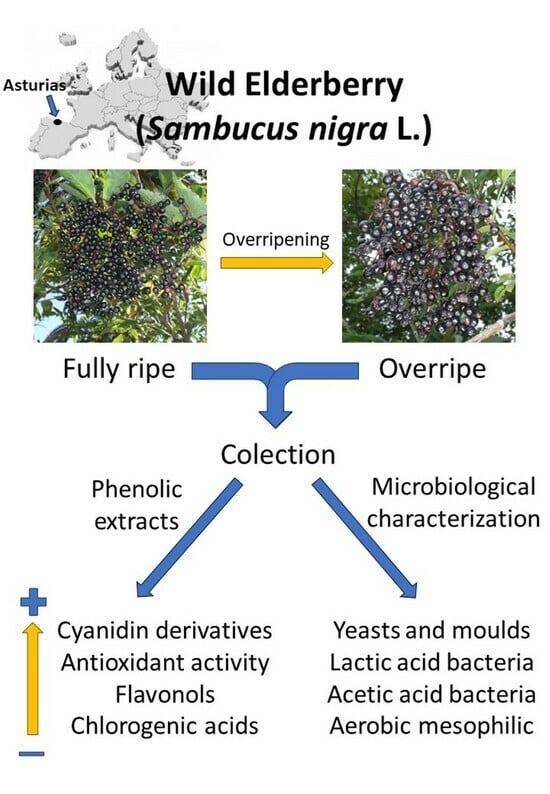
The Phenolic Composition, Antioxidant Activity and Microflora of Wild Elderberry in Asturias (Northern Spain): An Untapped Resource of Great Interest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material
2.3. Phenolic Extracts
2.4. Phenolic Composition
2.4.1. HPLC Analysis
2.4.2. Total Phenolic Content (TPC)
2.4.3. Monomeric Anthocyanin Content (MAC)
2.5. Antioxidant Activity
2.5.1. Reducing Power
2.5.2. Radical Scavenging Activity
DPPH Assay
ABTS Assay
2.6. Microbiological Assays
- −
- Total number of aerobic mesophilic microorganisms on PCA agar, incubation at 37 °C for 24–48 h;
- −
- Total number of moulds and yeasts on Sabouraud Chloramphenicol Agar (Sharlau), incubation at 25 °C for 24–48 h;
- −
- Total number of lactic acid bacteria (LAB) on MRS agar with pimaricin (50 mg/L) and streptomycin (100 mg/L) at 30 °C for 48–96 h;
- −
- Total number of acetic acid bacteria (AAB) on GYC medium [31] with pimaricin (50 mg/L) and penicillin (25 mg/L) at 30 °C for 72–96 h.
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic and Antioxidant Characterization of Locally Collected Samples
3.1.1. Phenolic Composition
3.1.2. Phenolic Profile
3.1.3. Antioxidant Activity
3.2. Comparison with Other Berries
3.3. Effect of Overripening on Phenolics and Antioxidant Properties of Elderberries
3.4. Microflora in Elderberries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. Fruits and Flowers: Chemical Composition and Related Bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Caruso, M.C.; Galgano, F.; Tolve, R.; Pecora, M.; Tedesco, I.; Favati, F.; Condelli, N. Nutraceutical Properties of Wild Berry Fruits from Southern Italy. J. Berry Res. 2016, 6, 321–332. [Google Scholar] [CrossRef]
- Diviš, P.; Vespalcová, M.; Pořízka, J.; Matějíček, A.; Kaplan, J. Elemental Composition of Fruits from Different Black Elder (Sambucus nigra L.) Cultivars Grown in the Czech Republic. J. Elem. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Vulic, J.; Vracar, L.; Sumic, Z. Chemical Characteristics of Cultivated Elderberry Fruit. Acta Period. Technol. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Sambucus nigra L., Fructus. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-sambucus-nigra-l-fructus_en.pdf (accessed on 10 October 2023).
- Mocanu, M.L.; Amariei, S. Elderberries—A Source of Bioactive Compounds with Antiviral Action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of Harvesting Year and Elderberry Cultivar on the Chemical Composition and Potential Bioactivity: A Three-Year Study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The Higher the Better? Differences in Phenolics and Cyanogenic Glycosides in Sambucus nigra Leaves, Flowers and Berries from Different Altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Maggio, A.; La Mantia, T.; Scordino, M.; Bruno, M.; Poli, F. Polyphenols Pattern and Correlation with Antioxidant Activities of Berries Extracts from Four Different Populations of Sicilian Sambucus nigra L. Nat. Prod. Res. 2014, 28, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Imenšek, N.; Ivančič, A.; Kraner Šumenjak, T.; Islamcevic Razboršek, M.; Kristl, J. The Effect of Maturation on Chemical Composition and Harvest of Fruits of Diverse Elderberry Interspecific Hybrids. Eur. J. Hortic. Sci. 2021, 86, 223–231. [Google Scholar] [CrossRef]
- Marțiș, G.S.; Mureșan, V.; Marc, R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.; Applequist, W.L.; Finley, J.; Lister, P.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of Select Flavonols and Chlorogenic Acid Content of Elderberry Collected throughout the Eastern United States. J. Food Compost. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef]
- Kiprovski, B.; Malenčić, Đ.; Ljubojević, M.; Ognjanov, V.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. Quality Parameters Change during Ripening in Leaves and Fruits of Wild Growing and Cultivated Elderberry (Sambucus nigra) Genotypes. Sci. Hortic. 2021, 277, 109792. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.G.; Giusti, M.M. Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and Its Impact on Color Expression. Plants 2020, 9, 1721. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-Products a Source of Anthocyanins and Antioxidant Polyphenols. Ind. Crop. Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, Multi-Functional Natural Products of Industrial Relevance: Recent Biotechnological Advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Tournas, V.H.; Katsoudas, E. Mould and Yeast Flora in Fresh Berries, Grapes and Citrus Fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Akbulut, M. Physico-Chemical Characteristics of Some Wild Grown European Elderberry (Sambucus nigra L.) Genotypes. Pharmacogn. Mag. 2009, 5, 320. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus nigra) in Food—A Review. J. Funct. Food 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Santos López, E.M.; Rodríguez, J.A.; Barros, L.; Lorenzo, J.M. Potential Use of Elderberry (Sambucus nigra L.) as Natural Colorant and Antioxidant in the Food Industry. A Review. Foods 2021, 10, 2713. [Google Scholar] [CrossRef] [PubMed]
- Imenšek, N.; Kristl, J.; Kraner Šumenjak, T.; Ivančič, A. Antioxidant Activity of Elderberry Fruits during Maturation. Agriculture 2021, 11, 555. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and Validation of Ultrasound Assisted Extraction (UAE) and HPLC-DAD Method for Determination of Polyphenols in Dry Beans (Phaseolus vulgaris). J. Food Compos. Anal. 2020, 85, 103334. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Color and pigment analyses in fruit products. Agric. Exp. Stn 1993, 5, 4–20. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and Antioxidant Composition of By-Products from the Cider Industry: Apple Pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Rasooli, I.; Beheshti-Maal, K. Isolation, Characterization and Optimization of Indigenous Acetic Acid Bacteria and Evaluation of Their Preservation Methods. Iran J. Microbiol. 2010, 2, 41–48. [Google Scholar]
- Goud, N.S.; Prasad, G. Antioxidant, Antimicrobial Activity and Total Phenol, and Flavonoids Analisys of Sambucus nigra (Elderberry). Int. J. Curr. Pharm. Sci. 2020, 12, 35–37. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and Other Polyphenolics in American Elderberry (Sambucus canadensis) and European Elderberry (S. nigra) Cultivars: Anthocyanins in American and European Elderberry Cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Csorba, V.; Tóth, M.; László, A.M.; Kardos, L.; Kovács, S. Cultivar and Year Effects on the Chemical Composition of Elderberry (Sambucus nigra L.) Fruits. Not. Bot. Horti. Agrobot. Clu-Na 2020, 48, 770–782. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit Phenolic Composition of Different Elderberry Species and Hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef] [PubMed]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Česlová, L.; Kalendová, P.; Dubnová, L.; Pernica, M.; Fischer, J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules 2023, 28, 6690. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef]
- Kaack, K.; Fretté, X.C.; Christensen, L.P.; Landbo, A.-K.; Meyer, A.S. Selection of Elderberry (Sambucus nigra L.) Genotypes Best Suited for the Preparation of Juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lovitt, R.W. Strategies for Enhanced Malolactic Fermentation in Wine and Cider Maturation. J. Chem. Technol. Biotechnol. 2006, 81, 1130–1140. [Google Scholar] [CrossRef]
- Barbe, J.-C.; de Revel, G.; Joyeux, A.; Bertrand, A.; Lonvaud-Funel, A. Role of Botrytized Grape Micro-Organisms in SO2 Binding Phenomena. J. Appl. Microbiol. 2001, 90, 34–42. [Google Scholar] [CrossRef]
- Torabian, G.; Bahramian, B.; Zambon, A.; Spilimbergo, S.; Adil, Q.; Schindeler, A.; Valtchev, P.; Dehghani, F. A Hybrid Process for Increasing the Shelf Life of Elderberry Juice. J. Supercrit. Fluids 2018, 140, 406–414. [Google Scholar] [CrossRef]
- Martins, G.; Miot-Sertier, C.; Lauga, B.; Claisse, O.; Lonvaud-Funel, A.; Soulas, G.; Masneuf-Pomarède, I. Grape Berry Bacterial Microbiota: Impact of the Ripening Process and the Farming System. Int. J. Food Microbiol. 2012, 158, 93–100. [Google Scholar] [CrossRef]
- Żebracka, A.; Winiarska-Mieczan, A.; Nowakowicz-Dębek, B.; Banach, M.; Drabik, A.; Pulit-Prociak, J.; Chmielowiec-Korzeniowska, A. Assessment of the Microbiological Quality and Bactericidal Properties of Flavoured Honey. Med. Weter. 2022, 78, 556–562. [Google Scholar] [CrossRef]

| Harvest Year | TPC 1 | MAC 2 | |
|---|---|---|---|
| 2021 (n = 31) | mean ± SD | 44.8 ± 8.8 a | 29.0 ± 8.2 a |
| min-max | 31.3–66.4 | 15.5–49.4 | |
| 2022 (n = 22) | mean ± SD | 41.2 ± 10.6 a | 23.1 ± 11.7 a |
| min-max | 28.4–76.1 | 6.3–58.3 | |
| 2023 (n = 26) | mean ± SD | 44.9 ± 11.9 a | 26.5 ± 11.5 a |
| min-max | 24.0–75.8 | 9.0–53.7 | |
| Total (n = 79) | mean ± SD | 43.8 ± 10.4 | 26.5 ± 10.5 |
| min-max | 24.0–76.1 | 6.3–58.3 |
| Harvest Year | Neochlorogenic * | Cryptochlorogenic * | Chlorogenic ** | C3G5G ** | C3S5G ** | C3G ** | C3S ** | C3R * | P3G * | P3MG * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 (n = 31) | mean ± SD | 388.5 ± 179.1 a | 104.8 ± 35.5 b | 1.6 ± 0.9 a | 0.2 ± 0.1 a | 1.3 ± 0.8 a | 14.3 ± 5.7 a | 1.6 ± 0.9 a | 27.5 ± 152.9 a | 0 ± 0 a | 16.9 ± 45.7 a |
| min-max | 47.6–676.2 | 42.3–165.4 | 0.5–3.6 | 0.1–0.4 | 0.5–3.5 | 4.9–26.8 | 0.5–3.6 | 0–851.1 | 0–0 | 0–167.2 | |
| 2022 (n = 22) | mean ± SD | 438.5 ± 338.4 a | 164.8 ± 62.5 a | 2.0 ± 1.0 a | 0.2 ± 0.1 a | 1.0 ± 0.4 a | 11.6 ± 6.0 a | 2.0 ± 1.0 a | 0 ± 0 a | 40.7 ± 143.6 a | 69.4 ± 149.9 a |
| min-max | 116.2–1597.5 | 78.4–347.9 | 0.7–4.0 | 0.1–0.3 | 0.3–2.1 | 3.2–28.5 | 0.7–4.0 | 0–0 | 0–665.8 | 0–668.4 | |
| 2023 (n = 26) | mean ± SD | 394.3 ± 304.9 a | 79.4 ± 55.8 b | 1.5 ± 0.8 a | 0.2 ± 0.1 a | 1.2 ± 0.7 a | 10.8 ± 5.8 a | 1.5 ± 0.8 a | 0 ± 0 a | 24.6 ± 78.8 a | 75.1 ± 115.7 a |
| min-max | 68.9–1207.6 | 21.5–256.8 | 0.5–4.1 | 0.1–0.4 | 0.3–3.0 | 3.1–22.5 | 0.5–4.1 | 0–0 | 0–365.4 | 0–487.1 | |
| Total (n = 79) | mean ± SD | 404.3 ± 271 | 113.2 ± 60.8 | 1.7 ± 0.9 | 0.2 ± 0.1 | 1.2 ± 0.7 | 12.4 ± 5.9 | 1.7 ± 0.9 | 10.8 ± 95.8 | 19.4 ± 88.5 | 50.7 ± 109 |
| min-max | 47.6–1597.5 | 21.5–347.9 | 0.5–4.1 | 0.1–0.4 | 0.3–3.5 | 3.1–28.5 | 0.5–4.1 | 0–851.1 | 0–665.8 | 0–668.4 |
| Harvest Year | QG | QR | QAcG | KG | KR | IsoRG | IsoRR | Q | |
|---|---|---|---|---|---|---|---|---|---|
| 2021 (n = 31) | mean ± SD | 336.5 ± 125.5 a | 3752.1 ± 1566.0 a | 185.9 ± 155 a | 3.1 ± 5.0 a | 63.6 ± 42.1 a | 3.2 ± 5.7 a | 31.8 ± 26.4 a | 12.4 ± 19.9 a |
| min-max | 118.2–538.9 | 1620.4–6786.0 | 0–668.2 | 0–12.5 | 17.9–188.9 | 0–17.5 | 0–110.8 | 0–92.6 | |
| 2022 (n = 22) | mean ± SD | 313.1 ± 96.3 a | 4340.5 ± 1681.2 a | 190.9 ± 78.0 a | 3.4 ± 6.5 a | 73.2 ± 31.6 a | 0 ± 0 b | 41.7 ± 29.5 a | 8.6 ± 12.3 a |
| min-max | 181.2–555.8 | 1724.7–6821.7 | 38.8–340.9 | 0–18.4 | 29.3–156.7 | 0–0 | 0–119.7 | 0–48.6 | |
| 2023 (n = 26) | mean ± SD | 353.0 ± 184.2 a | 4842.5 ± 2210.2 a | 213.6 ± 126.2 a | 2 ± 4.2 a | 65.9 ± 30.4 a | 1.3 ± 3.6 ab | 44.3 ± 31.7 a | 8.7 ± 19.3 a |
| min-max | 165.2–1106.7 | 1621.1–11,304.4 | 52.5–639.3 | 0–11.6 | 20.2–126.5 | 0–13.0 | 11–128.6 | 0–70.8 | |
| Total (n = 79) | mean ± SD | 335.4 ± 140.3 | 4274.8 ± 1867.4 | 196.4 ± 127 | 2.8 ± 5.2 | 67 ± 35.5 | 1.7 ± 4.3 | 38.7 ± 29.3 | 10.1 ± 17.7 |
| min-max | 118.2–1106.7 | 1620.4–11,304.4 | 0–668.2 | 0–18.4 | 17.9–188.9 | 0–17.5 | 0–128.6 | 0–92.6 |
| Harvest Year | DPPH 1 | ABTS 1 | FRAP 2 | |
|---|---|---|---|---|
| 2021 (n = 31) | mean ± SD | 297.9 ± 31.5 a | 428.3 ± 82.3 a | 671.8 ± 133.5 b |
| min–max | 239.7–363.2 | 276.1–602.2 | 420.4–986.2 | |
| 2022 (n = 22) | mean ± SD | 264.1 ± 43.1 b | 355.9 ± 109.8 b | 664.8 ± 212.3 b |
| min–max | 200.3–377.7 | 210.5–658.1 | 377.3–1271.8 | |
| 2023 (n = 26) | mean ± SD | 243.9 ± 38.3 b | 326.9 ± 100.4 b | 793.4 ± 245.5 a |
| min–max | 184.0–328.1 | 155.1–584.5 | 410.2–1487.5 | |
| Total (n = 79) | mean ± SD | 270.7 ± 43.6 | 374.7 ± 105.3 | 709.9 ± 204.4 |
| min–max | 187.9–328.1 | 170.3–584.5 | 410.2–1487.5 |
| DPPH (r = 0.942) | ABTS (r = 0.960) | FRAP (r = 0.810) | |
|---|---|---|---|
| Neochlorogenic | −0.086 | −0.069 | 0.063 |
| Cryptochlorogenic | 0.140 * | 0.128 ** | −0.193 |
| Chlorogenic | −0.024 | 0.001 | 0.162 |
| C3G5G | −0.074 | 0.061 | 0.003 |
| C3S5G | 0.276 *** | 0.127 | −0.007 |
| C3G | 0.597 *** | 0.379 *** | 0.029 |
| C3S | 0.261 ** | 0.402 *** | 0.527 ** |
| C3R | 0.106 * | 0.017 | −0.029 |
| P3G | 0.092 | 0.227 ** | 0.047 |
| P3MG | −0.380 *** | −0.372 *** | 0.148 |
| QG | 0.023 | 0.072 | 0.197 |
| QR | −0.077 | −0.082 | 0.317 * |
| QAcG | −0.062 | −0.102 | −0.012 |
| KG | 0.178 ** | 0.155 ** | −0.110 |
| KR | 0.040 | −0.045 | −0.249 * |
| IsoRG | 0.121 ** | 0.199 *** | −0.045 |
| IsoRR | −0.067 | −0.068 | −0.067 |
| Q | 0.085 | 0.132 ** | 0.046 |
| TPC 1 | MAC 2 | DPPH 3 | ABTS 3 | FRAP 4 | |
|---|---|---|---|---|---|
| Commercial elderberry | 21.4 | 4.0 | 120.9 | 235.7 | 326.8 |
| Raspberry | 11.5 | 3.7 | 117.7 | 118.1 | 376.6 |
| Blackberry | 32.7 | 17.0 | 319.5 | 417.9 | 560.4 |
| Blueberry (V. virgatum) | 13.2 | 6.1 | 112.0 | 138.4 | 206.6 |
| Blueberry (V. corymbosum) | 15.5 | 4.6 | 119.5 | 15.00 | 316.0 |
| Blueberry (V. sp. hybrid) | 17.2 | 9.1 | 122.5 | 169.9 | 291.1 |
| Fully Ripe | Overripe | |
|---|---|---|
| TPC 1 | 43.7 ± 10.9 | 50.3 ± 14.3 |
| MAC 2 | 24.9 ± 12.1 | 25.5 ± 12.8 |
| DPPH 3 | 253.5 ± 47.9 | 256.0 ± 41.4 |
| ABTS 3 | 340.2 ± 117.3 | 352.2 ± 119.7 |
| FRAP 4 | 747.0 ± 235.1 | 802.9 ± 277.2 |
| Neochlorogenic 5 | 365.0 ± 270.7 | 295.8 ± 230.9 |
| Cryptochlorogenic 5 | 104.1 ± 70.4 | 87.4 ± 54 |
| Chlorogenic 5 | 1696 ± 1028.7 | 1659.8 ± 925 |
| C3G5G 5 | 175.2 ± 63.6 | 171.0 ± 55.9 |
| C3S5G 5 | 1093.2 ± 417.8 | 1210.1 ± 524.2 |
| C3G 5 | 10,933.9 ± 6594.9 | 9991.5 ± 5820 |
| C3S 5 | 15,084.9 ± 7624.9 | 15,578.9 ± 8398.4 |
| C3R 5 | 0 ± 0 | 0 ± 0 |
| P3G 5 | 33.2 ± 137.4 | 28.6 ± 140.2 |
| P3MG 5 | 91.5 ± 148.6 | 82.4 ± 150.9 |
| QG 5 | 307.7 ± 103.5 | 360.1 ± 116.4 |
| QR 5 | 4167.3 ± 1830.6 | 3877.3 ± 1353 |
| QAcG 5 | 191.0 ± 90.9 | 165.9 ± 68.9 |
| KG 5 | 3.4 ± 6.3 | 5.8 ± 7.6 |
| KR 5 | 69.5 ± 36.1 | 67.7 ± 24.9 |
| IsoRG 5 | 0.4 ± 2.0 | 0.8 ± 2.9 |
| IsoRR 5 | 34.6 ± 25.8 | 32.3 ± 21.2 |
| Q 5,* | 10.7 ± 17.3 | 35.2 ± 43.7 |
| Fully Ripe | Overripe | ||
|---|---|---|---|
| Aerobic mesophilic | Range a | 4.0–7.7 | 4.3–8.2 |
| Percentage (%) of samples in the indicated interval | <2 | ||
| 2–3 | |||
| 3–4 | 8.3 | ||
| 4–5 | 4.2 | 12.5 | |
| 5–6 | 29.2 | 8.3 | |
| 6–7 | 37.5 | 16.7 | |
| 7–8 | 20.8 | 54.2 | |
| 8–9 | 8.3 | ||
| Lactic acid bacteria | Range a | <2.0–7.8 | <2.0–7.5 |
| Percentage (%) of samples in the indicated interval | <2 | 20.8 | 4.2 |
| 2–3 | 4.2 | 16.7 | |
| 3–4 | 12.5 | 8.3 | |
| 4–5 | 8.3 | 4.2 | |
| 5–6 | 29.2 | 20.8 | |
| 6–7 | 16.7 | 20.8 | |
| 7–8 | 8.3 | 25.0 | |
| Acetic acid bacteria | Range a | 3.7–8.0 | 5.1–8.3 |
| Percentage (%) of samples in the indicated interval | < 2 | ||
| 2–3 | |||
| 3–4 | 4.2 | ||
| 4–5 | 12.5 | ||
| 5–6 | 16.7 | 25.0 | |
| 6–7 | 41.7 | 41.7 | |
| 7–8 | 25.0 | 29.2 | |
| 8–9 | 4.2 | ||
| Yeasts | Range a | <2.0–8.0 | <2.0–8.5 |
| Percentage (%) of samples in the indicated interval | <2 | 4.2 | 4.2 |
| 2–3 | |||
| 3–4 | 16.7 | 4.2 | |
| 4–5 | 4.2 | ||
| 5–6 | 8.3 | 12.5 | |
| 6–7 | 12.5 | 4.2 | |
| 7–8 | 58.3 | 45.8 | |
| 8–9 | 25.0 | ||
| Moulds | Range a | <2.0–6.0 | <2.0–6.5 |
| Percentage (%) of samples in the indicated interval | <2 | 29.2 | 33.3 |
| 2–3 | |||
| 3–4 | |||
| 4–5 | 41.7 | 8.3 | |
| 5–6 | 29.2 | 45.8 | |
| 6–7 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Madrera, R.; Pando Bedriñana, R. The Phenolic Composition, Antioxidant Activity and Microflora of Wild Elderberry in Asturias (Northern Spain): An Untapped Resource of Great Interest. Antioxidants 2023, 12, 1986. https://doi.org/10.3390/antiox12111986
Rodríguez Madrera R, Pando Bedriñana R. The Phenolic Composition, Antioxidant Activity and Microflora of Wild Elderberry in Asturias (Northern Spain): An Untapped Resource of Great Interest. Antioxidants. 2023; 12(11):1986. https://doi.org/10.3390/antiox12111986
Chicago/Turabian StyleRodríguez Madrera, Roberto, and Rosa Pando Bedriñana. 2023. "The Phenolic Composition, Antioxidant Activity and Microflora of Wild Elderberry in Asturias (Northern Spain): An Untapped Resource of Great Interest" Antioxidants 12, no. 11: 1986. https://doi.org/10.3390/antiox12111986