Senescent Endothelial Cells Sustain Their Senescence-Associated Secretory Phenotype (SASP) through Enhanced Fatty Acid Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Senescence-Associated Beta-Galactosidase
2.3. MTT Assay
2.4. Oxidative Damage Assay
2.5. Immunoassays
2.6. Nuclear Magnetic Resonance Analysis
2.7. Telomere Length
- tel 1, GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT;
- tel 2, TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA;
- 36B4u, CAGCAAGTGGGAAGGTGTAATCC;
- 36B4d, CCCATTCTATCATCAACGGGTACAA.
2.8. Total RNA Extraction
2.9. mRNA Expression
2.10. Glucose and Lactate Measurement
2.11. ATP Assessment
2.12. Fatty Acid Oxidation Assay and Extracellular O2 Consumption Assay
2.13. Western Blot Analysis
2.14. Transmission Electron Microscopy
2.15. Statistical Analysis
3. Results
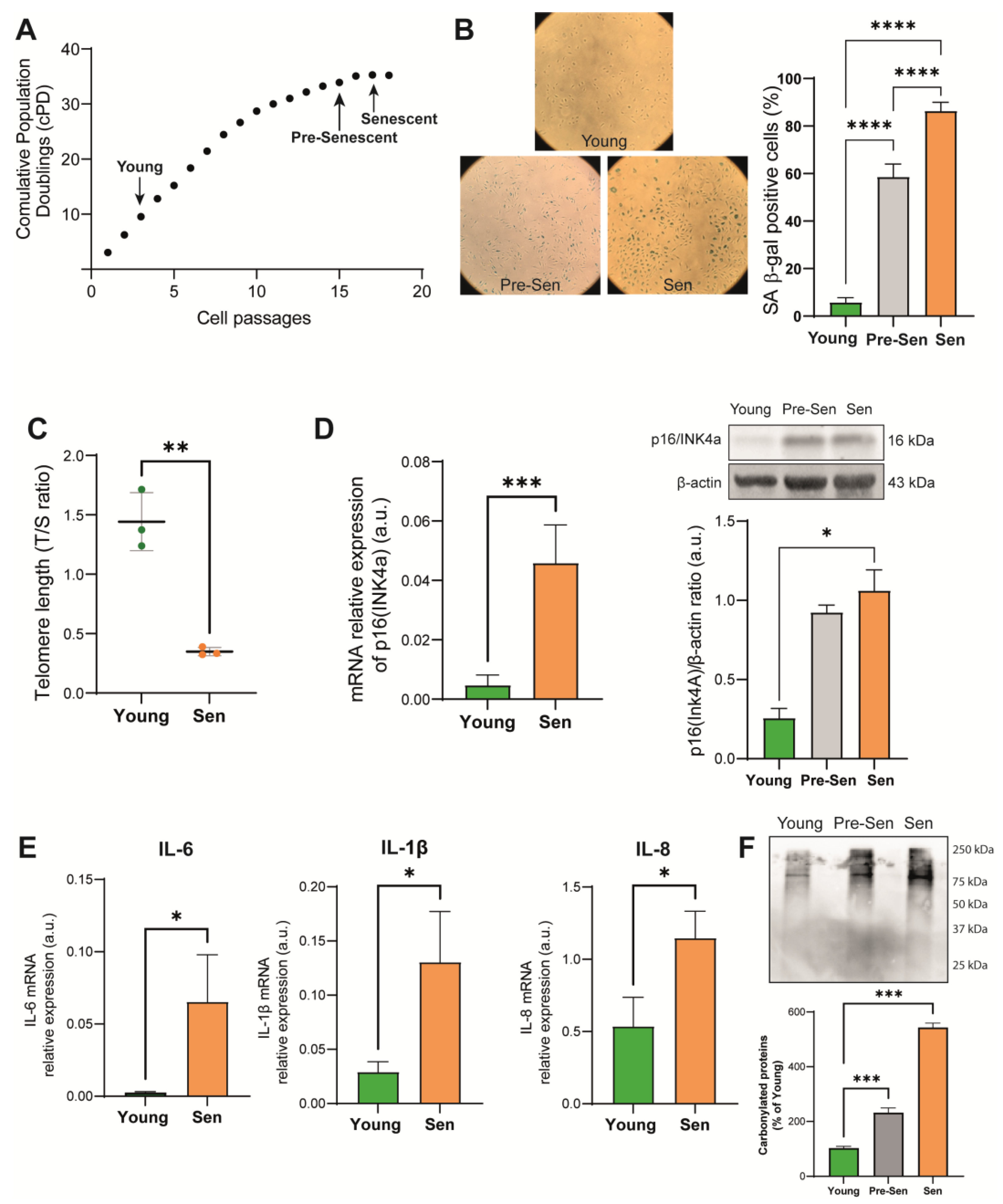
3.1. Characterization of the Senescence Status of HUVECs
3.2. Characteristics of the Basal Metabolism of Young and Senescent HUVECs
3.3. Lipid Metabolism in Senescent Endothelial Cells
3.4. Blockade of Glycolysis Increases the Expression of Proinflammatory Cytokines in Endothelial Cells
3.5. Blockade of Fatty Acid Oxidation Reduces the Expression of Proinflammatory Cytokines in Senescent Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Ramini, D.; Giuliani, A.; Recchioni, R.; Spazzafumo, L.; Olivieri, F. Connecting vascular aging and frailty in Alzheimer’s disease. Mech. Ageing Dev. 2021, 195, 111444. [Google Scholar] [CrossRef]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Pratico, D.; Vinciguerra, M.; Lip, G.Y.H.; Franceschi, C.; Ren, J. Inflammaging: Mechanisms and role in the cardiac and vasculature. Trends Endocrinol. Metab. 2023, 34, 373–387. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Bober, E.A.; Henson, S.M. Divergent mechanisms of metabolic dysfunction drive fibroblast and T-cell senescence. Ageing Res. Rev. 2018, 47, 24–30. [Google Scholar] [CrossRef]
- Yuan, X.; Logan, T.M.; Ma, T. Metabolism in Human Mesenchymal Stromal Cells: A Missing Link Between hMSC Biomanufacturing and Therapy? Front. Immunol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Barilani, M.; Lovejoy, C.; Piras, R.; Abramov, A.Y.; Lazzari, L.; Angelova, P.R. Age-related changes in the energy of human mesenchymal stem cells. J. Cell. Physiol. 2022, 237, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- James, E.L.; Michalek, R.D.; Pitiyage, G.N.; de Castro, A.M.; Vignola, K.S.; Jones, J.; Mohney, R.P.; Karoly, E.D.; Prime, S.S.; Parkinson, E.K. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome Res. 2015, 14, 1854–1871. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Carmeliet, P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell. Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Zwerschke, W.; Mazurek, S.; Stockl, P.; Hutter, E.; Eigenbrodt, E.; Jansen-Durr, P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003, 376, 403–411. [Google Scholar] [CrossRef]
- Kuosmanen, S.M.; Sihvola, V.; Kansanen, E.; Kaikkonen, M.U.; Levonen, A.L. MicroRNAs mediate the senescence-associated decline of NRF2 in endothelial cells. Redox Biol. 2018, 18, 77–83. [Google Scholar] [CrossRef]
- Giuliani, A.; Cirilli, I.; Prattichizzo, F.; Mensa, E.; Fulgenzi, G.; Sabbatinelli, J.; Graciotti, L.; Olivieri, F.; Procopio, A.D.; Tiano, L.; et al. The mitomiR/Bcl-2 axis affects mitochondrial function and autophagic vacuole formation in senescent endothelial cells. Aging (Albany NY) 2018, 10, 2855–2873. [Google Scholar] [CrossRef]
- Kostidis, S.; Addie, R.D.; Morreau, H.; Mayboroda, O.A.; Giera, M. Quantitative NMR analysis of intra- and extracellular metabolism of mammalian cells: A tutorial. Anal. Chim. Acta 2017, 980, 1–24. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Aird, K.M.; Zhang, R. Metabolic alterations accompanying oncogene-induced senescence. Mol. Cell. Oncol. 2014, 1, e963481. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Tanaka, H.; Koga, T. Cellular Senescence Variation by Metabolic and Epigenomic Remodeling. Trends Cell Biol. 2020, 30, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Gitenay, D.; Wiel, C.; Lallet-Daher, H.; Vindrieux, D.; Aubert, S.; Payen, L.; Simonnet, H.; Bernard, D. Glucose metabolism and hexosamine pathway regulate oncogene-induced senescence. Cell. Death Dis. 2014, 5, e1089. [Google Scholar] [CrossRef] [PubMed]
- Stabenow, L.K.; Zibrova, D.; Ender, C.; Helbing, D.L.; Spengler, K.; Marx, C.; Wang, Z.Q.; Heller, R. Oxidative Glucose Metabolism Promotes Senescence in Vascular Endothelial Cells. Cells 2022, 11, 2213. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell. Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Quijano, C.; Cao, L.; Fergusson, M.M.; Romero, H.; Liu, J.; Gutkind, S.; Rovira, I.I.; Mohney, R.P.; Karoly, E.D.; Finkel, T. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell. Cycle 2012, 11, 1383–1392. [Google Scholar] [CrossRef]
- Kaplon, J.; Zheng, L.; Meissl, K.; Chaneton, B.; Selivanov, V.A.; Mackay, G.; van der Burg, S.H.; Verdegaal, E.M.; Cascante, M.; Shlomi, T.; et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013, 498, 109–112. [Google Scholar] [CrossRef]
- Maeda, M.; Scaglia, N.; Igal, R.A. Regulation of fatty acid synthesis and Delta9-desaturation in senescence of human fibroblasts. Life Sci. 2009, 84, 119–124. [Google Scholar] [CrossRef]
- Flor, A.C.; Wolfgeher, D.; Wu, D.; Kron, S.J. A signature of enhanced lipid metabolism, lipid peroxidation and aldehyde stress in therapy-induced senescence. Cell. Death Discov. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Chong, M.; Yin, T.; Chen, R.; Xiang, H.; Yuan, L.; Ding, Y.; Pan, C.C.; Tang, Z.; Alexander, P.B.; Li, Q.J.; et al. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 2018, 19, e45274. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, V.; Cisneros, A.; Sica, V.; Deryagin, O.; Lai, Y.; Jung, S.; Andres, E.; An, J.; Segales, J.; Ortet, L.; et al. Author Correction: Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 2023, 614, E45. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; Cabodevilla, A.G.; Samovski, D.; Pietka, T.; Basu, D.; Goldberg, I.J. Endothelial Cell Receptors in Tissue Lipid Uptake and Metabolism. Circ. Res. 2021, 128, 433–450. [Google Scholar] [CrossRef]
- Fafian-Labora, J.; Carpintero-Fernandez, P.; Jordan, S.J.D.; Shikh-Bahaei, T.; Abdullah, S.M.; Mahenthiran, M.; Rodriguez-Navarro, J.A.; Niklison-Chirou, M.V.; O’Loghlen, A. FASN activity is important for the initial stages of the induction of senescence. Cell. Death Dis. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Schneider, J.G.; Shenouda, S.M.; Lee, A.; Towler, D.A.; Chakravarthy, M.V.; Vita, J.A.; Semenkovich, C.F. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J. Biol. Chem. 2011, 286, 2933–2945. [Google Scholar] [CrossRef] [PubMed]
- Bruning, U.; Morales-Rodriguez, F.; Kalucka, J.; Goveia, J.; Taverna, F.; Queiroz, K.C.S.; Dubois, C.; Cantelmo, A.R.; Chen, R.; Loroch, S.; et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell. Metab. 2018, 28, 866–880.e815. [Google Scholar] [CrossRef]
- Veitch, S.; Njock, M.S.; Chandy, M.; Siraj, M.A.; Chi, L.; Mak, H.; Yu, K.; Rathnakumar, K.; Perez-Romero, C.A.; Chen, Z.; et al. MiR-30 promotes fatty acid beta-oxidation and endothelial cell dysfunction and is a circulating biomarker of coronary microvascular dysfunction in pre-clinical models of diabetes. Cardiovasc. Diabetol. 2022, 21, 31. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Hsieh, W.Y.; Minarrieta, L.; Duong, T.N.; Kim, K.K.O.; Desousa, B.R.; Andreyev, A.Y.; Bowman, C.E.; Caradonna, K.; Dranka, B.P.; et al. Etomoxir Inhibits Macrophage Polarization by Disrupting CoA Homeostasis. Cell Metab. 2018, 28, 490–503.e497. [Google Scholar] [CrossRef]
- Yao, C.H.; Liu, G.Y.; Wang, R.; Moon, S.H.; Gross, R.W.; Patti, G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of beta-oxidation. PLoS Biol. 2018, 16, e2003782. [Google Scholar] [CrossRef]
- Kalucka, J.; Bierhansl, L.; Conchinha, N.V.; Missiaen, R.; Elia, I.; Bruning, U.; Scheinok, S.; Treps, L.; Cantelmo, A.R.; Dubois, C.; et al. Quiescent Endothelial Cells Upregulate Fatty Acid beta-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018, 28, 881–894.e813. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Guo, L.; Ghassemi, S.; Snyder, N.W.; Worth, A.J.; Weng, L.; Kam, Y.; Philipson, B.; Trefely, S.; Nunez-Cruz, S.; et al. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 2018, 8, 6289. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 2827. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, C.C.; Lavis, C.J.; Croft, A.; Dong, L.; Tseng, H.Y.; Yang, F.; Tay, K.H.; Hersey, P.; Zhang, X.D. 2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human melanoma cells through XBP-1-mediated up-regulation of TRAIL-R2. Mol. Cancer 2009, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Pruss, M.; Dwucet, A.; Tanriover, M.; Hlavac, M.; Kast, R.E.; Debatin, K.M.; Wirtz, C.R.; Halatsch, M.E.; Siegelin, M.D.; Westhoff, M.A.; et al. Dual metabolic reprogramming by ONC201/TIC10 and 2-Deoxyglucose induces energy depletion and synergistic anti-cancer activity in glioblastoma. Br. J. Cancer 2020, 122, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Pradhan, P.; Braud, L.; Fuchs, H.R.; Gueler, F.; Motterlini, R.; Foresti, R.; Immenschuh, S. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide—A divergent role for glycolysis. Redox Biol. 2019, 22, 101147. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; de Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]




| Metabolic Pathways | Total | Expected | Hits | Raw p | −Log10(p) | FDR |
|---|---|---|---|---|---|---|
| Glycolysis/gluconeogenesis | 26 | 0.17 | 5 | 2.11 × 10−7 | 6.67 | 1.78 × 10−5 |
| Glyoxylate and dicarboxylate metabolism | 32 | 0.21 | 4 | 2.89 × 10−5 | 4.54 | 1.21 × 10−3 |
| Aminoacyl tRNA biosynthesis | 48 | 0.31 | 4 | 1.49 × 10−4 | 3.83 | 4.16 × 10−3 |
| Pyruvate metabolism | 22 | 0.14 | 3 | 2.80 × 10−4 | 3.55 | 5.87 × 10−3 |
| Valine, leucine, and isoleucine biosynthesis | 8 | 0.05 | 2 | 1.03 × 10−3 | 2.99 | 1.73 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, A.; Giudetti, A.M.; Vergara, D.; Del Coco, L.; Ramini, D.; Caccese, S.; Sbriscia, M.; Graciotti, L.; Fulgenzi, G.; Tiano, L.; et al. Senescent Endothelial Cells Sustain Their Senescence-Associated Secretory Phenotype (SASP) through Enhanced Fatty Acid Oxidation. Antioxidants 2023, 12, 1956. https://doi.org/10.3390/antiox12111956
Giuliani A, Giudetti AM, Vergara D, Del Coco L, Ramini D, Caccese S, Sbriscia M, Graciotti L, Fulgenzi G, Tiano L, et al. Senescent Endothelial Cells Sustain Their Senescence-Associated Secretory Phenotype (SASP) through Enhanced Fatty Acid Oxidation. Antioxidants. 2023; 12(11):1956. https://doi.org/10.3390/antiox12111956
Chicago/Turabian StyleGiuliani, Angelica, Anna Maria Giudetti, Daniele Vergara, Laura Del Coco, Deborah Ramini, Sara Caccese, Matilde Sbriscia, Laura Graciotti, Gianluca Fulgenzi, Luca Tiano, and et al. 2023. "Senescent Endothelial Cells Sustain Their Senescence-Associated Secretory Phenotype (SASP) through Enhanced Fatty Acid Oxidation" Antioxidants 12, no. 11: 1956. https://doi.org/10.3390/antiox12111956






