Novel Fluorometric Assay of Antiglycation Activity Based on Methylglyoxal-Induced Protein Carbonylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
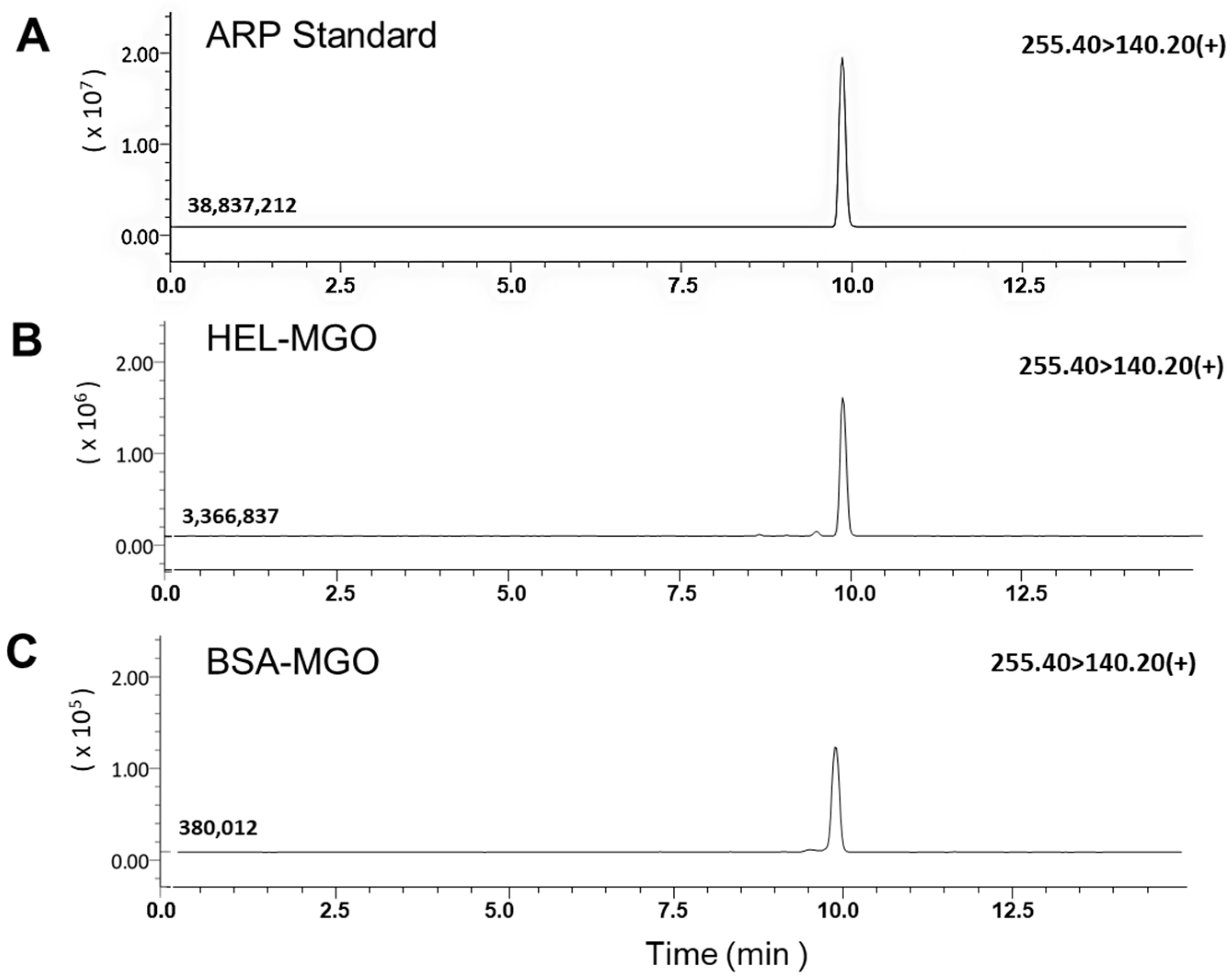
2.2. Identification of Argpyrimidine by LC–MS/MS
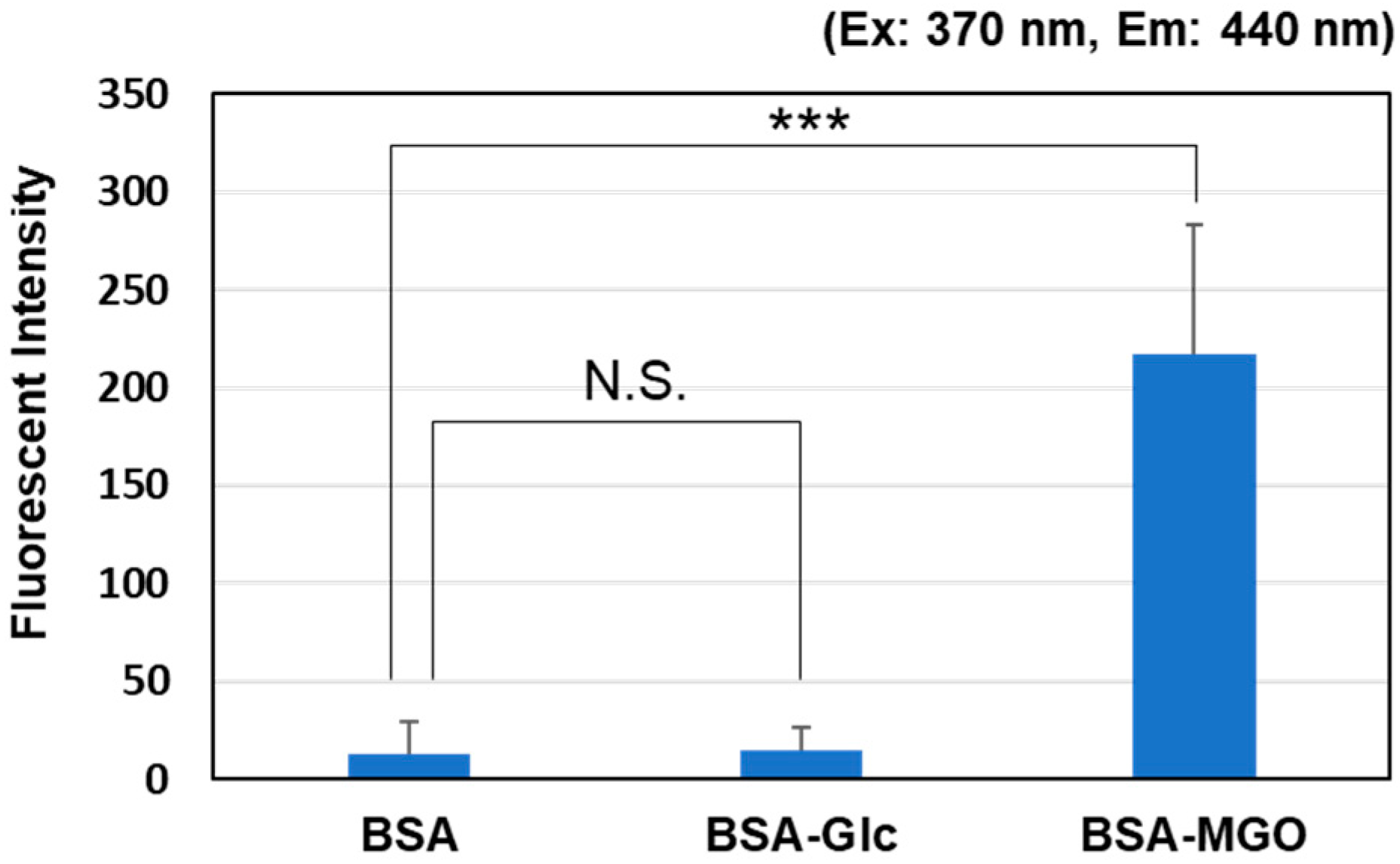
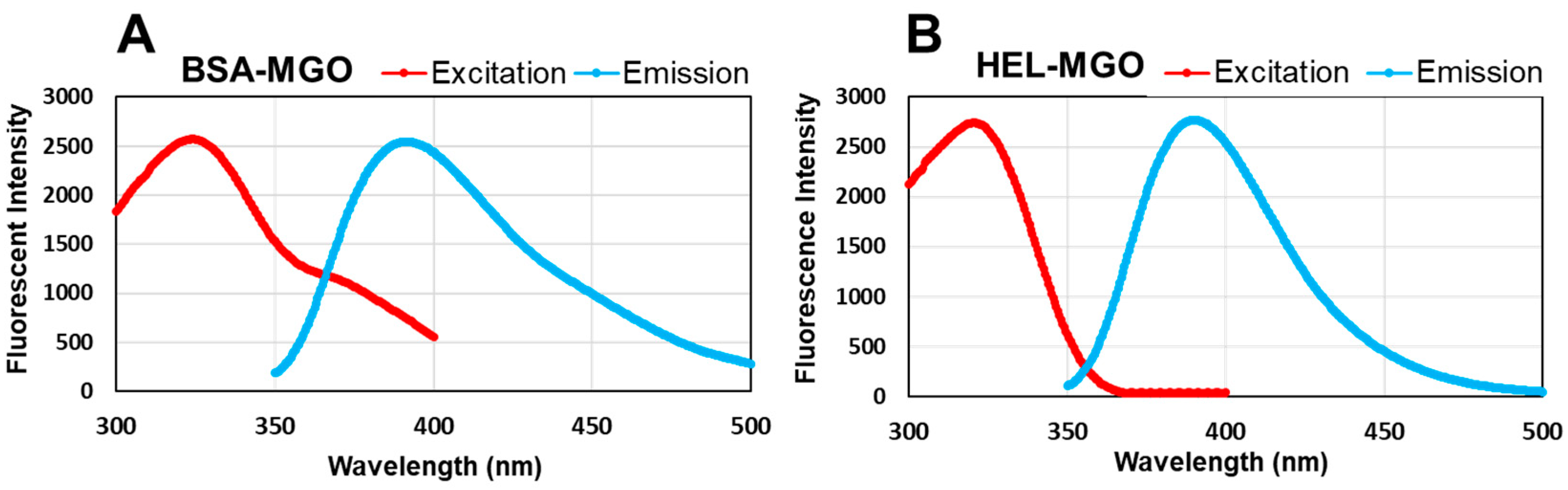
2.3. Detection of Intrinsic Fluorescent AGEs by Fluorescence Spectroscopy
2.4. Western Blotting Analysis
2.5. BSA–Glucose Assay
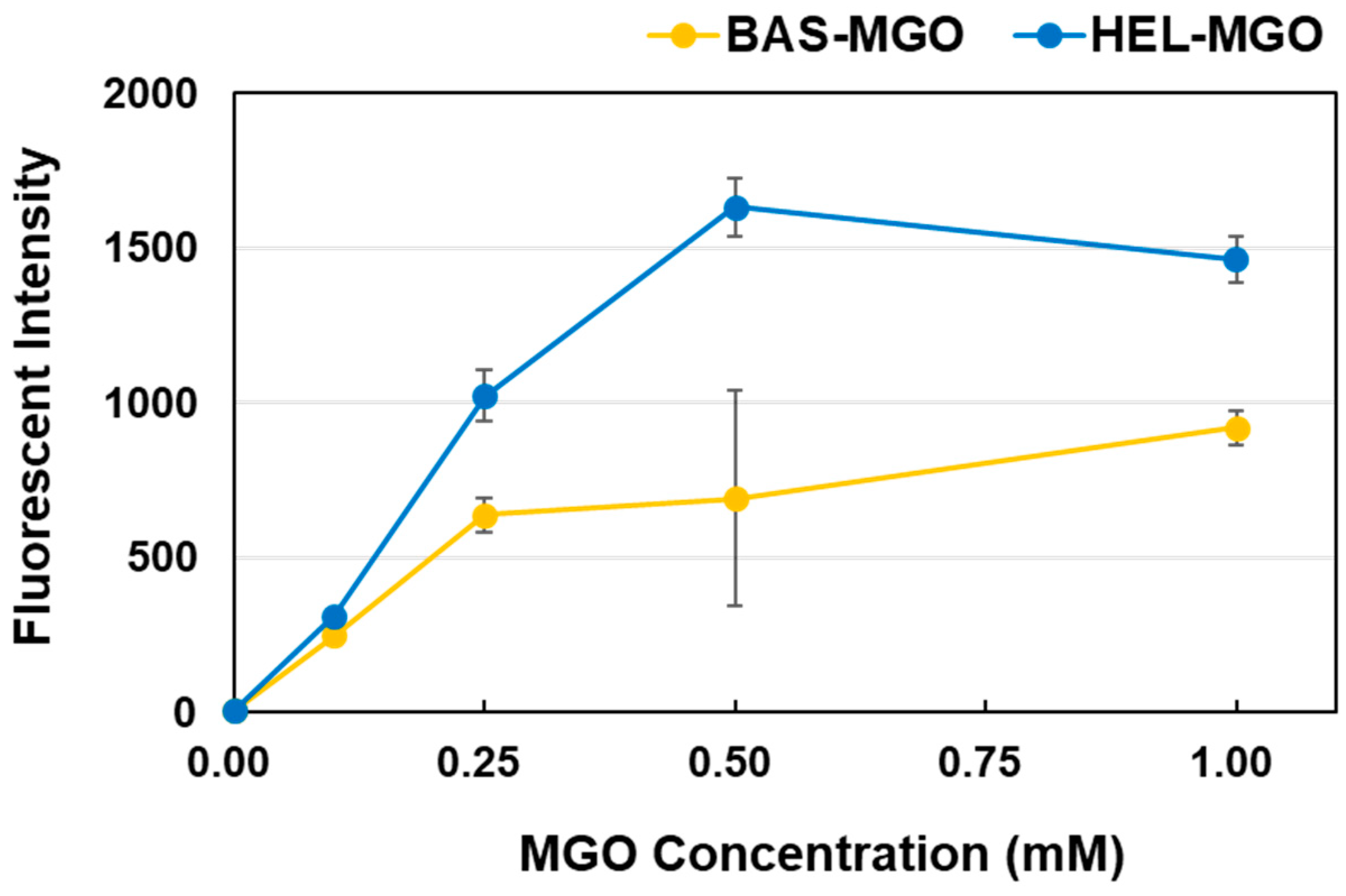
2.6. HEL–Methylglyoxal (MGO) Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glycation research in amino acids: A place to call home. Amino Acids 2012, 42, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Ashour, A.; Thornalley, P.J. Mass spectrometric determination of early and advanced glycation in biology. Gly-coconj. J. 2016, 33, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.; Alvi, S.S.; Khan, R.H.; Ahmad, S.; Ahmad, S.; Khan, M.S. Antiglycation study of HMG-R inhibitors and tocotrienol against glycated BSA and LDL: A comparative study. Int. J. Biol. Macromol. 2018, 116, 983–992. [Google Scholar] [CrossRef]
- Nabi, R.; Alvi, S.S.; Shah, M.S. A biochemical & biophysical study on in-vitro anti-glycating potential of iridin against D-Ribose modified BSA. Arch. Biochem. Biophys. 2020, 686, 108373. [Google Scholar]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Schmitt, A.; Schmitt, J.; Münch, G.; Gasic-Milencovic, J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal. Biochem. 2005, 338, 201–215. [Google Scholar] [CrossRef]
- Fraisse, D.; Bred, A.; Felgines, C.; Senejoux, F. Screening and Characterization of Antiglycoxidant Anthocyanins from Vaccinium myrtillus Fruit Using DPPH and Methylglyoxal Pre-Column HPLC Assays. Antioxidants 2020, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Cavaille, J.P.; Graziani, F.; Robin, M.; Ouari, O.; Pietri, S.; Stocker, P. High throughput assay for evaluation of reactive carbonyl scavenging capacity. Redox Biol. 2014, 2, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.C.; Pyke, S.M. Hydralazine inhibits rapid acrolein-induced protein oligomerization: Role of aldehydes scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006, 69, 1056–1065. [Google Scholar] [CrossRef]
- Galvanic, S.; Coatrieux, C.; Elbaz, M.; Grazide, M.H.; Thiers, J.C.; Parini, A.; Uchida, K.; Kamar, N.; Rostaing, L.; Baltas, M.; et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 2008, 45, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef]
- Sekowski, S.; Olchowik-Grabarek, E.; Dubis, A.T.; Sharan, L.; Kumar, A.; Abdulladjanova, N.; Markiewicz, P.; Zamaraeva, M. Inhibition of AGEs formation, antioxidative, and cytoprotective activity of Sumac (Rhus typhina L.) tannin under hyperglycemia: Molecular and cellular study. Mol. Cell. Biochem. 2023, 478, 443–457. [Google Scholar] [CrossRef]
- Shoda, H.; Miyata, S.; Liu, B.F.; Yamada, H.; Ohara, T.; Suzuki, K.; Oimomi, M.; Kasuga, M. Inhibitory effects of tenilsetam on the Maillard reaction. Endocrinology 1997, 138, 1886–1892. [Google Scholar] [CrossRef]
- Onorato, J.M.; Jenkins, A.J.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. J. Biol. Chem. 2000, 275, 21177–21184. [Google Scholar] [CrossRef]
- Ruggiero-Lopez, D.; Lecomte, M.; Moinet, G.; Patereau, G.; Lagarde, M.; Wiernsperger, N. Reaction of metformin with dicarbonyl compounds: Possible implications in the inhibition of advanced glycation end product formation. Biochem. Pharmacol. 1999, 58, 1765–1773. [Google Scholar] [CrossRef]
- Wu, J.T.; Tu, M.C.; Zhung, P. Advanced glycation end product (AGE): Characterization of the products from the reaction between D-glucose and serum albumin. J. Clin. Lab. Anal. 1996, 10, 21–34. [Google Scholar] [CrossRef]
- Pongor, S.; Ulrich, P.C.; Bencsath, F.A.; Cerami, A. Aging of proteins: Isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc. Natl. Acad. Sci. USA 1984, 812, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Shipanova, I.N.; Glomb, M.A.; Nagaraj, R.H. Protein modification by methylglyoxal: Chemical nature and synthetic mechanism of a major fluorescent adduct. Arch. Biochem. Biophys. 1997, 344, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.C.; Chellan, P.; Arnold, B.M.; Nagaraj, R.H. Chromatographic quantification of argpyrimidine, a methylglyoxal-derived product in tissue proteins: Comparison with pentosidine. Anal. Biochem. 2001, 290, 353–358. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, G.; Xiao, L.; Mitchell, A.E. Determination of advanced glycation endproducts by LC-MS/MS in raw and roasted almonds (Prunus dulcis). J. Agric. Food Chem. 2011, 59, 12037–12046. [Google Scholar] [CrossRef]
- Alhadid, A.; Bustanji, Y.; Harb, A.; Al-Hiari, Y.; Abdalla, S. Vanillic Acid Inhibited the Induced Glycation Using In Vitro and In Vivo Models. Evid. Based Complement Alternt. Med. 2022, 2022, 7119256. [Google Scholar] [CrossRef]
- Atta, A.; Shahid, M.; Kanwal, Z.; Jafri, S.A.; Riaz, M.; Xiao, H.; Abbas, M.; Egbuna, C.; Simal-Gandara, J. Inhibition of oxidative stress and advanced glycation end-product formation in purified BSA/glucose glycation system by polyphenol extracts of selected nuts from Pakistan. Food Sci. Nutr. 2023, 11, 3414–3421. [Google Scholar] [CrossRef]
- Jinnouchi, Y.; Sano, H.; Nagai, R.; Hakamata, H.; Kodama, T.; Suzuki, H.; Yoshida, M.; Ueda, S.; Horiuchi, S. Glycolaldehyde-modified low density lipoprotein leads macrophages to foam cells via the macrophage scavenger receptor. J. Biochem. 1998, 123, 1208–1217. [Google Scholar] [CrossRef]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef]
- Iwamoto, K.; Kanno, K.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Tazuma, S.; Chayama, K. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J. Gastroenterol. 2008, 43, 298–304. [Google Scholar] [CrossRef]
- Yang, C.W.; Vlassara, H.; Peten, E.P.; He, C.J.; Striker, G.E.; Striker, L.J. Advanced glycation end products up-regulate gene expression found in diabetic glomerular disease. Proc. Natl. Acad. Sci. USA 1994, 91, 9436–9440. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Meyers, S.W.; Basinger, J.B.; Green, D.T.; Shew, R.L. Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci. 1994, 55, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Uribarri, J.; Vlassara, H. Aging and glycoxidant stress. Hormones 2008, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Uruska, A.; Gandecka, A.; Araszkiewicz, A.; Zozulinska-Ziolkiewicz, D. Accumulation of advanced glycation end products in the skin is accelerated in relation to insulin resistance in people with Type 1 diabetes mellitus. Diabet. Med. 2019, 36, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Shimizu, T.; Sugawara, H.; Watanabe, H.; Nakamura, H.; Choei, H.; Sasaki, N.; Yamagishi, S.; Takeuchi, M.; Shimizu, H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J. Invest. Dermatol. 2004, 122, 461–467. [Google Scholar] [CrossRef]
- Koike, S.; Toriumi, K.; Kasahara, S.; Kibune, Y.; Ishida, Y.-I.; Dan, T.; Miyata, T.; Arai, M.; Ogasawara, Y. Accumulation of Carbonyl Proteins in the Brain of Mouse Model for Methylglyoxal Detoxification Deficits. Antioxidants 2021, 10, 574. [Google Scholar] [CrossRef]
- Kimzey, M.J.; Kinsky, O.R.; Yassine, H.N.; Tsaprailis, G.; Stump, C.S.; Monks, T.J.; Lau, S.S. Site specific modification of the human plasma proteome by methylglyoxal. Toxicol. Appl. Pharmacol. 2015, 289, 155–162. [Google Scholar] [CrossRef]
- Zhao, D.; Le, T.T.; Larsen, L.B.; Li, L.; Qin, D.; Su, G.; Li, B. Effect of glycation from α-dicarbonyl compounds on the in vitro digestibility of β-casein and β-lactoglobulin: A model study with glyoxal, methylglyoxal and butanedione. Food Res. Int. 2017, 102, 313–322. [Google Scholar] [CrossRef]
- Alouffi, S.; Shahab, U.; Khan, S.; Khan, M.; Khanam, A.; Akasha, R.; Shahanawaz, S.D.; Arif, H.; Tahir, I.K.; Rehman, S.; et al. Glyoxal induced glycative insult suffered by immunoglobulin G and fibrinogen proteins: A comparative physicochemical characterization to reveal structural perturbations. Int. J. Biol. Macromol. 2022, 205, 283–296. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Pragada, R.R.; Nammi, S. Attenuation of Glucose-Induced Myoglobin Glycation and the Formation of Advanced Glycation End Products (AGEs) by (R)-α-Lipoic Acid In Vitro. Biomolecules 2018, 8, 9. [Google Scholar] [CrossRef]
- Muraoka, M.Y.; Justino, A.B.; Caixeta, D.C.; Queiroz, J.S.; Sabino-Silva, R.; Salmen Espindola, F. Fructose and methylglyoxal-induced glycation alters structural and functional properties of salivary proteins, albumin and lysozyme. PLoS ONE 2022, 17, e0262369. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakazawa, Y.; Ienaga, K. Acid-stable fluorescent advanced glycation end products: Vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem. Biophys. Res. Commun. 1997, 232, 227–230. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, J.H.; Ahn, J.; Hong, M.J.; Kim, J.; Kim, S.Y. Isosamidin from Peucedanum japonicum Roots Prevents Methylglyoxal-Induced Glucotoxicity in Human Umbilical Vein Endothelial Cells via Suppression of ROS-Mediated Bax/Bcl-2. Antioxidants 2020, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.A.; Kim, J.H.; Kim, E.H.; Bae, O.N. Methylglyoxal-Induced Dysfunction in Brain Endothelial Cells via the Suppression of Akt/HIF-1α Pathway and Activation of Mitophagy Associated with Increased Reactive Oxygen Species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chan, W.H. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol. Vitr. 2007, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- An, S.H.; Lee, M.S.; Kang, J.H. Oxidative modification of ferritin induced by methylglyoxal. BMB Rep. 2012, 45, 147–152. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Yadav, N.; Palkhede, J.D.; Kim, S.Y. Anti-Glucotoxicity Effect of Phytoconstituents via Inhibiting MGO-AGEs Formation and Breaking MGO-AGEs. Int. J. Mol. Sci. 2023, 24, 7672. [Google Scholar] [CrossRef]
- Wang, T.; Douglass, E.F., Jr.; Fitzgerald, K.J.; Spiegel, D.A. A “turn-on” fluorescent sensor for methylglyoxal. J. Am. Chem. Soc. 2013, 135, 12429–12433. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Li, S.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Black garlic melanoidins inhibit in vitro α-amylase and α-glucosidase activity: The role of high-molecular-weight fluorescent compounds. J. Sci. Food Agric. 2023, 103, 5376–5387. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In Vitro Antioxidant and Anti-Glycation Activity of Resveratrol and Its Novel Triester with Trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Pugachenko, I.S.; Novikova, N.N.; Topunov, A.F. Antiglycation and Antioxidant Effect of Nitroxyl towards Hemoglobin. Antioxidants 2022, 11, 2007. [Google Scholar] [CrossRef]
- Tang, D.; Zhu, J.X.; Wu, A.G.; Xu, Y.H.; Duan, T.T.; Zheng, Z.G.; Wang, R.S.; Li, D.; Zhu, Q. Pre-column incubation followed by fast liquid chromatography analysis for rapid screening of natural methylglyoxal scavengers directly from herbal medicines: Case study of Polygonum cuspidatum. J. Chromatogr. A 2013, 1286, 102–110. [Google Scholar] [CrossRef]
- Liu, H.; Huo, X.; Wang, S.; Yin, Z. The inhibitory effects of natural antioxidants on protein glycation as well as aggregation induced by methylglyoxal and underlying mechanisms. Colloids Surf. B Biointerfaces 2022, 212, 112360. [Google Scholar] [CrossRef]
- Alvi, S.S.; Nabi, R.; Khan, M.S.; Akhter, F.; Ahmad, S.; Khan, M.S. Glycyrrhizic Acid Scavenges Reactive Carbonyl Species and Attenuates Glycation-Induced Multiple Protein Modification: An In Vitro and In Silico Study. Oxid. Med. Cell. Longev. 2021, 2021, 7086951. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, S.; Saito, Y.; Ogasawara, Y. Novel Fluorometric Assay of Antiglycation Activity Based on Methylglyoxal-Induced Protein Carbonylation. Antioxidants 2023, 12, 2030. https://doi.org/10.3390/antiox12122030
Koike S, Saito Y, Ogasawara Y. Novel Fluorometric Assay of Antiglycation Activity Based on Methylglyoxal-Induced Protein Carbonylation. Antioxidants. 2023; 12(12):2030. https://doi.org/10.3390/antiox12122030
Chicago/Turabian StyleKoike, Shin, Yuna Saito, and Yuki Ogasawara. 2023. "Novel Fluorometric Assay of Antiglycation Activity Based on Methylglyoxal-Induced Protein Carbonylation" Antioxidants 12, no. 12: 2030. https://doi.org/10.3390/antiox12122030






