D. thouarsii (leaf) | Bacillus cereus, B. megaterium, S. aureus, E. faecalis, Vibrio harveyi, V. fisheri, Salmonella enterica, S. antarctica, E. coli, K. pneumoniae | 12.5–>100 mg/mL | [135] |
| Hakea sericea (fruit) | S. aureus, Methicillin-resistant S. aureus | 0.31 mg/mL | [131] |
| M. integriflora (flower) | Aeromonas hydrophilia, Citrobacter freundi, E. coli, Proteus mirabilis, Pseudomonas flurosecens, Serratia marcenscens, C. albicans, Saccharomyces cerevisiae | 2.9–19.9 µg/mL | [130] |
| M. integriflora (leaf) | A. hydrophilia, C. freundi, E. coli, P. mirabilis, S. marcenscens, C. albicans, S. cerevisiae, B. cereus. | 2.4–22.1 µg/mL | |
| M. integrifolia (nut) | P. mirabilis | 15 µg/mL | [141] |
| M. integrifolia (leaf) | 2790 µg/mL | ||
| E. coccineum (leaf) | E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, S. aureus, Streptococcus pyogenes | 31.125–500 μg/mL | [136] |
| E. coccineum (bark, leaf) | P. aeruginosa, E. coli | No inhibition | [139] |
| G. avellana (aerial parts) | 64–>512 μg/mL | ||
| Banksia integrifolia (bark) | P. aeruginosa, E. coli, B. cereus, S. aureus, Streptococcus pneumoniae, C. albicans | 78–1250 µg/mL | [143] |
| Bleasdalia bleasdalei (bark) | 78–624 µg/mL | ||
| Buckinghamia celsissima (bark) | 312–624 µg/mL | ||
| Cardwellia sublimis (bark) | <19.5–>2500 µg/mL | ||
| Darlingia darlingiana (bark) | 39–312 µg/mL | ||
| D. thouarsii (bark) | S. pyogenes, S. aureus, Clostridium perfringens, Listeria monocytogenes, P. mirabilis | 0.197–0.31 mg/mL | [144] |
| Knightia excelsa (honey) | E. coli | 22.0 ± 4.1 mg/mL | [145] |
| Lomatia hirsute (leaf) | C. albicans | 8 μg/mL | [146] |
| O. grandiflora (aerial parts) | S. aureus | 2 mg/mL | [147] |
| Roupala sp. (stem) | S. aureus, E. faecalis | 60–100 μg/mL | [148] |
| B. celsissima (leaf) | A. hydrophilia, B. cereus, B. subtilis, Citrobacter freundii, E. coli, C. albicans, S. cerevisiae | 8.3–13.6 mm inhibition zone at 0.02 mg/mL | [149] |
| Protea rotundifolia (herb) | S. aureus, Micrococcus luteus | 20.5–30.0 μM | [150] |
| Toronia toru (leaf and stem) | B. subtilis, E. coli, P. aeruginosa, T. mentagrophytes | Inhibition zone of 4-hydroxyphenyl 6-O-[(3R)-3,4-dihydroxy-2-methylenebutanoyl]-β -d-glucopyranoside: 3–5 mm | [151] |
| P. linearis (fruit) | B. subtilis, P. cinnamomic, E. coli | 4-hydroxyphenyl 6-O-[(3R)-3,4-dihydroxy-2-methylenebutanoyl]-β-d-glucopyranoside: 6.25–12.5 μg/disk | [25] |
| M. integrifolia (kernel) | Alternariu heliarzthi, Botrytis cinerea, Ceratocystis purodoxa, Colletotrichum falcutum, Fusurium oxysporum, Leptosphaeria maculans, Macrophomina phaseolinu, Phytophthoru cryptogeu, Pyrhium grunzinicolu, Sclerotinia sclerotiorum, Sclerotinia sclerotiorum, Sclerotium rolfsii, Verticilium dahlia, Clavibacter michigunensis, P. yeudomonus rubrilineans, Aspergillus fumigatus, Candidu nlhicuns, Microsyorum gypseum, E. coli, Saccharonzyces cerevisiae, Colletotrichum gloeosporioides. | MiAMPl peptide: 2–>100 μg/mL | [152] |
| MiAMP2c peptide: 5–>50 μg/mL | [153] | ||
| G. pteridifolia (stem) | B. anthraci, S. simulans, Enterococcus faecali, Enterococcus faecium, L. monocytogenes, Shigella dysenteriae, S. epidermidis, S. aureus, S. pneumoniae. | <0.0325–4.0 µg/mL in Kakadumycin A <0.0325–8.0 µg/mL in Echinomycin 0.125–4.0 µg/mL in Vancomycin | [154] |
| D. thouarsii (leaf) | P. aeruginosa; V. harveyi; V. fischeri; Salmonella enterica; S. antarctica; E. coli; K. pneumoniae; B. cereus; B. megaterium; E. faecalis; S. aureus. | 7–19 mm inhibition zone at 1 mg/disc | [155] |
| F. saligna | Propionibacterium acnes | 500 µg/mL | [109] |
| G. juncifolia (leaf) | Alcaligenes faecalis, Pseudomonas fluorescens, Yersinia entercolitica, B. cereus, B. subtilis, S. aureus, S. epiedermidis, Artemia nauplii | 62–1387 µg/mL | [156] |
| G. juncifolia (flower) | A. hydrophilia, P. fluorescens, Y. entercolitica, B. cereus, B. subtilis, S. aureus, S. epiedermidis, A. nauplii | 226–1055 µg/mL | |
| G. robusta (leaf) | A. hydrophilia, A. faecalis, P. fluorescens, Y. entercolitica, B. cereus, B. subtilis, S. aureus, S. epiedermidis, A. nauplii, S. Salford, K. pneumoniae | 83–1788 µg/mL | |
| G. robusta (flower) | B. cereus, A. nauplii | 880–2360 µg/mL | |
| G. banksia (inflorescence) | E. coli | 5.0 ± 0.1% inhibition at 250 µg/mL | [157] |
| Hakea spp. (leaf) | L. monocytogenes, M. luteus, S. aureus, E. coli, K. pneumoniae, P. aeruginosa | Neutral to very inhibitory | [129] |
| G. avellana (aerial parts) | MRSA, Methicillin-Sensitive S. aureus | >512 µg/mL | [158] |
| E. coccineum (cortex and folium) | >512 µg/mL | ||
| F. saligna (leaf) | M. tuberculosis | >1000 µg/mL | [159] |
| H. sericea (stem) | S. aureus, B. cereus, L. monocytogenes, E. coli, P. aeruginosa, K. pneumoniae, methicillin-resistant S. aureus, C. albicans, C. tropicalis | 0.315–2.5 mg/mL | [160] |
| H. sericea (leaf) | 0.315–2.5 mg/mL | ||
| H. sericea (fruit) | 0.04–2.5 mg/mL | ||
| B. menziesii (floral) | S. aureus, E. faecalis, E. coli, P. aeruginosa | 26.8% w/v | [104] |
| B. sessilis (floral) | 23.4% w/v | ||
| A. sericeus (stem) | B. subtilis, S. aureus, E. coli, Salmonella sp. | 10–16 mm inhibition zone at 100 mg/mL | [107] |
| Alloxylon flammeum (bark) | B. cereus, S. aureus, P. aeruginosa, E. coli, C. albicans, A. niger | 78–1250 µg/mL | [137] |
| Athertonia diversifolia (bark) | 312–1250 µg/mL | ||
| Austromuelleria trinervia (bark) | 156–1250 µg/mL | ||
| Carnrvonia araliifolia (bark) | <19.5–1250 µg/mL | ||
| Darlingia ferruginea (bark) | 156–1250 µg/mL | ||
| G. baileyanna (bark) | 78–1250 µg/mL | ||
| G. hilliang (bark) | <19.5–625 µg/mL | ||
| Helicia australasica (bark) | 312–1250 µg/mL | ||
| Lomatia fraxinifolia (bark) | 39–625 µg/mL | ||
| M. grandis (bark) | 156–625 µg/mL | ||
| Opisthiolepis heterophylla (bark) | 78–1250 µg/mL | ||
| Placospermum coriaceum (bark) | 156–1250 µg/mL | ||
| Stenocarpus sinuatus (bark) | 78–1250 µg/mL | ||
| Triunia erythrocarpa (bark) | 39–1250 µg/mL | ||
| E. coccineum (leaf and bark) | E. coli, P. aeruginosa | No activity | [139] |
| G. avellana (leaf, stem, and fruit) | >512 μg/mL | ||
| L. hirsuta (leaf and stem) | No activity | ||
| Protea caffra | E. coli, E. faecalis, K. pneumoniae, S. aureus, Penicillin-resistant S. aureus. | 0.31–>2.5 mg/mL | [161] |
6.3. Cytotoxicity
| Species: Compounds/Extract | Cell Line | IC50 | References | |
|---|---|---|---|---|
| B. bleasdalei (bark): (24E)-3β-hydroxy-7,24-euphadien-26-oic acid | P388 | About 80% of viable cells at 25 µM | [173] | |
| L. hirsute (leaf): 2-methoxyjuglone | HepG2 | 3.8 µg/mL | [174] | |
| F. saligna (leaf) | U937 | 202.4 µg/mL | [159] | |
| Kermadecia elliptica (bark): Kermadecin A–D | L1210 | 4.1–18.5 µM | [175] | |
| KB | 3.6–>10 µM | |||
| T. toru (leaf): 4-hydroxyphenyl 6-O-(4-hydroxy-2-methylenebutanoyl)-β-d-glucopyranoside, 4-hydroxyphenyl 6-O-[(3R)-3,4-dihydroxy-2-methylenebutanoyl]-β-d-glucopyranoside, arbutin | P388 | 50–100 µg/mL | [151] | |
| BSC | 3–>25 µg/mL | |||
| G. robusta (leaf): Graviquinone, cis-3-hydroxy-5-pentadecylcyclohexanone, methyl 5-ethoxy-2-hydroxycinnamate, methyl 2,5-dihydroxycinnamate | MCF-7 | 15.0–>50 µM | [180] | |
| NCI-H460 | 10.8–>50 µM | |||
| SF-268 | 5.9–>50 µM | |||
| B. integrifolia (bark) | HepG2 | 20.66 µg/mL | [143] | |
| B. bleasdalei (bark) | HepG2 | 46.20 µg/mL | ||
| MDA-MB-231 | 61.23 µg/mL | |||
| B. celsissima (bark) | HepG2 | 4.43 µg/mL | ||
| Cardwellia sublimis (bark) | HepG2 | 94.62 µg/mL | ||
| MDA-MB-231 | 100 µg/mL | |||
| Human 5637 | 32.57 µg/mL | |||
| D. darlingiana (bark) | HepG2 | 42.20 µg/mL | ||
| 5637 | 12.40 µg/mL | |||
| H. lobata (leaf): 6′-((E)2-methoxy-5-hydroxycinnamoyl) arbutin, 2′-((E)2, 5-dihydroxycinnamoyl) arbutin, 6′-[(E)-2″-hydroxymethyl-2″-butenylacyl] arbutin, 6′-[(E)-4″-hydroxycinnamoyl] arbutin, 6′-[(E)-2″, 5″-dihydroxycinnamoyl] arbutin, grevillic acid, hydroquinone. | MGC-803 | 11.3 ± 2.1–>50 µg/mL | [176] | |
| HEEC | 19.4 ± 1.9–>50 µg/mL | |||
| K. elliptica (bark): kermadecin A, 17-methoxykermadecin A, 22-methoxykermadecin A, 17,22-methoxykermadecin A, 17,19,22-trimethoxykermadecin A, (±)-cis-27,28-dihydroxy-17,19,22-trimethoxy-27,28-dihydrokermadecin A, (±)-28-hydroxy-17,19,22-trimethoxy-27-oxo-27,28-dihydrokermadecin A, (±)-cis-27,28-diacetoxy-17,19,22-trimethoxy-27,28-dihydrokermadecin A, 27,28-dihydrokermadecin A, 17,19,22-trimethoxy-27,28-dihydrokermadecin A | U937 | 3.86–>100 µM | [181] | |
| HL60 | 2.29–100 µM | |||
| KB | 2.78–100 µM | |||
| D. thouarsii (leaf): Dilobenol A–G | FcB1 | 15.8 ± 1.4–34.3 ± 0.6 µM | [155] | |
| L-6 | 58.8 ± 0.4–>137 µM | |||
| G. robusta (leaf): Gravicycle, Dehydrogravicycle, Bisgravillol, Dehydrobisgravillol, Dehydrograviphane, Methyldehydrograviphane, Graviphane, Methylgraviphane, Robustol, dehydrorobustol A, bis-norstriatol, 5-[14′-(3″,5″-dihydroxyphenyl)-cis-tetradec-6′-en-1-yl] resorcinol, cis-5-n-pentadecylresorcinol, cis-5-n-pentadec-8′-enylresorcinol | MCF-7 | 28.6 ± 3.2–37.1 ± 1.9 µM | [182] | |
| NCI-H460 | 22.8 ± 1.3–35.4 ± 1.7 µM | |||
| SF-268 | 27.7 ± 1.5–39.2 ± 0.7 µM | |||
| O. grandiflora (leaf) | HL-60 | 3.12–6.25 µg/mL | [38] | |
| O. grandiflora (flower) | 50–100 µg/mL | |||
| Protea madiensis (root and bark) | LOCE-MM001 | 10.0–>500 µg/mL | [177] | |
| LOCE-MM028 | 10.0–>500 µg/mL | |||
| R. brasiliensis (stem) | BS | 197.7–>1000 µg/mL | [138] | |
| G. robusta (leaf) | BS | 0.45 ± 0.04–191.14 ± 0.19 µg/mL | [183] | |
| K. excelsa (inner stem) | P388 | <1 µg/mL | [187] | |
| H. erratica (seed) | BS | >1000 µg/mL | [184] | |
| G. robusta (aerial part) | WI-38 | 249.5 ± 10.7 µg/mL | [178] | |
| MCF-7 | 89.5 ± 6.3 µg/mL | |||
| HepG2 | 199.1 ± 25.7 µg/mL | |||
| G. whiteana: NP-011694, NP-013296, NP-013330, NP-013378, NP-014428 | L6 | 15.5 ± 1.8–54.2 ± 0.5 µM | [196] | |
| H. salicifolia (leaf) | RAW 264.7 | >900 µg/mL | [185] | |
| Telopea speciossissima (leaf) | RAW 264.7 | >900 µg/mL | ||
| C. teretifolium/C. brownie (root): 3-geranyllawsone | U373 | 48 μM | [179] | |
| Hs683 | 12 μM | |||
| A549 | 11 μM | |||
| PC-3 | 28 μM | |||
| SKMEL-28 | 12 μM | |||
| LoVo | 7 μM | |||
| H. terminalis (trunk) | HepG2 | 99.6 ± 5.0% inhibition (b) | [98] | |
| M. integrifolia (nut): MiAMPl peptide | Hela | >1 mg/mL | [152] | |
| Bark | A. flammeum | Hs578T | 100% (a) | [137] |
| A. diversifolia | SK-MEL-28 | 38.56% (a) | ||
| A. trinervia | SK-MEL-28 | 58.58% (a) | ||
| C. araliifolia | MDA-MB-231, 5637 | 100% (b) | ||
| D. ferruginea | SK-MEL-28 | 19.86% (a) | ||
| G. baileyanna | 5637 | 40.20% (b) | ||
| G. hilliang | Hs578T | 99.41% (a) | ||
| H. australasica | HepG2 | 55.32% (a) | ||
| L. fraxinifolia | Hs578T | 99.47% (a) | ||
| M. grandis | MDA-MB-231 | 44.50% (b) | ||
| O. heterophylla | MDA-MB-231 | 45.03% (b) | ||
| P. coriaceum | HepG2 | 35.61% (a) | ||
| S. sinuatus | 5637 | 99.94% (b) | ||
| T. erythrocarpa | HepG2 | 90.11% (a) | ||
6.4. Anti-Inflammatory Activity
| Species | Study Mode | IC50 | References |
|---|---|---|---|
| O. grandiflora (leaf) | ROS | 4.1 ± 0.07 µg/mL | [38] |
| O. grandiflora (flower) | 5.87 ± 1.48 µg/mL | ||
| F. speciosa (leaf) | Animal | 55.50 ± 0.78% inhibition at 100 mg/kg | [102] |
| F. saligna (bark) | NO | 21.0 ± 0.7 µg/mL | [110] |
| H. terminalis (trunk): Bisresorcinol | NO | 71.15 ± 6.66 mg/mL | [98] |
| P. simplex (bark) | COX-1 | 86.1–94.2% inhibition at 250 μg/mL | [202] |
| COX-2 | 16.7–41.0% inhibition at 250 μg/mL | ||
| P. simplex (leaf) | COX-1 | 57.8–100.1% inhibition at 250 μg/mL | |
| COX-2 | 20.9–72.4% inhibition at 250 μg/mL | ||
| L. hirsuta (leaf) | Animal | 17.1 ± 0.8% inhibition at 4.0 mg/kg | [58] |
| G. robusta (leaf) | Animal | 6.2 mm thickness at 400 mg/kg | [207] |
| G. robusta (bark) | 6.8 mm thickness at 400 mg/kg | ||
| L. hieronymi (stem): oleanolic acid, epi-oleanolic acid, epi-Maslinic acid, p-Hydroxyacetophenone, p-Hydroxyacetophenone-β-glucoside | Animal | 24–30% inhibition at 80 mg/kg | [203] |
| P. falcata (leaf) | XO | 2.24 ± 1.72% inhibition at 100 µg/mL | [208] |
| K. excelsa (honey) | 15-LOX | >2000 µg/mL | [209] |
| H. salicifolia (leaf) | NO | 195.9 ± 30.7 µg/mL | [185] |
| TNF-α | 697.7 ± 185.3 µg/mL | ||
| T. speciossissima (leaf) | NO | 116.5 ± 20.1 µg/mL | |
| TNF-α | 555.1 ± 87.5 µg/mL | ||
| H. terminalis (root) | NO | 11.98 ± 0.71 µg/mL | [210] |
| G. avellana (nut) | COX-1 | 50.1–79.9% inhibition at 100 µg/mL | [99] |
| COX-2 | 15.7–33.8% inhibition at 100 µg/mL | ||
| LOX | 9.1–26.0% inhibition at 100 µg/mL |
6.5. Antiviral Activity
6.6. Other Bioactivities
7. Bioactive Properties of the Persoonia Genus
8. Opportunities and Challenges
9. Conclusions and Recommendations for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, F.; Douglas, J. No bush foods without people: The essential human dimension to the sustainability of trade in native plant products from desert Australia. Rangel. J. 2011, 33, 395–416. [Google Scholar] [CrossRef]
- Pearson, G.J. Managing the landscapes of the Australian Northern Territory for sustainability: Visions, issues and strategies for successful planning. Futur. J. Policy Plan. Futures Stud. 2010, 42, 711–722. [Google Scholar] [CrossRef]
- Ferguson, M.; Brown, C.; Georga, C.; Miles, E.; Wilson, A.; Brimblecombe, J. Traditional food availability and consumption in remote Aboriginal communities in the Northern Territory, Australia. Aust. New Zealand J. Public Health 2017, 41, 294–298. [Google Scholar] [CrossRef]
- AIHW. Poor Diet. Available online: https://www.aihw.gov.au/reports/food-nutrition/poor-diet/contents/dietary-guidelines (accessed on 20 June 2023).
- Prakash, V.; Martín-Belloso, O.; Keener, L.; Astley, S.; Braun, S.; McMahon, H.; Lelieveld, H. Regulating Safety of Traditional and Ethnic Foods; Academic Press: Cambridge, MA, USA, 2016; pp. 1–6. [Google Scholar]
- Richmond, R.; Bowyer, M.; Vuong, Q. Australian native fruits: Potential uses as functional food ingredients. J. Funct. Foods 2019, 62, 103547. [Google Scholar] [CrossRef]
- Kubitzki, K. Flowering Plants: Eudicots; Springer: Berlin, Germany, 2014; pp. 1–332. [Google Scholar]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Carthew, S.M. Breeding and Mating Systems of Australian Proteaceae. Aust. J. Bot. 1998, 46, 421–437. [Google Scholar] [CrossRef]
- Jussieu, A.L.d. Genera Plantarum Secundum Ordines Naturales Disposita; Theophilum Barrois: Paris, France, 1789. [Google Scholar]
- Weston, P.H. Flora of Australia Volume 16, Elaeagnaceae, Proteaceae 1; CSIRO Australia: Melbourne, Australia, 1995. [Google Scholar]
- Westonand, P.; Barker, N. A new suprageneric classification of the Proteaceae, with an annotated checklist of genera. Telopea 2006, 11, 314–344. [Google Scholar] [CrossRef]
- Offord, A.; Rollason, A.; Frith, C.A. Tissue culture of Persoonia species for horticulture and restoration. Acta Hortic. 2015, 1101, 75–80. [Google Scholar] [CrossRef]
- Baines, J.A. Australian Plant Genera: An Etymological Dictionary of Australian Plant Gener; Society for Growing Australian Plants: Sydney, Australia, 1981. [Google Scholar]
- Weston, P.H. Proteaceae Subfamily Persoonioideae. Aust. Plants 2003, 22, 62–78. [Google Scholar]
- Emery, N.J.; Offord, C.A. Managing Persoonia (Proteaceae) species in the landscape through a better understanding of their seed biology and ecology. Cunninghamia 2018, 18, 89–107. [Google Scholar]
- Bellard, C.; Leclerc, C.; Hoffmann, B.D.; Courchamp, F. Vulnerability to climate change and sea-level rise of the 35th biodiversity hotspot, the Forests of East Australia. Environ. Conserv. 2015, 43, 79–89. [Google Scholar] [CrossRef]
- WAH. Persoonia Sm. Available online: https://florabase.dpaw.wa.gov.au/browse/profile/21322 (accessed on 30 January 2023).
- Rymer, P.D. Plant Rarity: Species Distributional Patterns, Population Genetics, Pollination Biology, and Seed Dispersal in Persoonia (Proteaceae). Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2006. [Google Scholar]
- DCCEEW. Species Profile and Threats Database: Persoonia. Available online: https://www.environment.gov.au/cgi-bin/sprat/public/spratlookupspecies.pl?proc=search&searchtype=wildcard&query=Persoonia&sortorder=epbc_status (accessed on 30 January 2023).
- Andres, S.E.; Powell, J.R.; Emery, N.J.; Rymer, P.D.; Gallagher, R.V. Does threatened species listing status predict climate change risk? A case study with Australian Persoonia (Proteaceae) species. Glob. Ecol. Conserv. 2021, 31, e01862. [Google Scholar] [CrossRef]
- Corbyn, L. Persoonia nutans R. Br (Nodding Geebung) Recovery Plan. 2006. Available online: https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Recovery-plans/nodding-geebung-persoonia-nutans-r-br-recovery-plan.pdf (accessed on 9 January 2023).
- ANBG. Aboriginal Plant Use. Available online: https://www.anbg.gov.au/gardens/visiting/exploring/aboriginal-trail/index.html (accessed on 20 June 2023).
- Atkinson, N. Antibotics in Australian plants and fungi. Med. J. Aust. 1949, 1, 605–610. [Google Scholar] [CrossRef]
- MacLeod, J.K.; Rasmussen, H.B.; Willis, A.C. A new glycoside antimicrobial agent from Persoonia linearia x pinifolia. J. Nat. Prod. 1997, 60, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Deans, B.J.; Kilah, N.L.; Jordan, G.J.; Bissember, A.C.; Smith, J.A. Arbutin Derivatives Isolated from Ancient Proteaceae: Potential Phytochemical Markers Present in Bellendena, Cenarrhenes, and Persoonia Genera. J. Nat. Prod. 2018, 81, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.B. The naturalist in medicine with particular reference to Australia. Med. J. Aust. 1950, 1, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.J. The Use of Plant Medicines and Poisons by Australian Aborigines. Mankind 1969, 7, 137–146. [Google Scholar] [CrossRef]
- Jones, P.J. Clinical nutrition: 7. Functional foods—More than just nutrition. CMAJ 2002, 166, 1555–1563. [Google Scholar]
- Weston, P.; Crisp, M. Trans-Pacific biogeographic patterns in the Proteaceae. In The Origin and Evolution of Pacific Island Biotas, New Guinea to Eastern Polynesia: Patterns and Processes; SPB Academic: Amsterdam, the Netherlands, 1996; pp. 215–232. [Google Scholar]
- Johnson, L.A.S.; Briggs, B.G. On the Proteaceae—The evolution and classification of a southern family. Bot. J. Linn. Soc. 2008, 70, 83–182. [Google Scholar] [CrossRef]
- Holmes, G.D.; Weston, P.H.; Murphy, D.J.; Connelly, C.; Cantrill, D.J. The genealogy of geebungs: Phylogenetic analysis of Persoonia (Proteaceae) and related genera in subfamily Persoonioideae. Aust. Syst. Bot. 2018, 31, 166–189. [Google Scholar] [CrossRef]
- Bernhardt, P.; Weston, P. The pollination ecology of Persoonia (Proteaceae) in eastern Australia. Telopea 1996, 6, 775–804. [Google Scholar] [CrossRef]
- AVH. Persoonia. Available online: https://avh.ala.org.au/occurrences/search?taxa=Persoonia (accessed on 5 May 2023).
- USDA. Tree Nuts Annual. 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Tree%20Nuts%20Annual_New%20Delhi_India_09-15-2020 (accessed on 20 February 2023).
- Insanu, M.; Hartati, R.; Bajri, F.; Fidrianny, I. Macadamia Genus: An Updated Review of Phytochemical Compounds and Pharmacological Activities. Biointerface Res. Appl. Chem. 2021, 11, 14898–14905. [Google Scholar]
- Carrillo, W.; Carpio, C.; Morales, D.; Vilcacundo, E.; Alvarez, M. Fatty acids composition in macadamia seed oil (Macadamia integrifolia) from Ecuador. Asian J. Pharm. Clin. Res. 2017, 10, 303–306. [Google Scholar] [CrossRef]
- Vinueza, D.; Yanza, K.; Tacchini, M.; Grandini, A.; Sacchetti, G.; Chiurato, M.A.; Guerrini, A. Flavonoids in Ecuadorian Oreocallis grandiflora (Lam.) R. Br.: Perspectives of Use of This Species as a Food Supplement. Evid.-Based Complement. Altern. Med. 2018, 2018, 1353129. [Google Scholar] [CrossRef] [PubMed]
- Rozas-Muñoz, E.; Gamé, D.; Mir-Bonafé, J.-F.; Piquero-Casals, J. Plant Contact Dermatitis in 2021. Curr. Treat. Options Allergy 2022, 9, 76–90. [Google Scholar] [CrossRef]
- Chia, K.A.; Koch, J.M.; Sadler, R.; Turner, S.R. Developmental phenology of Persoonia longifolia (Proteaceae) and the impact of fire on these events. Aust. J. Bot. 2015, 63, 415–425. [Google Scholar] [CrossRef]
- Criley, R.A. Proteaceae: Beyond the big three. Acta Hortic. 2000, 545, 79–85. [Google Scholar] [CrossRef]
- Wightman, G.M.; Jackson, D.M.; Williams, L.L.V. Alawa Ethnobotany: Aboriginal Plant Use from Minyerri, Northern Australia; Conservation Commission of the Northern Territory: Palmerston City, Australia, 1991.
- Florin, S.A.; Fairbairn, A.S.; Nango, M.; Djandjomerr, D.; Marwick, B.; Fullagar, R.; Smith, M.; Wallis, L.A.; Clarkson, C. The first Australian plant foods at Madjedbebe, 65,000–53,000 years ago. Nat. Commun. 2020, 11, 924. [Google Scholar] [CrossRef]
- Bauer, L.M.; Johnston, M. Propagation of Persoonia Virgarta for the Development of a New Floricultural Export Crop; The Australian Flora Foundation: Willoughby, Australia, 1999. [Google Scholar]
- Stewart, K.; Percival, B. Bush Foods of New South Wales: A Botanic Record and an Aboriginal Oral History; Royal Botanic Gardens: Sydney, Australia, 1997. [Google Scholar]
- Renwick, C. Geebungs and Snake Whistles: Koori People and Plants of Wreck Bay; Aboriginal Studies Press: Canberra, Australia, 2000. [Google Scholar]
- Robinson, L. Field Guide to the Native Plants of Sydney; Simon & Schuster Australia: Cammeray, Australia, 2003. [Google Scholar]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Isaacs, J. Bush Food: Aboriginal Food; Weldons: McMahons Point, Australia, 1987. [Google Scholar]
- ACNT. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse Publications: Melbourne, Australia, 1988. [Google Scholar]
- Turbet, P. The Aborigines of the Sydney District before 1788; Langaroo Press: Sydney, Australia, 1989. [Google Scholar]
- Nash, D. Aboriginal Plant Use in South-Eastern Australia; Australian National Botanic Gardens: Canberra, Australia, 2004.
- BGPA. Grevillea Eriostachya. Available online: https://www.bgpa.wa.gov.au/about-us/information/our-plants/plants-in-focus/grevillea-eriostachya (accessed on 30 January 2023).
- Hansen, V.; Horsfall, J. Noongar Bush Medicine: Medicinal Plants of the South-West of Western Australia; UWA Publishing: Perth, Australia, 2016. [Google Scholar]
- Wrigley, J.W.; Fagg, M. Banksias, Waratahs & Grevilleas, and All Other Plants in the Australian Proteaceae Family; Collins Australia: Sydney, Australia, 1989. [Google Scholar]
- Cock, I.E. Medicinal and aromatic plants—Australia. In Ethnopharmacology, Encyclopedia of Life Support Systems; EOLSS Publishers: Abu Dhabi, United Arab Emirates, 2011. [Google Scholar]
- Yalo, M.; Makhaba, M.; Hussein, A.A.; Sharma, R.; Koki, M.; Nako, N.; Mabusela, W.T. Characterization of Four New Compounds from Protea cynaroides Leaves and Their Tyrosinase Inhibitory Potential. Plants 2022, 11, 1751. [Google Scholar] [CrossRef]
- Erazo, S.; García, R.; Backhouse, N.; Lemus, I.I.; Delporte, C.; Andrade, C. Phytochemical and biological study of Radal Lomatia hirsuta (Proteaceae). J. Ethnopharmacol. 1997, 57, 81–83. [Google Scholar] [CrossRef]
- Setzer, M.C. Green Gold from Down Under: Bioprospecting for Phytopharmaceuticals from Paluma, North Queensland, Australia. Master’s Thesis, The University of Alabama in Huntsville, Ann Arbor, MI, USA, 2000. [Google Scholar]
- Twilley, D.; Lall, N. 16—African Plants with Dermatological and Ocular Relevance. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, the Netherlands, 2014; pp. 493–512. [Google Scholar]
- Chinsembu, K.C.; Syakalima, M.; Semenya, S.S. Ethnomedicinal plants used by traditional healers in the management of HIV/AIDS opportunistic diseases in Lusaka, Zambia. South Afr. J. Bot. 2019, 122, 369–384. [Google Scholar] [CrossRef]
- Tlau, L.; Lalawmpuii, L. Commonly used medicinal plants in N. Mualcheng, Mizoram, India. Sci. Vis. 2020, 20, 156–161. [Google Scholar] [CrossRef]
- Ray, D.S.; Saini, M.K. Impending threats to the plants with medicinal value in the Eastern Himalayas Region: An analysis on the alternatives to its non-availability. Phytomed. Plus 2022, 2, 100151. [Google Scholar] [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. J. Ethnopharmacol. 2001, 77, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Liang, Q.; Zhang, Y.J.; Zhao, P. Naturally occurring arbutin derivatives and their bioactivities. Chem. Biodivers. 2015, 12, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T. Protea Atlas Project—A spectacular year of atlassing. Veld Flora 1993, 79, 26–27. [Google Scholar]
- Chia, K. Ecology, Seed Dormancy and Germination Biology of Persoonia longifolia for Use in Land Restoration and Horticulture. Ph.D. Thesis, The University of Western Australia, Crawley, Australia, 2016. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism, 1st ed.; Kluwer Academic Publishers: Boston, MA, USA, 1998. [Google Scholar]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical Changes of Bioactive Phytochemicals during Thermal Processing. In Reference Module in Food Science; Elsevier: Amsterdam, the Netherlands, 2016. [Google Scholar]
- Gadea, A.; Khazem, M.; Gaslonde, T. Current knowledge on chemistry of Proteaceae family, and biological activities of their bis-5-alkylresorcinol derivatives. Phytochem. Rev. 2022, 21, 1969–2005. [Google Scholar] [CrossRef]
- Yang, F. Chemotaxonomy Study of Plants from the Family Proteaceae Based on Its Natural Product Profile (Alkaloid). Ph.D. Thesis, Griffith University, Brisbane, Australia, 2017. [Google Scholar]
- Tang, C.; Xie, B.; Sun, Z. Antibacterial activity and mechanism of B-type oligomeric procyanidins from lotus seedpod on enterotoxigenic Escherichia coli. J. Funct. Foods 2017, 38, 454–463. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Kupeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; König, G.M.; Angerhofer, C.K.; Greenidge, P.; Linden, A.; Desqueyroux-Faúndez, R. Antimalarial Activity: The Search for Marine-Derived Natural Products with Selective Antimalarial Activity. J. Nat. Prod. 1996, 59, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Ooshiro, A.; Hiradate, S.; Kawano, S.; Takushi, T.; Fujii, Y.; Natsume, M.; Abe, H. Identification and activity of ethyl gallate as an antimicrobial compound produced by Geranium carolinianum. Weed Biol. Manag. 2009, 9, 169–172. [Google Scholar] [CrossRef]
- Tareq, S.M.; Islam, M.; Shahadat, S.; Guha, B.; Azad, M.; Ikram, M.; Royhan, M.; Paul, A.; Kabir, M. Anticancer potential of isolated phytochemicals from grevillea robustra against breast cancer: In slico molecular docking approach. World J. Pharm. Res. 2016, 5, 1358–1365. [Google Scholar]
- Ryu, B.; Park, E.-J.; Doan, T.-P.; Cho, H.-M.; An, J.-P.; Pham, T.-L.-G.; Pham, H.-T.-T.; Oh, W.-K. Heliciopsides A–E, Unusual Macrocyclic and Phenolic Glycosides from the Leaves of Heliciopsis terminalis and Their Stimulation of Glucose Uptake. Pharmaceuticals 2022, 15, 1315. [Google Scholar]
- Saechan, C.; Nguyen, U.H.; Wang, Z.; Sugimoto, S.; Yamano, Y.; Matsunami, K.; Otsuka, H.; Phan, G.M.; Pham, V.H.; Tipmanee, V.; et al. Potency of bisresorcinol from Heliciopsis terminalis on skin aging: In vitro bioactivities and molecular interactions. PeerJ 2021, 9, e11618. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Hawary, S.S.; Abubaker, M.; Abd El-Kader, E.M.; Mahrous, E.A. Phytochemical constituents and anti-tyrosinase activity of Macadamia integrifolia leaves extract. Nat. Prod. Res. 2022, 36, 1089–1094. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Masike, K.; de Villiers, A.; Hoffman, E.W.; Brand, D.J.; Causon, T.; Stander, M.A. Detailed Phenolic Characterization of Protea Pure and Hybrid Cultivars by Liquid Chromatography–Ion Mobility–High Resolution Mass Spectrometry (LC-IM-HR-MS). J. Agric. Food Chem. 2020, 68, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Makboul, M.A. Chemical investigation of Grevillea robusta A.Cunn flowers. Sphinx J. Pharm. Med. Sci. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Medina, J.C.; Suárez, A.I.; Cumbicus, N.; Morocho, V. Estudio fitoquímico de roupala montana aubl. de la provincia de Loja. Axioma 2018, 19, 5–11. [Google Scholar]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999, 70, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Roulle, P. Nutritional properties of commercially grown native Australian fruits: Lipophilic antioxidants and minerals. Food Res. Int. 2011, 44, 2339–2344. [Google Scholar] [CrossRef]
- Salvin, S.; Bourke, M.; Byrne, T. The New Crop Industry Handbook; Rural Industries Research & Development Corporation: Barton, Australia, 2004; Volume 4, pp. 417–426. [Google Scholar]
- Dissanayake, I.H.; Zak, V.; Kaur, K.; Jaye, K.; Ayati, Z.; Chang, D.; Li, C.G.; Bhuyan, D.J. Australian native fruits and vegetables: Chemical composition, nutritional profile, bioactivity and potential valorization by industries. In Critical Reviews In Food Science and Nutrition; Taylor & Francis: Abingdon, UK, 2022; pp. 1–34. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Vinueza, D.; Cajamarca, D.; Acosta, K.; Pilco, G. Oreocallis grandiflora photoprotective effect against ultraviolet B radiation-induced cell death. Asian J. Pharm. Clin. Res. 2018, 11, 276. [Google Scholar] [CrossRef]
- Vinueza, D.; Allauca, A.A.; Bonilla, G.A.; León, K.L.; López, S.P. Assessment of the diuretic and urinary electrolyte effects of hydroalcoholic extract of Oreocallis grandiflora (Lam.) R. Br. in Wistar albino rats. Pharmacologyonline 2018, 1, 117–127. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Giang, P.M.; Thao, D.T.; Nga, N.T.; Van Trung, B.; Anh, D.H.; Viet, P.H. Evaluation of the Antioxidant, Hepatoprotective, and Anti-Inflammatory Activities of Bisresorcinol Isolated from the Trunk of Heliciopsis Terminalis. Pharm. Chem. J. 2019, 53, 628–634. [Google Scholar] [CrossRef]
- Pino Ramos, L.L.; Jiménez-Aspee, F.; Theoduloz, C.; Burgos-Edwards, A.; Domínguez-Perles, R.; Oger, C.; Durand, T.; Gil-Izquierdo, Á.; Bustamante, L.; Mardones, C.; et al. Phenolic, oxylipin and fatty acid profiles of the Chilean hazelnut (Gevuina avellana): Antioxidant activity and inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Food Chem. 2019, 298, 125026. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Somwongin, S.; Sirilun, S.; Chantawannakul, P.; Anuchapreeda, S.; Chaiyana, W. Potential antioxidant, anti-aging enzymes, and anti-tyrosinase properties of Macadamia (Macadamia integrifolia) pericarp waste products. Asia-Pac. J. Sci. Technol. 2022, 27, 1–8. [Google Scholar] [CrossRef]
- Ayisi, F.; Mensah, C.N.; Borquaye, L.S. In Vivo Antiplasmodial Activity and Toxicological Analyses of the Ethanolic Leaf and Twig Extract of Faurea speciosa Welw. J. Parasitol. Res. 2021, 2021, 7347532. [Google Scholar] [CrossRef]
- León, F.; Alfayate, C.; Batista, C.V.; López, A.; Rico, M.; Brouard, I. Phenolic compounds, antioxidant activity and ultrastructural study from Protea hybrid ‘Susara’. Ind. Crops Prod. 2014, 55, 230–237. [Google Scholar] [CrossRef]
- Green, K.J.; Islam, M.K.; Lawag, I.; Locher, C.; Hammer, K.A. Honeys derived from plants of the coastal sandplains of Western Australia: Antibacterial and antioxidant activity, and other characteristics. J. Apic. Res. 2022, 62, 909–922. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Optimisation of Ultrasonic Conditions as an Advanced Extraction Technique for Recovery of Phenolic Compounds and Antioxidant Activity from Macadamia (Macadamia tetraphylla) Skin Waste. Technologies 2015, 3, 302–320. [Google Scholar] [CrossRef]
- Ramos, R.F.d.A. Constituintes Químicos e Atividade Antioxidante de Roupala Paulensis Sleumer (Proteaceae); Universidade Federal da Paraíba: João Pessoa, Brazil, 2015. [Google Scholar]
- Abba, J.; Ekwumemgbo, P.; Dallatu, Y.; Micheal, A.M. Phytochemical Screening, Antioxidant and Antimicrobial Activities of the Stem Extracts of Woolly Bush (Adenanthos sericeus). J. Appl. Sci. Environ. Manag. 2016, 22, 1849. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of Hakea sericea Fruits Regarding Chemical Composition and Extract Properties. Waste Biomass Valorization 2020, 11, 4859–4870. [Google Scholar] [CrossRef]
- Canha, M.N. Antimicrobial and Anti-Inflammatory Effect of Southern African Plants Against Propionibacterium acnes. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2014. [Google Scholar]
- Lawal, F.; Bapela, M.J.; Adebayo, S.A.; Nkadimeng, S.M.; Yusuf, A.A.; Malterud, K.E.; McGaw, L.J.; Tshikalange, T.E. Anti-inflammatory potential of South African medicinal plants used for the treatment of sexually transmitted infections. South Afr. J. Bot. 2019, 125, 62–71. [Google Scholar] [CrossRef]
- Leyton, M.; Mellado, M.; Jara, C.; Montenegro, I.; González, S.; Madrid, A. Free radical-scavenging activity of sequential leaf extracts of Embothrium coccineum. Open Life Sci. 2015, 10, 260–268. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A. Bioactive Compounds, RP-HPLC Analysis of Phenolics, and Antioxidant Activity of Some Portuguese Shrub Species Extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Srzednicki, G.; Konczak, I. Composition and inhibitory activities towards digestive enzymes of polyphenolic-rich fractions of Davidson’s plum and quandong. LWT Food Sci. Technol. 2014, 57, 366–375. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Williams, D.; Chaliha, M.; Konczak, I.; Smyth, H. Changes in Quality and Bioactivity of Native Food during Storage; Rural Industries Research and Development Corporation: Barton, Australia, 2015. [Google Scholar]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Zhang, J.; Seididamyeh, M.; Srivarathan, S.; Netzel, M.E.; Sivakumar, D.; Sultanbawa, Y. Hydrolysable tannins, physicochemical properties, and antioxidant property of wild-harvested Terminalia ferdinandiana (exell) fruit at different maturity stages. Front. Nutr. 2022, 9, 961679. [Google Scholar] [CrossRef]
- Cherikoff, V. Wild Foods. In An Overview of the Health Attributes of Wild Foods; Cherikoff, V., Konczak, I., Eds.; New Holland Publishers: Wahroonga, Australia, 2015; pp. 98–106. [Google Scholar]
- Lim, V.; Gorji, S.G.; Daygon, V.D.; Fitzgerald, M. Untargeted and Targeted Metabolomic Profiling of Australian Indigenous Fruits. Metabolites 2020, 10, 114. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P.; Roulfe, P.; Pavan, A. Health Benefits of Australian Native Foods—An Evaluation of Health-Enhancing Compounds; Rural Industries Research and Development Corporation: Wagga Wagga, Australia, 2009; pp. 1–41. [Google Scholar]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef]
- Mani, J.; Johnson, J.; Hosking, H.; Hoyos, B.E.; Walsh, K.B.; Neilsen, P.; Naiker, M. Bioassay Guided Fractionation Protocol for Determining Novel Active Compounds in Selected Australian Flora. Plants 2022, 11, 2886. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Zabaras, D.; Sze, D.M.Y. Potential Antioxidant, Antiinflammatory, and Proapoptotic Anticancer Activities of Kakadu Plum and Illawarra Plum Polyphenolic Fractions. Nutr. Cancer 2011, 63, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Netzel, M.E.; Tinggi, U.; Osborne, S.A.; Fletcher, M.T.; Sultanbawa, Y. Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Konczak, I.; Ramzan, I.; Sze, D.M.Y. Antioxidant and cytoprotective activities of native Australian fruit polyphenols. Food Res. Int. 2011, 44, 2034–2040. [Google Scholar] [CrossRef]
- NSW Food Authority. NSW Government Food Safety Strategy 2015–2021; NSW Food Authority: Newington, Australia, 2021.
- Zhang, J.; Phan, A.D.T.; Srivarathan, S.; Akter, S.; Sultanbawa, Y.; Cozzolino, D. Proximate composition, functional and antimicrobial properties of wild harvest Terminalia carpentariae fruit. J. Food Meas. Charact. 2022, 16, 582–589. [Google Scholar] [CrossRef]
- Courtney, R.; Sirdaarta, J.; Matthews, B.; Cock, I.E. Tannin components and inhibitory activity of Kakadu plum leaf extracts against microbial triggers of autoimmune inflammatory diseases. Pharmacogn. J. 2015, 7, 18–31. [Google Scholar] [CrossRef]
- Wigmore, S.; Naiker, M.; Bean, D. Antimicrobial Activity of Extracts from Native Plants of Temperate Australia. Pharmacogn. Commun. 2016, 6, 80–84. [Google Scholar] [CrossRef]
- Boyer, H.; Cock, I.E. Evaluation of the potential of Macadamia integriflora extracts as antibacterial food agents. Pharmacogn. Commun. 2013, 3, 53. [Google Scholar]
- Luís, Â.; Cruz, C.; Duarte, A.P.; Domingues, F. An Alkenylresorcinol Derivative from Hakea sericea Fruits and their Antimicrobial Activity. Nat. Prod. Commun. 2013, 8, 1934578X1300801031. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Toledo, M.d.G.T.; Rossa, L.S.; Mengarda, M.; Stofella, N.C.F.; Oliveira, L.J.; Gonçalves, A.G.; Murakami, F.S. Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC50%, and IC90% of antimicrobial compounds. J. Microbiol. Methods 2019, 162, 50–61. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Razafintsalama, V.; Sarter, S.; Mambu, L.; Randrianarivo, R.; Petit, T.; Rajaonarison, J.F.; Mertz, C.; Rakoto, D.; Jeannoda, V. Antimicrobial activities of Dilobeia thouarsii Roemer and Schulte, a traditional medicinal plant from Madagascar. South Afr. J. Bot. 2013, 87, 1–3. [Google Scholar] [CrossRef]
- Canales, N.; Montenegro, I.; Párraga, M.; Olguín, Y.; Godoy, P.; Werner, E.; Madrid, A. In Vitro Antimicrobial Activity of Embothrium coccineum Used as Traditional Medicine in Patagonia against Multiresistant Bacteria. Molecules 2016, 21, 1441. [Google Scholar] [CrossRef]
- Setzer, M.C.; Schmidt, J.M.; Irvine, A.K.; Jackes, B.; Setzer, W. Biological activity of rainforest plant extracts from Far North Queensland, Australia. Aust. J. Med. Herbal 2006, 18, 6–20. [Google Scholar]
- Violante, I.M.; Hamerski, L.; Garcez, W.S.; Batista, A.L.; Chang, M.R.; Pott, V.J.; Garcez, F.R. Antimicrobial activity of some medicinal plants from the cerrado of the centralwestern region of Brazil. Braz. J. Microbiol. 2012, 43, 1302–1308. [Google Scholar] [CrossRef]
- Mølgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef]
- Madureira, A.M.; Duarte, A.; Teixeira, G. Antimicrobial activity of selected extracts from Hakea salicifolia and H. sericeae (Proteaceae) against Staphylococcus aureus multiresistant strains. South Afr. J. Bot. 2012, 81, 40–43. [Google Scholar] [CrossRef]
- Cock, I.E.; Winnett, V.; Sirdaarta, J.; Matthews, B. The potential of selected Australian medicinal plants with anti-Proteus activity for the treatment and prevention of rheumatoid arthritis. Pharmacogn. Mag. 2015, 11, 190–208. [Google Scholar] [CrossRef]
- Qongqo, A.; Nchu, F.; Geerts, S. Relationship of alien species continues in a foreign land: The case of Phytophthora and Australian Banksia (Proteaceae) in South African Fynbos. Ecol. Evol. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Setzer, M.C.; Setzer, W.N.; Jackes, B.R.; Gentry, G.A.; Moriarity, D.M. The Medicinal Value of Tropical Rainforest Plants from Paluma, North Queensland, Australia. Pharm. Biol. 2001, 39, 67–78. [Google Scholar] [CrossRef]
- Razafintsalama, V.E.; Ralambonirina Rasoarivelo, S.T.; Randriamialinoro, F.; Ranarivelo, L.; Rakotonandrasana, S.R.; Petit, T.; Sarter, S. Antibacterial activities of fourteen medicinal plants from the endemic plant diversity of Madagascar. South Afr. J. Bot. 2017, 112, 303–306. [Google Scholar] [CrossRef]
- Brady, N.; Molan, P.; Bang, L. A survey of non-manuka New Zealand honeys for antibacterial and antifungal activities. J. Apic. Res. 2004, 43, 47–52. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Adsersen, A.; Berthelsen, L.; Christensen, S.B.; Guzmán, A.; Mølgaard, P. Ethnopharmacological evaluation of radal (leaves of Lomatia hirsuta) and isolation of 2-methoxyjuglone. BMC Complement. Med. Ther. 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef]
- Suffredini, I.; Paciencia, M.; Varella, A.; Younes, R. Antibacterial activity of Brazilian Amazon plant extracts. Braz. J. Infect. Dis. 2007, 10, 400–402. [Google Scholar] [CrossRef]
- Cock, I. Antibacterial and Antifungal Activity of Buckinghamia Celsissima Leaf Extracts. Internet J. Microbiol. 2008, 6, 6008–6018. [Google Scholar]
- Chang, J.; Inui, T. Novel Phenolic Glycoside Dimer and Trimer from the Whole Herb of Pyrola rotundifolia. Chem. Pharm. Bull. 2005, 53, 1051–1053. [Google Scholar] [CrossRef]
- Perry, N.B.; Brennan, N.J. Antimicrobial and cytotoxic phenolic glycoside esters from the New Zealand tree Toronia toru. J. Nat. Prod. 1997, 60, 623–626. [Google Scholar] [CrossRef]
- Marcus, J.P.; Goulter, K.C.; Green, J.L.; Harrison, S.J.; Manners, J.M. Purification, Characterisation and cDNA Cloning of an Antimicrobial Peptide from Macadamia integrifolia. Eur. J. Biochem. 1997, 244, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Castillo, U.; Harper, J.K.; Strobel, G.A.; Sears, J.; Alesi, K.; Ford, E.; Lin, J.; Hunter, M.; Maranta, M.; Ge, H.; et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol. Lett. 2003, 224, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Razafintsalama, V.; Girardot, M.; Randrianarivo, R.; Rakoto, D.; Sarter, S.; Petit, T.; Ralambonirina, S.; Deville, A.; Grellier, P.; Jeannoda, V.; et al. Dilobenol A–G, Diprenylated Dihydroflavonols from the Leaves of Dilobeia thouarsii. Eur. J. Org. Chem. 2013, 2013, 1929–1936. [Google Scholar] [CrossRef]
- Cock, I. Grevillea juncifolia Hook. and Grevillea robusta A. Cunn. Ex. R. Br. Methanolic Leaf and Flower Extracts Inhibit the Growth of Gram Positive and Gram Negative Bacteria. Pharmacogn. Commun. 2019, 9, 112–117. [Google Scholar] [CrossRef]
- Almeida, R.; Silva, O.; Candido, E.; Moreira, J.; Jojoa, D.; Gomes, D.; Freire, M.; Burgel, P.; Oliveira Júnior, N.; Arboleda Valencia, J.; et al. Screening and isolation of antibacterial proteinaceous compounds from flower tissues: Alternatives for treatment of healthcare-associated infections. Tang Humanit. Med. 2014, 4, 0026. [Google Scholar] [CrossRef]
- Holler, J.G.; Søndergaard, K.; Slotved, H.C.; Gúzman, A.; Mølgaard, P. Evaluation of the antibacterial activity of Chilean plants traditionally used for wound healing therapy against multidrug-resistant Staphylococcus aureus. Planta Medica 2012, 78, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Gasa, N. Antibiofilm Activity of South African Plant Extracts Against Mycobacterium spp. and Their Mechanism of Action Using Mycothiol Reductase. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2015. [Google Scholar]
- Luís, A.; Breitenfeld, L.; Ferreira, S.; Duarte, A.P.; Domingues, F. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharmacogn. Mag. 2014, 10, 6–13. [Google Scholar] [CrossRef]
- Vambe, M.; Aremu, A.O.; Chukwujekwu, J.C.; Gruz, J.; Luterová, A.; Finnie, J.F.; Van Staden, J. Antibacterial, Mutagenic Properties and Chemical Characterisation of Sugar Bush (Protea caffra Meisn.): A South African Native Shrub Species. Plants 2020, 9, 1331. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Nizet, V.; Klein, J.O. CHAPTER 6—Bacterial Sepsis and Meningitis. In Infectious Diseases of the Fetus and Newborn, 7th ed.; Remington, J.S., Klein, J.O., Wilson, C.B., Nizet, V., Maldonado, Y.A., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 222–275. [Google Scholar]
- Jahani, S.; Bazi, S.; Shahi, Z.; Sheykhzade Asadi, M.; Mosavi, F.; Sohil Baigi, G. Antifungal Effect of the Extract of the Plants Against Candida albicans. Int. J. Infect. Dis. 2017, 4, e36807. [Google Scholar] [CrossRef]
- Seok, H.; Huh, K.; Cho, S.Y.; Kang, C.-I.; Chung, D.R.; Huh, W.S.; Park, J.B.; Peck, K.R. Invasive Fungal Diseases in Kidney Transplant Recipients: Risk Factors for Mortality. J. Clin. Med. 2020, 9, 1824. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.; Maen, A. Inhibitory activity of high antioxidant Australian native fruits against the bacterial triggers of selected autoimmune diseases. Pharmacogn. Commun. 2015, 5, 48–58. [Google Scholar] [CrossRef]
- Cheesman, M.J.; White, A.; Matthews, B.; Cock, I.E. Terminalia ferdinandiana Fruit and Leaf Extracts Inhibit Methicillin-Resistant Staphylococcus aureus Growth. Planta Medica 2019, 85, 1253–1262. [Google Scholar] [CrossRef]
- Noé, W.; Murhekar, S.; White, A.; Davis, C.; Cock, I.E. Inhibition of the growth of human dermatophytic pathogens by selected australian and asian plants traditionally used to treat fungal infections. J. Mycol. Médicale 2019, 29, 331–344. [Google Scholar] [CrossRef]
- WHO. Cancer Over Time. Available online: https://gco.iarc.fr/overtime/en (accessed on 30 January 2023).
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating Medicinal Plants for Anticancer Activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef]
- Larramendy, M.; Soloneski, S. Genotoxicity: A Predictable Risk to Our Actual World; IntechOpen: London, UK, 2018. [Google Scholar]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-Based Assays for Measurement of Antiproliferative Activity of Green Tea Polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Deng, J.Z.; Starck, S.R.; Sun, D.A.; Sabat, M.; Hecht, S.M. A new 7,8-euphadien-type triterpenoid from Brackenridgea nitida and Bleasdalea bleasdalei that inhibits DNA polymerase beta. J. Nat. Prod. 2000, 63, 1356–1360. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Li, X.; Zeng, F.; Ruan, H. 2-Methoxyjuglone Induces Apoptosis in HepG2 Human Hepatocellular Carcinoma Cells and Exhibits in Vivo Antitumor Activity in a H22 Mouse Hepatocellular Carcinoma Model. J. Nat. Prod. 2013, 76, 889–895. [Google Scholar] [CrossRef]
- Jolly, C.; Thoison, O.; Martin, M.-T.; Dumontet, V.; Gilbert, A.; Pfeiffer, B.; Léonce, S.; Sévenet, T.; Guéritte, F.; Litaudon, M. Cytotoxic turrianes of Kermadecia elliptica from the New Caledonian rainforest. Phytochemistry 2008, 69, 533–540. [Google Scholar] [CrossRef]
- Qi, W.; Ou, N.; Wu, X.; Xu, H. New arbutin derivatives from the leaves of Heliciopsis lobata with cytotoxicity. Chin. J. Nat. Med. 2016, 14, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Kamagaju, L.; Morandini, R.; Bizuru, E.; Nyetera, P.; Nduwayezu, J.B.; Stévigny, C.; Ghanem, G.; Duez, P. Tyrosinase modulation by five Rwandese herbal medicines traditionally used for skin treatment. J. Ethnopharmacol. 2013, 146, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.; Osman, S.; Haffez, H.; Hassan, Z. In-vitro screening of some plant extracts for their potential anticancer activity. Afr. J. Tradit. Complement. Altern. Med. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Bruyère, C.; Mathieu, V.; Kiss, R.; Genovese, S. Growth inhibitory activity for cancer cell lines of lapachol and its natural and semi-synthetic derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 454–457. [Google Scholar] [CrossRef]
- Chuang, T.-H.; Chan, H.-H.; Wu, T.-S.; Li, C.-F. Chemical Constituents and Biological Studies of the Leaves of Grevillea robusta. Molecules 2011, 16, 9331–9339. [Google Scholar] [CrossRef]
- Khazem, M.; Gaslonde, T.; Dumontet, V.; Poullain, C.; Litaudon, M.; Michel, S. Cytotoxic turrianes from Kermadecia elliptica: Hemisynthesis and biological activities of kermadecin A derivatives. Phytochem. Lett. 2014, 10, 249–254. [Google Scholar] [CrossRef]
- Chuang, T.; Wu, P. Cytotoxic 5-Alkylresorcinol Metabolites from the Leaves of Grevillea robusta. J. Nat. Prod. 2007, 70, 319–323. [Google Scholar] [CrossRef]
- Ullah, M.; Sikder, M.A.A.; Sharmin, T.; Rashid, M. Pharmacological Activities of Grevillea robusta, a Medicinal Plant of Bangladesh. Bangladesh Phramaceutical J. 2014, 17, 135–137. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Berhow, M.A.; Phillips, B.S.; Duval, S.M.; Weisleder, D.; Vaughn, S.F. Bioactive crude plant seed extracts from the NCAUR oilseed repository. Phytomedicine 2003, 10, 325–333. [Google Scholar] [CrossRef]
- Akhtar, M.A. Australian Native Plants—A Source of Novel Anti-Inflammatory Compounds. Ph.D. Thesis, Western Sydney University, Sydney, Australia, 2018. [Google Scholar]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021.
- Lang, G.; Cole, A.L.J.; Blunt, J.W.; Robinson, W.T.; Munro, M.H.G. Excelsione, a Depsidone from an Endophytic Fungus Isolated from the New Zealand Endemic Tree Knightia excelsa. J. Nat. Prod. 2007, 70, 310–311. [Google Scholar] [CrossRef]
- Yu, H.-y.; Liu, L.; Li, J.; Liu, D.; Ruan, H.-l. 2-Methoxyjuglone, a Promising Bioactive Compound for Pharmaceutical and Agricultural Purposes: A Review. Curr. Med. Sci. 2022, 42, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Brotz, E.; Herrmann, J.; Wiese, J.; Zinecker, H.; Maier, A.; Kelter, G.; Imhoff, J.F.; Müller, R.; Paululat, T. Synthesis and Cytotoxic Activity of a Small Naphthoquinone Library: First Synthesis of Juglonbutin. Eur. J. Org. Chem. 2014, 2014, 5318–5330. [Google Scholar] [CrossRef]
- Fási, L.; Di Meo, F.; Kuo, C.-Y.; Stojkovic Buric, S.; Martins, A.; Kúsz, N.; Béni, Z.; Dékány, M.; Balogh, G.T.; Pesic, M.; et al. Antioxidant-Inspired Drug Discovery: Antitumor Metabolite Is Formed in Situ from a Hydroxycinnamic Acid Derivative upon Free-Radical Scavenging. J. Med. Chem. 2019, 62, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Ziauddin Sultani, S.; Jabbar, A.; Iqbal Choudhary, M. Cinnamates and coumarins from the leaves of Murraya paniculata. Phytochemistry 1997, 44, 683–685. [Google Scholar] [CrossRef]
- Ng, M.K.; Abdulhadi-Noaman, Y.; Cheah, Y.K.; Yeap, S.K.; Alitheen, N. Bioactivity studies and chemical constituents of Murraya paniculata (Linn) Jack. Int. Food Res. J. 2012, 19, 1306–1312. [Google Scholar]
- Whysner, J.; Verna, L.; English, J.C.; Williams, G.M. Analysis of Studies Related to Tumorigenicity Induced by Hydroquinone. Regul. Toxicol. Pharmacol. 1995, 21, 158–176. [Google Scholar] [CrossRef]
- Bertanha, C.S.; Januário, A.H.; Alvarenga, T.A.; Pimenta, L.P.; Silva, M.L.A.e.; Cunha, W.R.; Pauletti, P.M. Quinone and Hydroquinone Metabolites from the Ascidians of the Genus Aplidium. Mar. Drugs 2014, 12, 3608–3633. [Google Scholar] [CrossRef]
- Sunassee, S.; Davies-Coleman, M. Cytotoxic and antioxidant marine prenylated quinones and hydroquinones. Nat. Prod. Rep. 2012, 29, 513–535. [Google Scholar] [CrossRef]
- Herrmann, F.C.; Lenz, M.; Jose, J.; Kaiser, M.; Brun, R.; Schmidt, T.J. In Silico Identification and in Vitro Activity of Novel Natural Inhibitors of Trypanosoma brucei Glyceraldehyde-3-phosphate-dehydrogenase. Molecules 2015, 20, 16154–16169. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Raju, R.; Beattie, K.D.; Bodkin, F.; Münch, G. Medicinal Plants of the Australian Aboriginal Dharawal People Exhibiting Anti-Inflammatory Activity. Evid.-Based Complement. Altern. Med. 2016, 2016, 2935403. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Hongzhi, D.; Xiaoying, H.; Yujie, G.; Le, C.; Yuhuan, M.; Dahui, L.; Luqi, H. Classic mechanisms and experimental models for the anti-inflammatory effect of traditional Chinese medicine. Anim. Models Exp. Med. 2022, 5, 108–119. [Google Scholar] [CrossRef]
- Fawole, O.A.; Ndhlala, A.R.; Amoo, S.O.; Finnie, J.F.; Van Staden, J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J. Ethnopharmacol. 2009, 123, 237–243. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Rotelli, A.E.; Pelzer, L.E.; Saad, J.R.; Giordano, O. Phytochemical study and anti-inflammatory properties of Lampaya hieronymi Schum. ex Moldenke. Il Farmaco 2000, 55, 502–505. [Google Scholar] [CrossRef]
- Wang, H.; Leach, D.N.; Thomas, M.C.; Blanksby, S.J.; Forster, P.I.; Waterman, P.G. Bisresorcinol Derivatives from Grevillea glauca. Helv. Chim. Acta 2011, 94, 1812–1819. [Google Scholar] [CrossRef]
- Chen, Y.-P. Recent developments of natural product chemistry in Taiwan. Korean J. Pharmacogn. 1980, 11, 163–172. [Google Scholar]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy. Drug. Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Ahmed, A.S. Phytochemical and biological study of Grevillea robusta A. Cunn cultivated in Egypt. Bull. Pharm. Sciences. Assiut 2006, 29, 272–288. [Google Scholar] [CrossRef]
- Sweeney, A.P.; Wyllie, S.G.; Shalliker, R.A.; Markham, J.L. Xanthine oxidase inhibitory activity of selected Australian native plants. J. Ethnopharmacol. 2001, 75, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Meloncelli, D. Authentication of Australian and New Zealand Honey Origins by Chromatography, and Their Anti-Inflammatory Properties. Ph.D. Thesis, University of the Sunshine Coast, Sippy Downs, Australia, 2019. [Google Scholar]
- Sangphum, A. Biological Activities of Thai Traditional Remedy Called Prasachangdaeng and Its Plant Ingredients. Ph.D. Thesis, Thammasat University, Bangkok, Thailand, 2016. [Google Scholar]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Decosterd, L.A.; Parsons, I.C.; Gustafson, K.R.; Cardellina, J.H., II; McMahon, J.B.; Cragg, G.M.; Murata, Y.; Pannell, L.K.; Steiner, J.R. HIV inhibitory natural products. 11. Structure, absolute stereochemistry, and synthesis of conocurvone, a potent, novel HIV-inhibitory naphthoquinone trimer from a Conospermum sp. J. Am. Chem. Soc. 1993, 115, 6673–6679. [Google Scholar] [CrossRef]
- Dhar, M.; Dhawan, B.N.; Prasad, C.; Rastogi, R.; Singh, K.; Tandon, J. Screening of Indian plants for biological activity: Part V. Indian J. Exp. Biol. 1974, 12, 512–523. [Google Scholar] [PubMed]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Cunha, N.L.; Uchôa, C.J.d.M.; Cintra, L.S.; Souza, H.C.d.; Peixoto, J.A.; Silva, C.P.; Magalhães, L.G.; Gimenez, V.M.M.; Groppo, M.; Rodrigues, V.; et al. In Vitro Schistosomicidal Activity of Some Brazilian Cerrado Species and Their Isolated Compounds. Evid.-Based Complement. Altern. Med. 2012, 2012, 173614. [Google Scholar] [CrossRef]
- Takahashi, M.; Fuchino, H.; Satake, M.; Agatsuma, Y.; Sekita, S. In Vitro Screening of Leishmanicidal Activity in Myanmar Timber Extracts. Biol. Pharm. Bull. 2004, 27, 921–925. [Google Scholar] [CrossRef]
- Aubouy, A.; Camara, A.; Haddad, M. Chapter 8—Medicinal plants from West Africa used as antimalarial agents: An overview. In Medicinal Plants as Anti-Infectives; Chassagne, F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 267–306. [Google Scholar]
- Laryea, M.K.; Borquaye, L.S. Antimalarial Efficacy and Toxicological Assessment of Extracts of Some Ghanaian Medicinal Plants. J. Parasitol. Res. 2019, 2019, 1630405. [Google Scholar] [CrossRef]
- Cock, I.E.; Selesho, M.I.; van Vuuren, S.F. A review of the traditional use of southern African medicinal plants for the treatment of malaria. J. Ethnopharmacol. 2019, 245, 112176. [Google Scholar] [CrossRef]
- Yamashita-Higuchi, Y.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Nakai, T. Grevillosides J-Q, arbutin derivatives from the leaves of Grevillea robusta and their melanogenesis inhibitory activity. Chem. Pharm. Bull. 2014, 62, 364–372. [Google Scholar] [CrossRef]
- Sonka, L. Exploring Anti-Tyrosinase Bioactive Compounds from the Cape Flora; University of the Western Cape: Cape Town, South Africa, 2018. [Google Scholar]
- Beniddir, M.A.; Simonin, A.-L.; Martin, M.-T.; Dumontet, V.; Poullain, C.; Guéritte, F.; Litaudon, M. Turrianes from Kermadecia rotundifolia as new acetylcholinesterase inhibitors. Phytochem. Lett. 2010, 3, 75–78. [Google Scholar] [CrossRef]
- Roufogalis, B.D.; Li, Q.; Tran, V.H.; Kable, E.P.W.; Duke, C.C. Investigation of plant-derived phenolic compounds as plasma membrane Ca2+-ATPase inhibitors with potential cardiovascular activity. Drug Dev. Res. 1999, 46, 239–249. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Masuda, T.; Mizuguchi, S.; Tanaka, T.; Iritani, K.; Takeda, Y.; Yonemori, S. Isolation and Structure Determination of New Antioxidative Ferulic Acid Glucoside Esters from the Rhizome of Alpinia speciosa, a Zingiberaceae Plant Used in Okinawan Food Culture. J. Agric. Food Chem. 2000, 48, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Gendaram, O.; Choi, Y.H.; Sun-young, K.; Ryu, S.Y. Anti-oxidative and antibacterial constituents from Sedum hybridum. Nat. Prod. Sci. 2011, 17, 279–284. [Google Scholar]
- Pavlović, D.R.; Branković, S.; Kovačević, N.; Kitić, D.; Veljković, S. Comparative Study of Spasmolytic Properties, Antioxidant Activity and Phenolic Content of Arbutus unedo from Montenegro and Greece. Phytother. Res. 2011, 25, 749–754. [Google Scholar] [CrossRef]
- Gascoigne, R.E.; White, D.E. A Survey of Anthocyanins in Australian Flora: Issued 23 November 1948. J. Proc. R. Soc. N. S. W. 1949, 82, 44–70. [Google Scholar]
- Lila, M.A. Anthocyanins and Human Health: An In Vitro Investigative Approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef]
- Cornforth, J.W. The glycoside of Persoonia salicina fruits. J. Proc. R. Soc. N. S. W. 1938, 72, 255–256. [Google Scholar] [CrossRef]
- Cannon, J.; Metcalf, B. Phenolic constituents of Persoonia elliptica (Proteaceae). Aust. J. Chem. 1971, 24, 1925–1931. [Google Scholar] [CrossRef]
- Bateman, P.W.; Pearlman, P.; Robertson, P.; Schultz, B.; Wardell-Johnson, G. Is the Biodiversity Conservation Act 2016 (WA) fit for purpose? Pac. Conserv. Biol. 2017, 23, 146–149. [Google Scholar] [CrossRef]
- Ward, M.S.; Simmonds, J.S.; Reside, A.E.; Watson, J.E.M.; Rhodes, J.R.; Possingham, H.P.; Trezise, J.; Fletcher, R.; File, L.; Taylor, M. Lots of loss with little scrutiny: The attrition of habitat critical for threatened species in Australia. Conserv. Sci. Pract. 2019, 1, 117. [Google Scholar] [CrossRef]

| No | Compounds | Molecular Formula | Accurate Mass (m/z) | Species | References |
|---|---|---|---|---|---|
| 1 | 3,4-bis(4-hydroxybenzoyl)-1, 5-anhydro-d-glucitol | C20H20O9 | 404.37 | P. cynaroides | [57] |
| 2 | 4-hydroxybenzoyl-1,5- anhydro-d-glucitol | C13H16O7 | 284.26 | ||
| 3 | 2-(hydroxymethyl)-4- oxo-4H-pyran-3-yl- 6-O-benzoate-β-d- glucopyranoside | C19H20O11 | 424.36 | ||
| 4 | 3-hydroxy-7,8-dihydro -β-ionone-3-O-β -d-glucopyranoside | C19H32O8 | 388.46 | ||
| 5 | 3,4-dihydroxybenzoic acid | C7H6O4 | 154.12 | ||
| 6 | 3-hydroxykojic acid | C6H6O5 | 158.11 | ||
| 7 | B-type Procyanidin | C30H26O12 | 578.53 | [84] | |
| 8 | Diosmetin | C16H12O6 | 300.27 | ||
| 9 | 3,5-dihydroxy cinnamate | C10H10O4 | 194.19 | G. robusta | [85] |
| 10 | 6-hydroxy coumarin | C9H6O3 | 162.14 | ||
| 11 | p-hydroxybenzeldahyde | C7H6O2 | 112.12 | ||
| 12 | Methyl gallate | C8H8O5 | 184.15 | ||
| 13 | Arbutin 6″-O-3,5- dihydroxycinnamic acid | C21H22O10 | 434.40 | ||
| 14 | Ethyl gallate | C9H10O5 | 198.17 | ||
| 15 | Icariside B1 | C19H30O8 | 386.44 | [78] | |
| 16 | Heliciopside A | C26H28O14 | 564.50 | H. terminalis | [79] |
| 17 | Heliciopside B | C26H28O14 | 564.50 | ||
| 18 | Heliciopside C | C28H31O16 | 624.55 | ||
| 19 | Heliciopside D | C28H36O16 | 628.58 | ||
| 20 | Heliciopside E | C42H42O22 | 898.78 | ||
| 21 | Clemochinenoside D | C27H30O15 | 594.52 | ||
| 22 | 3,4,5-trimethoxyphenyl-β -d-glucopyranoside | C15H22O9 | 346.33 | ||
| 23 | Kusukuenol B1 | C30H44O4 | 468.68 | ||
| 24 | Ursolic acid | C30H48O3 | 456.71 | [80] | |
| 25 | 3,7,11,15-Tetramethyl-2-hexadecene | C20H40 | 280.54 | Stenocarpus sinuatus | [81] |
| 26 | Neophytadiene | C20H38 | 278.52 | ||
| 27 | Phytol | C20H40O | 296.54 | ||
| 28 | α-Tocospiro A | C29H50O4 | 462.71 | ||
| 29 | 2-Methyloctacosane | C29H60 | 408.8 | ||
| 30 | β-tocopherol | C28H48O2 | 416.69 | ||
| 31 | Hentriacontane | C31H64 | 436.85 | ||
| 32 | γ-sitosterol | C29H50O | 414.72 | ||
| 33 | β-sitosterol | C29H50O | 414.72 | M. integrifolia | [82] |
| 34 | Monogalactosyl diacylglycerol | C38H72O10 | 688.51 | ||
| 35 | Protochatechuic acid | C7H6O4 | 154.12 | ||
| 36 | Kaur-16-ene | C20H32 | 272.48 | R. montana | [86] |
| 37 | Kaur-15-ene | C20H32 | 272.48 | ||
| 38 | Nerolidol | C15H26O | 222.37 | ||
| 39 | Farnesylacetone | C18H30O | 262.44 |
| Bioactive Properties | Findings | References |
|---|---|---|
| Treatment | Sore eyes, sore throats, colds, diarrhea, and chest infections treated by leaves and wood of P. falcata | [28,49,56] |
| Antimicrobial activity | Potential antimicrobial activity against a wide range of bacteria found in the leaves of P. gunnii, the fruit of P. pinifolia, and P. linearis | [24,25,26] |
| Antibiotic activity of P. juniperina against typhoid bacilli, staphylococci, and Mycobacterium phlei | [27] | |
| Toxicity | Mice died injected with the smallest dose of 500 milligrams per kilogram of body weight of chloroform extract of P. pinifolia | [24] |
| Anti-inflammatory activity | P. falcata leaves can against XO given 2.24 ± 1.72% inhibition at 100 µg/mL | [208] |
| Phytochemicals | Arbutin derivatives and pyroside found in the leaves of P. gunnii and Arbutin derivatives found in the ripening fruit of P. linearis × pinifolia and P. salicina | [25,26,230] |
| (Z)-5-undec-3-enylresorcinol compound extracted from the wood of P. elliptica | [231] | |
| Anthocyanins found in P. linearis, P. pinifolia, and P. myrtilloides | [228] | |
| Saponins and tannins screened in P. falcata | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Netzel, M.E.; Pengelly, A.; Sivakumar, D.; Sultanbawa, Y. A Review of Phytochemicals and Bioactive Properties in the Proteaceae Family: A Promising Source of Functional Food. Antioxidants 2023, 12, 1952. https://doi.org/10.3390/antiox12111952
Zhang J, Netzel ME, Pengelly A, Sivakumar D, Sultanbawa Y. A Review of Phytochemicals and Bioactive Properties in the Proteaceae Family: A Promising Source of Functional Food. Antioxidants. 2023; 12(11):1952. https://doi.org/10.3390/antiox12111952
Chicago/Turabian StyleZhang, Jiale, Michael E. Netzel, Andrew Pengelly, Dharini Sivakumar, and Yasmina Sultanbawa. 2023. "A Review of Phytochemicals and Bioactive Properties in the Proteaceae Family: A Promising Source of Functional Food" Antioxidants 12, no. 11: 1952. https://doi.org/10.3390/antiox12111952