Investigation of a UPR-Related Gene Signature Identifies the Pro-Fibrotic Effects of Thrombospondin-1 by Activating CD47/ROS/Endoplasmic Reticulum Stress Pathway in Lung Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Administration of Bleomycin and Drug Treatment
2.2. Masson’s Trichrome Staining
2.3. The Determination of Hydroxyproline Content
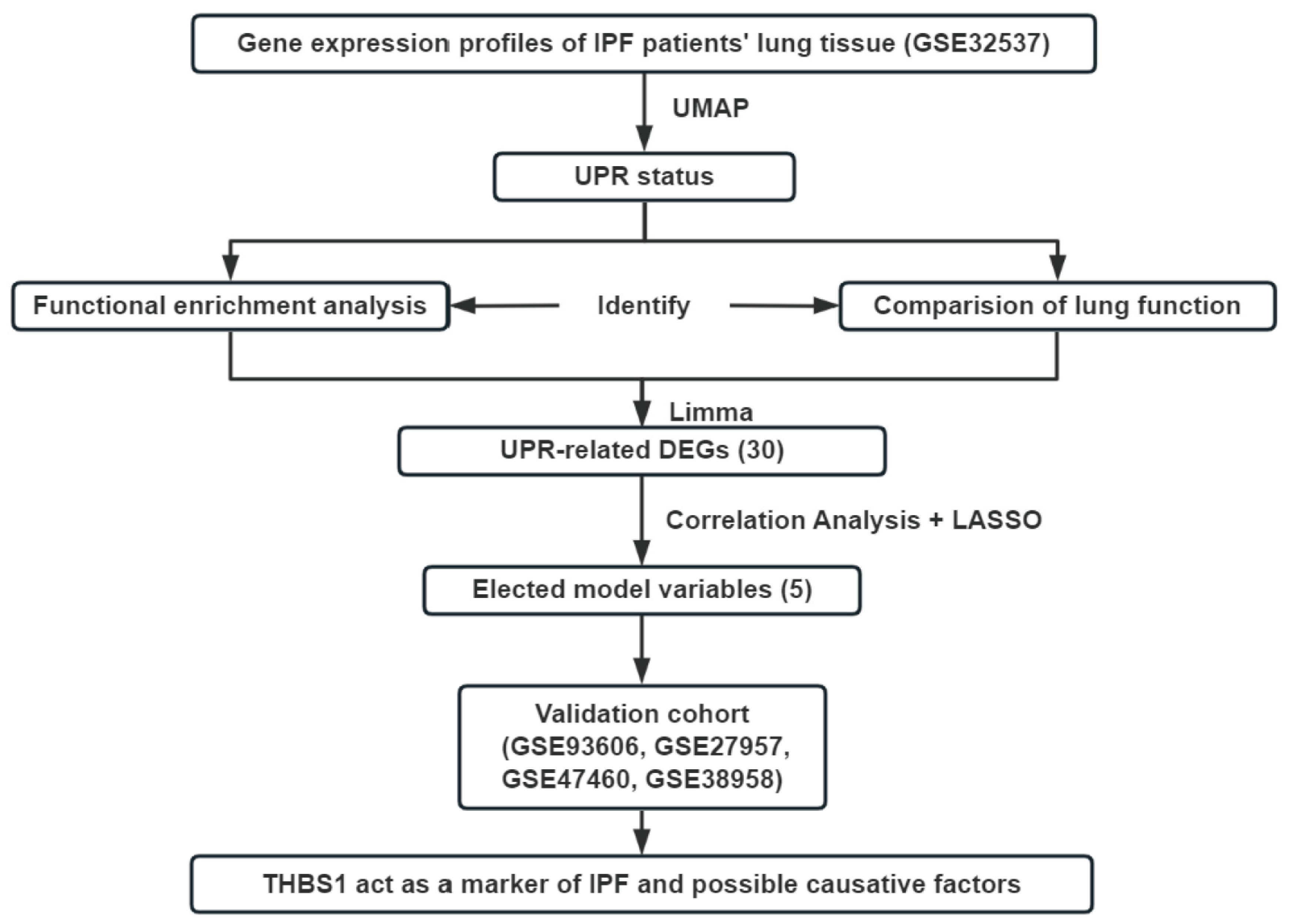
2.4. The Analysis of Publicly Available Gene Expression Data and High Throughput Sequencing Data
2.5. The Identification of UPR Status and DEGs
2.6. Correlation Analysis and LASSO Regression
2.7. Functional Analysis and Survival Analysis
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. Cell Culture and Stable Transfection
2.10. Immunofluorescence and Immunohistochemistry Staining
2.11. Western Blot
2.12. Statistical Analysis
3. Results
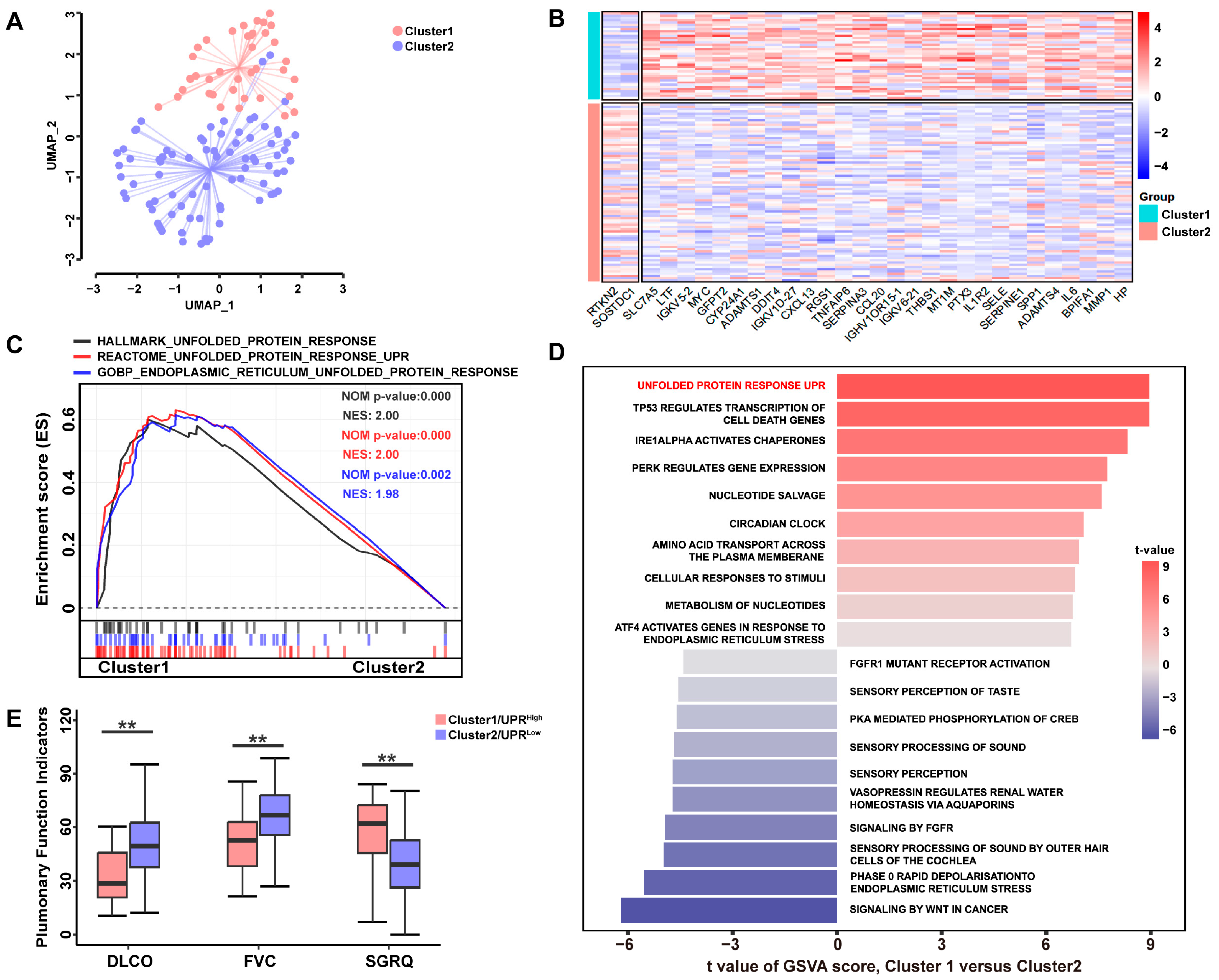
3.1. A High UPR Status Is Associated with Poorer Pulmonary Function in Patients with IPF
3.2. UPR-Related Gene Signature Predicts the Pro-Fibrotic Effects of TSP-1
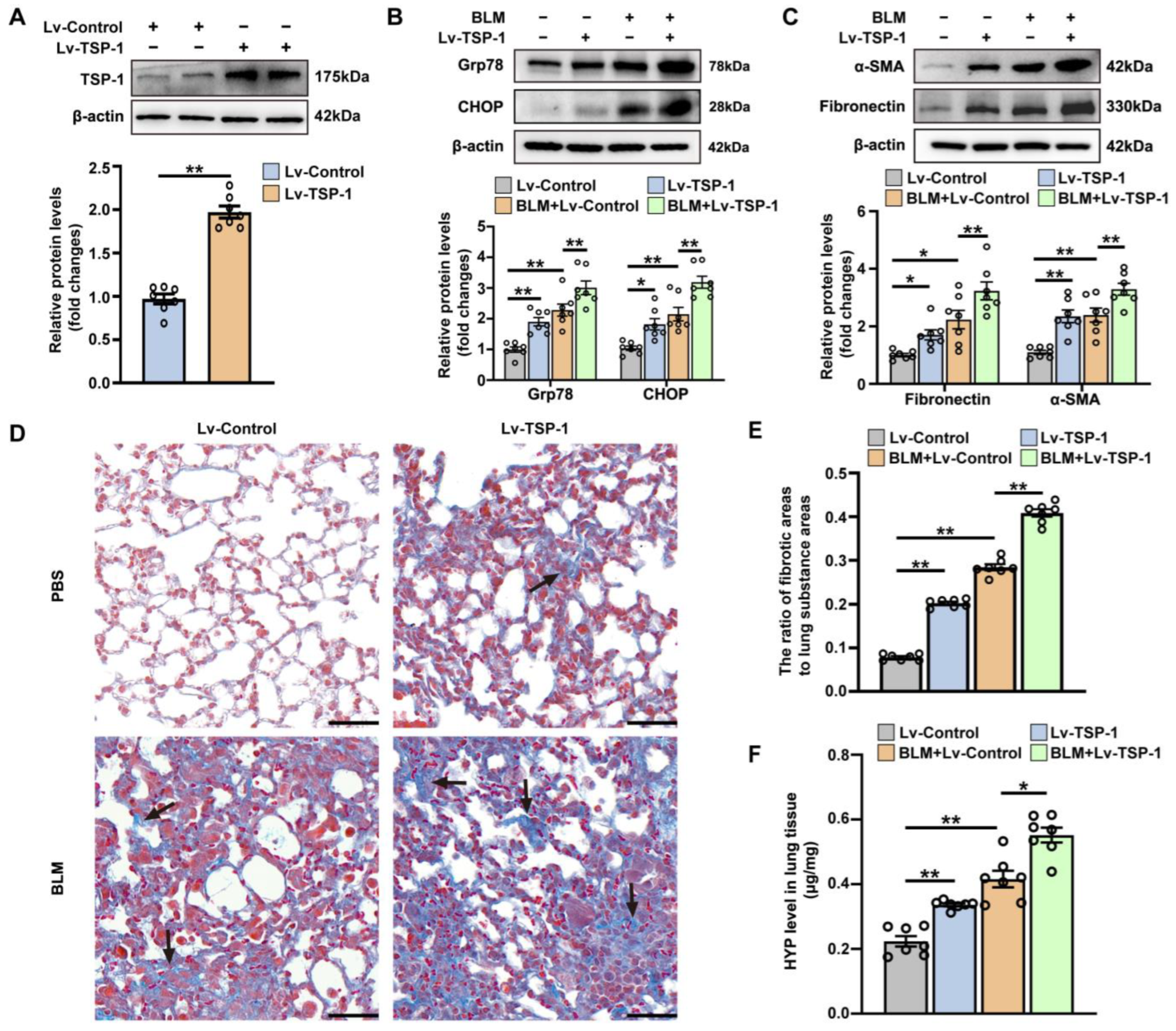
3.3. The Effect of Intrapulmonary TSP1 Overexpression on the Development of ER Stress and Pulmonary Fibrosis in Mice
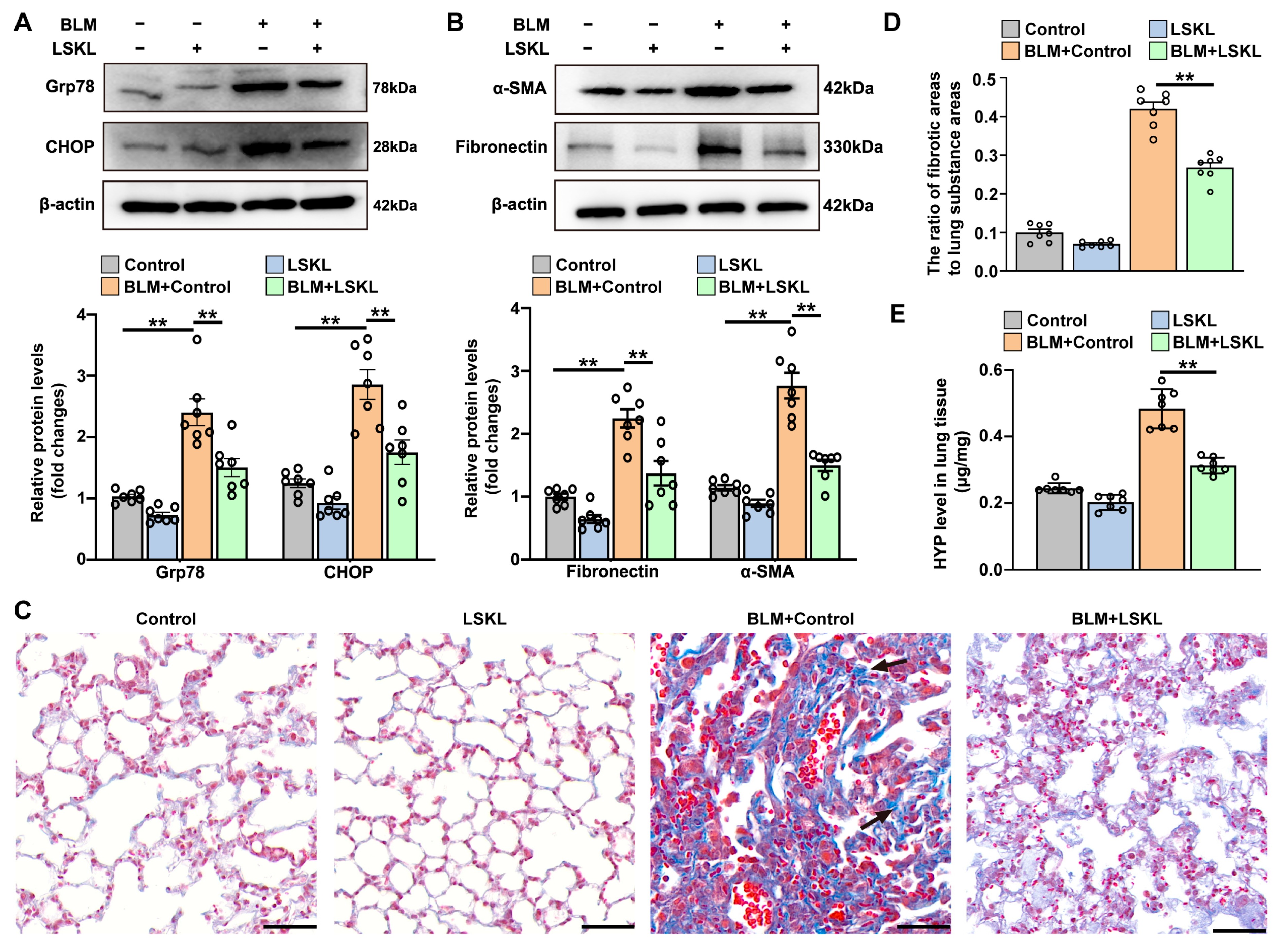
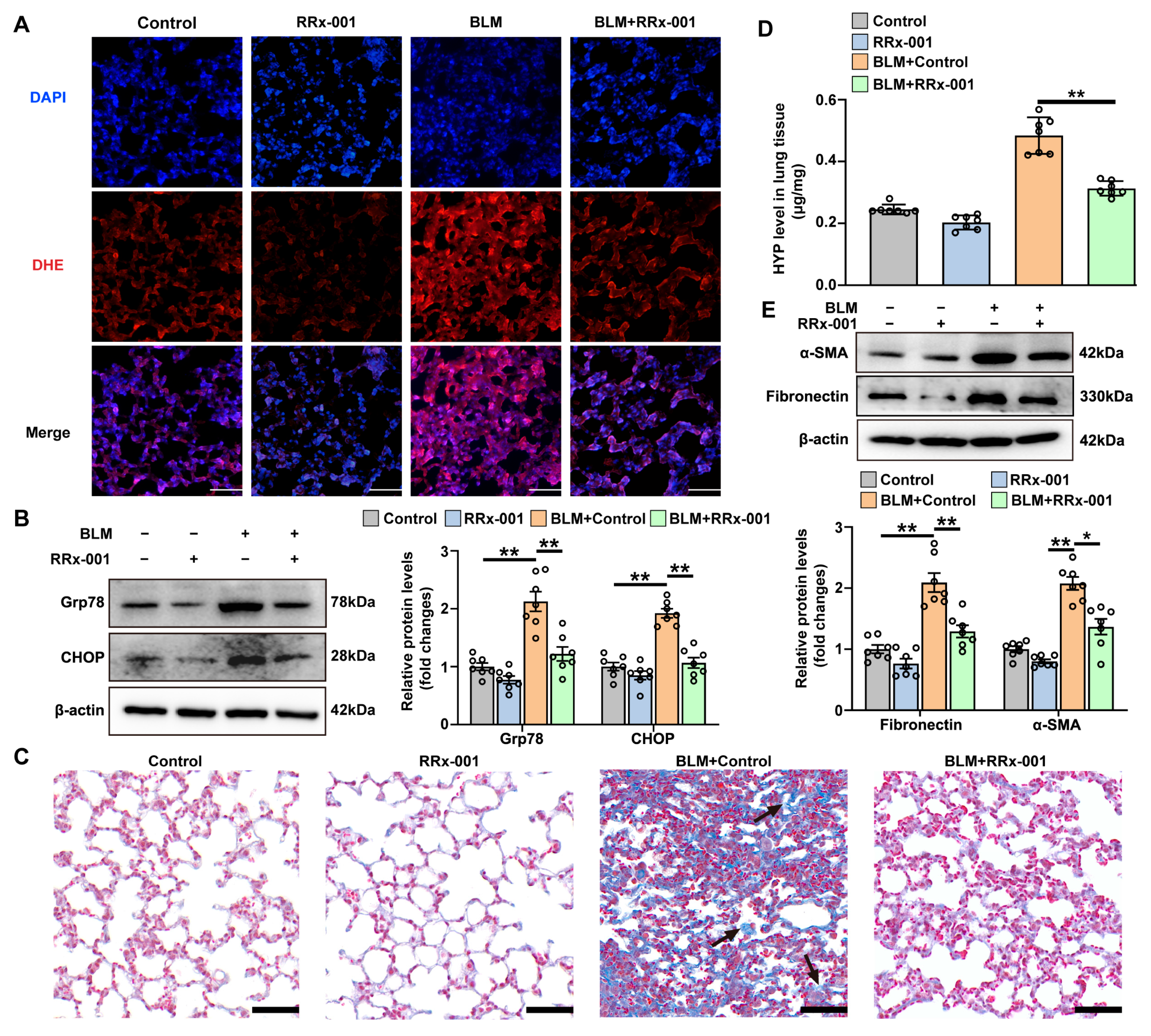
3.4. TSP-1 Inhibitor Attenuates Bleomycin-Induced ER Stress and Pulmonary Fibrosis
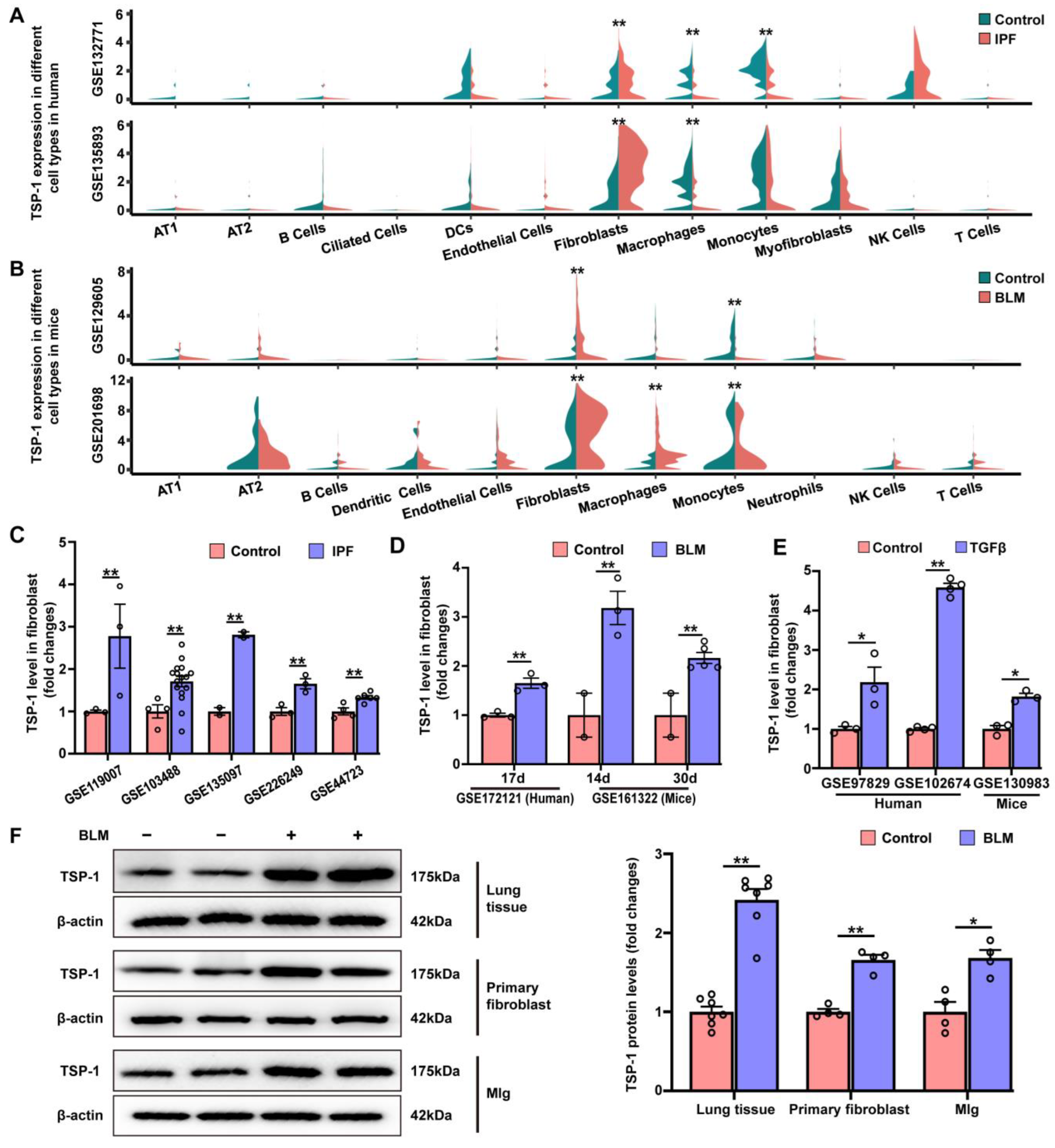
3.5. TSP-1 Is Upregulated in Lung Fibroblasts during Pulmonary Fibrosis
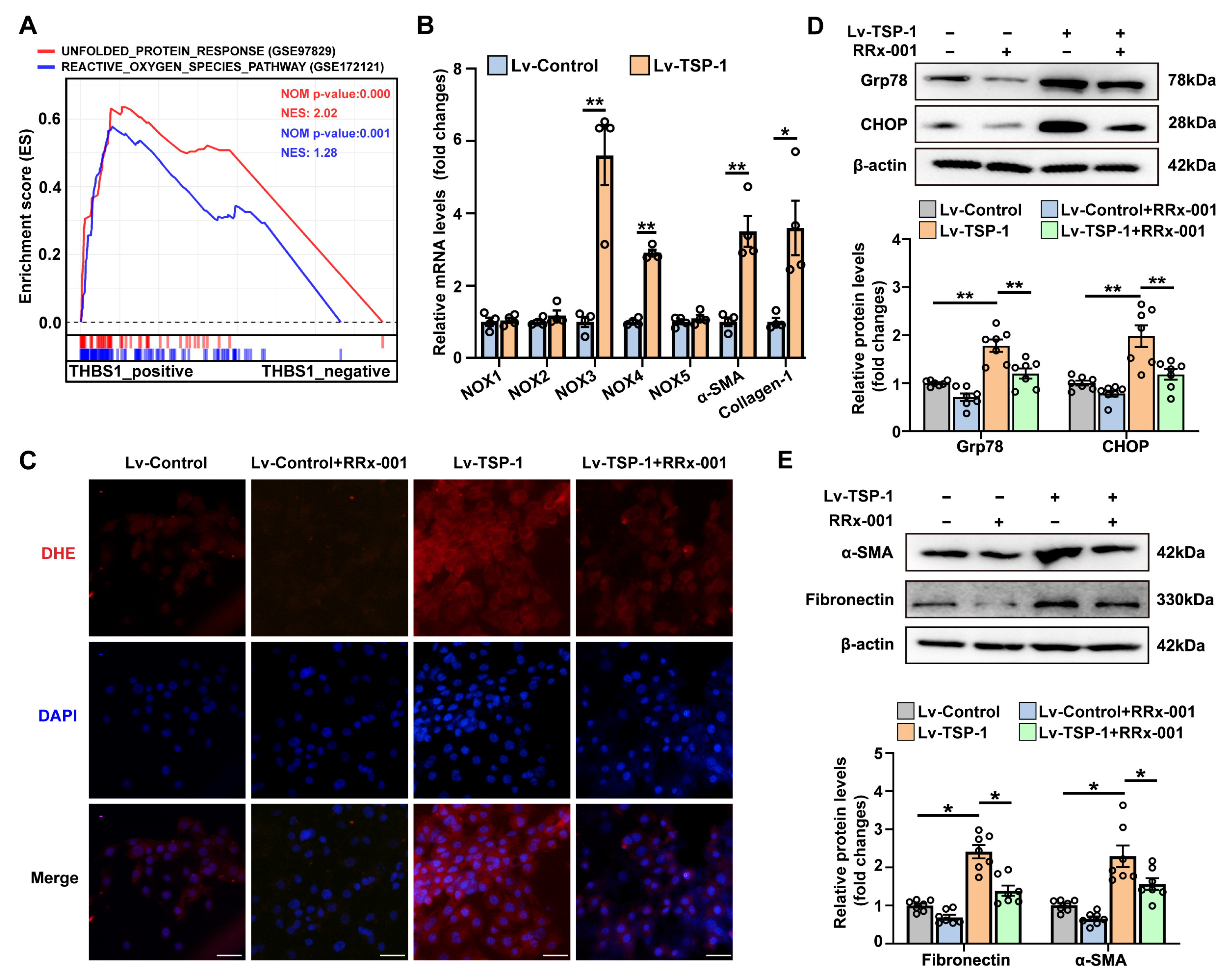
3.6. Stable Overexpression of TSP-1 Promotes Fibroblast Activation by CD47/ROS/ER Stress Signaling Pathway
3.7. CD47 Inhibitor Attenuates BLM-Induced ROS Production, ER Stress, and Pulmonary Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Hult, E.M.; Gurczynski, S.J.; O’Dwyer, D.N.; Zemans, R.L.; Rasky, A.; Wang, Y.; Murray, S.; Crawford, H.C.; Moore, B.B. Myeloid- and Epithelial-derived Heparin-Binding Epidermal Growth Factor-like Growth Factor Promotes Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 641–653. [Google Scholar] [CrossRef]
- Liang, J.; Huang, G.; Liu, X.; Liu, N.; Taghavifar, F.; Dai, K.; Yao, C.; Deng, N.; Wang, Y.; Chen, P.; et al. Reciprocal interactions between alveolar progenitor dysfunction and aging promote lung fibrosis. Elife 2023, 12, e85415. [Google Scholar] [CrossRef]
- Pitre, T.; Mah, J.; Helmeczi, W.; Khalid, M.F.; Cui, S.; Zhang, M.; Husnudinov, R.; Su, J.; Banfield, L.; Guy, B.; et al. Medical treatments for idiopathic pulmonary fibrosis: A systematic review and network meta-analysis. Thorax 2022, 77, 1243–1250. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Bueno, M.; Brands, J.; Voltz, L.; Fiedler, K.; Mays, B.; St, C.C.; Sembrat, J.; Mallampalli, R.K.; Rojas, M.; Mora, A.L. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell 2018, 17, e12720. [Google Scholar] [CrossRef]
- Baek, H.A.; Kim, D.S.; Park, H.S.; Jang, K.Y.; Kang, M.J.; Lee, D.G.; Moon, W.S.; Chae, H.J.; Chung, M.J. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 731–739. [Google Scholar] [CrossRef]
- Fu, L.; Zhao, H.; Xiang, Y.; Xiang, H.X.; Hu, B.; Tan, Z.X.; Lu, X.; Gao, L.; Wang, B.; Wang, H.; et al. Reactive oxygen species-evoked endoplasmic reticulum stress mediates 1-nitropyrene-induced epithelial-mesenchymal transition and pulmonary fibrosis. Environ. Pollut. 2021, 283, 117134. [Google Scholar] [CrossRef]
- Kaneshige, A.; Kaji, T.; Zhang, L.; Saito, H.; Nakamura, A.; Kurosawa, T.; Ikemoto-Uezumi, M.; Tsujikawa, K.; Seno, S.; Hori, M.; et al. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell 2022, 29, 265–280.e6. [Google Scholar] [CrossRef]
- Vanhoutte, D.; Schips, T.G.; Vo, A.; Grimes, K.M.; Baldwin, T.A.; Brody, M.J.; Accornero, F.; Sargent, M.A.; Molkentin, J.D. Thbs1 induces lethal cardiac atrophy through PERK-ATF4 regulated autophagy. Nat. Commun. 2021, 12, 3928. [Google Scholar] [CrossRef]
- Sun, J.; Ge, X.; Wang, Y.; Niu, L.; Tang, L.; Pan, S. USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol. Res. 2022, 176, 105962. [Google Scholar] [CrossRef]
- Wahab, N.A.; Schaefer, L.; Weston, B.S.; Yiannikouris, O.; Wright, A.; Babelova, A.; Schaefer, R.; Mason, R.M. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia 2005, 48, 2650–2660. [Google Scholar] [CrossRef]
- Maloney, J.P.; Stearman, R.S.; Bull, T.M.; Calabrese, D.W.; Tripp-Addison, M.L.; Wick, M.J.; Broeckel, U.; Robbins, I.M.; Wheeler, L.A.; Cogan, J.D.; et al. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L541–L554. [Google Scholar] [CrossRef]
- Kumar, R.; Mickael, C.; Kassa, B.; Sanders, L.; Hernandez-Saavedra, D.; Koyanagi, D.E.; Kumar, S.; Pugliese, S.C.; Thomas, S.; McClendon, J.; et al. Interstitial macrophage-derived thrombospondin-1 contributes to hypoxia-induced pulmonary hypertension. Cardiovasc. Res. 2020, 116, 2021–2030. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, Z.; Lechner, E.J.; Klenotic, P.A.; Hamburg, B.J.; Hulver, M.; Khare, A.; Oriss, T.; Mangalmurti, N.; Chan, Y.; et al. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014, 7, 440–448. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Olonisakin, T.F.; Bain, W.G.; Qu, Y.; van der Geest, R.; Zupetic, J.; Hulver, M.; Xiong, Z.; Newstead, M.W.; Zou, C.; et al. Thrombospondin-1 Restricts Interleukin-36γ-Mediated Neutrophilic Inflammation during Pseudomonas aeruginosa Pulmonary Infection. Mbio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Qu, Y.; Olonisakin, T.; Bain, W.; Zupetic, J.; Brown, R.; Hulver, M.; Xiong, Z.; Tejero, J.; Shanks, R.M.; Bomberger, J.M.; et al. Thrombospondin-1 protects against pathogen-induced lung injury by limiting extracellular matrix proteolysis. JCI Insight 2018, 3, e96914. [Google Scholar] [CrossRef]
- Li, S.R.; Tan, Z.X.; Chen, Y.H.; Hu, B.; Zhang, C.; Wang, H.; Zhao, H.; Xu, D.X. Vitamin D deficiency exacerbates bleomycin-induced pulmonary fibrosis partially through aggravating TGF-β/Smad2/3-mediated epithelial-mesenchymal transition. Respir. Res. 2019, 20, 266. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.W.; Jose, R.J.; Biros, E.; Moran, C.S.; Wang, Y.; Clancy, P.; Golledge, J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 389–398. [Google Scholar] [CrossRef]
- Niu, S.; Cheng, K.; Jia, L.; Liang, J.; Mu, L.; Wang, Y.; Yang, X.; Yang, C.; Zhang, Y.; Wang, C.; et al. Lineage tracing of mutant granulosa cells reveals in vivo protective mechanisms that prevent granulosa cell tumorigenesis. Cell Death Differ. 2023, 30, 1235–1246. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Lin, B.; Chen, Y.; Deng, X.; Jiang, W.; Zhou, R. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell. Mol. Immunol. 2021, 18, 1425–1436. [Google Scholar] [CrossRef]
- Wei, J.; Zhan, J.; Ji, H.; Xu, Y.; Xu, Q.; Zhu, X.; Liu, Y. Fibroblast Upregulation of Vitamin D Receptor Represents a Self-Protective Response to Limit Fibroblast Proliferation and Activation during Pulmonary Fibrosis. Antioxidants 2023, 12, 1634. [Google Scholar] [CrossRef]
- Guo, L.; Li, S.; Zhao, Y.; Qian, P.; Ji, F.; Qian, L.; Wu, X.; Qian, G. Silencing Angiopoietin-Like Protein 4 (ANGPTL4) Protects Against Lipopolysaccharide-Induced Acute Lung Injury Via Regulating SIRT1 /NF-kB Pathway. J. Cell. Physiol. 2015, 230, 2390–2402. [Google Scholar] [CrossRef]
- Du, J.K.; Yu, Q.; Liu, Y.J.; Du, S.F.; Huang, L.Y.; Xu, D.H.; Ni, X.; Zhu, X.Y. A novel role of kallikrein-related peptidase 8 in the pathogenesis of diabetic cardiac fibrosis. Theranostics 2021, 11, 4207–4231. [Google Scholar] [CrossRef]
- Du, S.F.; Wang, X.L.; Ye, C.L.; He, Z.J.; Li, D.X.; Du, B.R.; Liu, Y.J.; Zhu, X.Y. Exercise training ameliorates bleomycin-induced epithelial mesenchymal transition and lung fibrosis through restoration of H(2) S synthesis. Acta Physiol. 2019, 225, e13177. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Xu, L.; Zhao, P.; Deng, Z.; Mo, X.; Li, P.; Qi, L.; Li, J.; Gao, J. Total extract of Yupingfeng attenuates bleomycin-induced pulmonary fibrosis in rats. Phytomedicine 2015, 22, 111–119. [Google Scholar] [CrossRef]
- Yang, I.V.; Coldren, C.D.; Leach, S.M.; Seibold, M.A.; Murphy, E.; Lin, J.; Rosen, R.; Neidermyer, A.J.; McKean, D.F.; Groshong, S.D.; et al. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 2013, 68, 1114–1121. [Google Scholar] [CrossRef]
- Herazo-Maya, J.D.; Noth, I.; Duncan, S.R.; Kim, S.; Ma, S.F.; Tseng, G.C.; Feingold, E.; Juan-Guardela, B.M.; Richards, T.J.; Lussier, Y.; et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2013, 5, 205ra136. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Willis-Owen, S.; Cox, M.J.; James, P.; Cowman, S.; Loebinger, M.; Blanchard, A.; Edwards, L.M.; Stock, C.; Daccord, C.; et al. Host-Microbial Interactions in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 1640–1650. [Google Scholar] [CrossRef]
- Huang, L.S.; Mathew, B.; Li, H.; Zhao, Y.; Ma, S.F.; Noth, I.; Reddy, S.P.; Harijith, A.; Usatyuk, P.V.; Berdyshev, E.V.; et al. The mitochondrial cardiolipin remodeling enzyme lysocardiolipin acyltransferase is a novel target in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2014, 189, 1402–1415. [Google Scholar] [CrossRef]
- Anathy, V.; Lahue, K.G.; Chapman, D.G.; Chia, S.B.; Casey, D.T.; Aboushousha, R.; van der Velden, J.; Elko, E.; Hoffman, S.M.; McMillan, D.H.; et al. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018, 24, 1128–1135. [Google Scholar] [CrossRef]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.I.; Taylor, C.J.; Jetter, C.; et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef]
- Tsukui, T.; Sun, K.H.; Wetter, J.B.; Wilson-Kanamori, J.R.; Hazelwood, L.A.; Henderson, N.C.; Adams, T.S.; Schupp, J.C.; Poli, S.D.; Rosas, I.O.; et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Peyser, R.; MacDonnell, S.; Gao, Y.; Cheng, L.; Kim, Y.; Kaplan, T.; Ruan, Q.; Wei, Y.; Ni, M.; Adler, C.; et al. Defining the Activated Fibroblast Population in Lung Fibrosis Using Single-Cell Sequencing. Am. J. Respir. Cell Mol. Biol. 2019, 61, 74–85. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sheng, W.; Michkov, A.; Sriarm, K.; Sun, R.; Dvorkin-Gheva, A.; Insel, P.A.; Janssen, L.J. Prostaglandin E(2) inhibits profibrotic function of human pulmonary fibroblasts by disrupting Ca(2+) signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L810–L821. [Google Scholar] [CrossRef]
- Habiel, D.M.; Camelo, A.; Espindola, M.; Burwell, T.; Hanna, R.; Miranda, E.; Carruthers, A.; Bell, M.; Coelho, A.L.; Liu, H.; et al. Divergent roles for Clusterin in Lung Injury and Repair. Sci. Rep. 2017, 7, 15444. [Google Scholar] [CrossRef]
- Negreros, M.; Hagood, J.S.; Espinoza, C.R.; Balderas-Martínez, Y.I.; Selman, M.; Pardo, A. Transforming growth factor beta 1 induces methylation changes in lung fibroblasts. PLoS ONE 2019, 14, e0223512. [Google Scholar] [CrossRef]
- Ma, H.Y.; Vander, H.J.; Uttarwar, S.; Xi, Y.; N’Diaye, E.N.; LaCanna, R.; Caplazi, P.; Gierke, S.; Moffat, J.; Wolters, P.J.; et al. Inhibition of MRTF activation as a clinically achievable anti-fibrotic mechanism for pirfenidone. Eur. Respir. J. 2023, 61, 2200604. [Google Scholar] [CrossRef]
- Peng, R.; Sridhar, S.; Tyagi, G.; Phillips, J.E.; Garrido, R.; Harris, P.; Burns, L.; Renteria, L.; Woods, J.; Chen, L.; et al. Bleomycin induces molecular changes directly relevant to idiopathic pulmonary fibrosis: A model for “active” disease. PLoS ONE 2013, 8, e59348. [Google Scholar] [CrossRef]
- Suezawa, T.; Kanagaki, S.; Moriguchi, K.; Masui, A.; Nakao, K.; Toyomoto, M.; Tamai, K.; Mikawa, R.; Hirai, T.; Murakami, K.; et al. Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 2021, 16, 2973–2987. [Google Scholar] [CrossRef]
- Tan, Q.; Link, P.A.; Meridew, J.A.; Pham, T.X.; Caporarello, N.; Ligresti, G.; Tschumperlin, D.J. Spontaneous Lung Fibrosis Resolution Reveals Novel Antifibrotic Regulators. Am. J. Respir. Cell Mol. Biol. 2021, 64, 453–464. [Google Scholar] [CrossRef]
- Savary, G.; Dewaeles, E.; Diazzi, S.; Buscot, M.; Nottet, N.; Fassy, J.; Courcot, E.; Henaoui, I.S.; Lemaire, J.; Martis, N.; et al. The Long Noncoding RNA DNM3OS Is a Reservoir of FibromiRs with Major Functions in Lung Fibroblast Response to TGF-β and Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 184–198. [Google Scholar] [CrossRef]
- Selvarajah, B.; Azuelos, I.; Platé, M.; Guillotin, D.; Forty, E.J.; Contento, G.; Woodcock, H.V.; Redding, M.; Taylor, A.; Brunori, G.; et al. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-β(1)-induced collagen biosynthesis. Sci. Signal. 2019, 12, eaav3048. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.W.; Ko, N.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef]
- Li, X.; Cai, H.; Cai, Y.; Zhang, Q.; Ding, Y.; Zhuang, Q. Investigation of a Hypoxia-Immune-Related Microenvironment Gene Signature and Prediction Model for Idiopathic Pulmonary Fibrosis. Front. Immunol. 2021, 12, 629854. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Tu, J.; Wan, J.; Zhang, J.; Wu, B.; Chen, S.; Zhou, J.; Mu, Y.; Wang, L. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat. Commun. 2014, 5, 5627. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Sun, S.; Xin, Q.; Liu, S.; Mu, X.; Yuan, X.; Chen, K.; Wang, H.; Varga, K.; et al. Catalytically potent and selective clusterzymes for modulation of neuroinflammation through single-atom substitutions. Nat. Commun. 2021, 12, 114. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, K.; Xu, S.; Garcia, J.; Wang, C.; Cai, H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox. Biol. 2020, 36, 101638. [Google Scholar] [CrossRef]
- Meijles, D.N.; Sahoo, S.; Al, G.I.; Amaral, J.H.; Bienes-Martinez, R.; Knupp, H.E.; Attaran, S.; Sembrat, J.C.; Nouraie, S.M.; Rojas, M.M.; et al. The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1. Sci. Signal. 2017, 10, eaaj1784. [Google Scholar] [CrossRef]
- Sharma, P.; Alizadeh, J.; Juarez, M.; Samali, A.; Halayko, A.J.; Kenyon, N.J.; Ghavami, S.; Zeki, A.A. Autophagy, Apoptosis, the Unfolded Protein Response, and Lung Function in Idiopathic Pulmonary Fibrosis. Cells 2021, 10, 1642. [Google Scholar] [CrossRef]
- Ide, M.; Ishii, H.; Mukae, H.; Iwata, A.; Sakamoto, N.; Kadota, J.; Kohno, S. High serum levels of thrombospondin-1 in patients with idiopathic interstitial pneumonia. Respir. Med. 2008, 102, 1625–1630. [Google Scholar] [CrossRef]
- Todd, J.L.; Neely, M.L.; Overton, R.; Durham, K.; Gulati, M.; Huang, H.; Roman, J.; Newby, L.K.; Flaherty, K.R.; Vinisko, R.; et al. Peripheral blood proteomic profiling of idiopathic pulmonary fibrosis biomarkers in the multicentre IPF-PRO Registry. Respir. Res. 2019, 20, 227. [Google Scholar] [CrossRef]
- Smadja, D.M.; Nunes, H.; Juvin, K.; Bertil, S.; Valeyre, D.; Gaussem, P.; Israel-Biet, D. Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol. Biol. 2014, 62, 391–394. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, W.L.; Liu, J.; Li, W.B.; Bai, L.L.; Yuan, Y.D.; Song, S.X. Plasminogen activator inhibitor-1 promotes the proliferation and inhibits the apoptosis of pulmonary fibroblasts by Ca(2+) signaling. Thromb. Res. 2013, 131, 64–71. [Google Scholar] [CrossRef]
- Shioya, S.; Masuda, T.; Senoo, T.; Horimasu, Y.; Miyamoto, S.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Hattori, N. Plasminogen activator inhibitor-1 serves an important role in radiation-induced pulmonary fibrosis. Exp. Ther. Med. 2018, 16, 3070–3076. [Google Scholar] [CrossRef]
- Chi, J.Y.; Hsiao, Y.W.; Liang, H.Y.; Huang, T.H.; Chen, F.W.; Chen, C.Y.; Ko, C.Y.; Cheng, C.C.; Wang, J.M. Blockade of the pentraxin 3/CD44 interaction attenuates lung injury-induced fibrosis. Clin. Transl. Med. 2022, 12, e1099. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, B.; Suo, Y.; Liu, C.; Yang, Q.; Zhang, K.; Yuan, M.; Yuan, M.; Zhang, Y.; Li, G. Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis. Elife 2020, 9, e49923. [Google Scholar] [CrossRef]
- Walsh, S.M.; Worrell, J.C.; Fabre, A.; Hinz, B.; Kane, R.; Keane, M.P. Novel differences in gene expression and functional capabilities of myofibroblast populations in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L697–L710. [Google Scholar] [CrossRef]
- Venkatraman, L.; Tucker-Kellogg, L. The CD47-binding peptide of thrombospondin-1 induces defenestration of liver sinusoidal endothelial cells. Liver Int. 2013, 33, 1386–1397. [Google Scholar] [CrossRef]
- Ruschkowski, B.A.; Esmaeil, Y.; Daniel, K.; Gaudet, C.; Yeganeh, B.; Grynspan, D.; Jankov, R.P. Thrombospondin-1 Plays a Major Pathogenic Role in Experimental and Human Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2022, 205, 685–699. [Google Scholar] [CrossRef]
- Ezzie, M.E.; Piper, M.G.; Montague, C.; Newland, C.A.; Opalek, J.M.; Baran, C.; Ali, N.; Brigstock, D.; Lawler, J.; Marsh, C.B. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 44, 556–561. [Google Scholar] [CrossRef]
- Tabary, M.; Gheware, A.; Peñaloza, H.F.; Lee, J.S. The matricellular protein thrombospondin-1 in lung inflammation and injury. Am. J. Physiol. Cell Physiol. 2022, 323, C857–C865. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.-H.; Wei, J.; Liu, L.; Xu, Y.-T.; Ji, H.; Wang, C.-N.; Liu, Y.-J.; Zhu, X.-Y. Investigation of a UPR-Related Gene Signature Identifies the Pro-Fibrotic Effects of Thrombospondin-1 by Activating CD47/ROS/Endoplasmic Reticulum Stress Pathway in Lung Fibroblasts. Antioxidants 2023, 12, 2024. https://doi.org/10.3390/antiox12122024
Zhan J-H, Wei J, Liu L, Xu Y-T, Ji H, Wang C-N, Liu Y-J, Zhu X-Y. Investigation of a UPR-Related Gene Signature Identifies the Pro-Fibrotic Effects of Thrombospondin-1 by Activating CD47/ROS/Endoplasmic Reticulum Stress Pathway in Lung Fibroblasts. Antioxidants. 2023; 12(12):2024. https://doi.org/10.3390/antiox12122024
Chicago/Turabian StyleZhan, Jun-Hui, Juan Wei, Lin Liu, Yi-Tong Xu, Hui Ji, Chang-Nan Wang, Yu-Jian Liu, and Xiao-Yan Zhu. 2023. "Investigation of a UPR-Related Gene Signature Identifies the Pro-Fibrotic Effects of Thrombospondin-1 by Activating CD47/ROS/Endoplasmic Reticulum Stress Pathway in Lung Fibroblasts" Antioxidants 12, no. 12: 2024. https://doi.org/10.3390/antiox12122024




