Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke
Abstract
1. Introduction
2. Development of New Pharmacological Neuroprotective Strategies for the Treatment of Ischemic Stroke
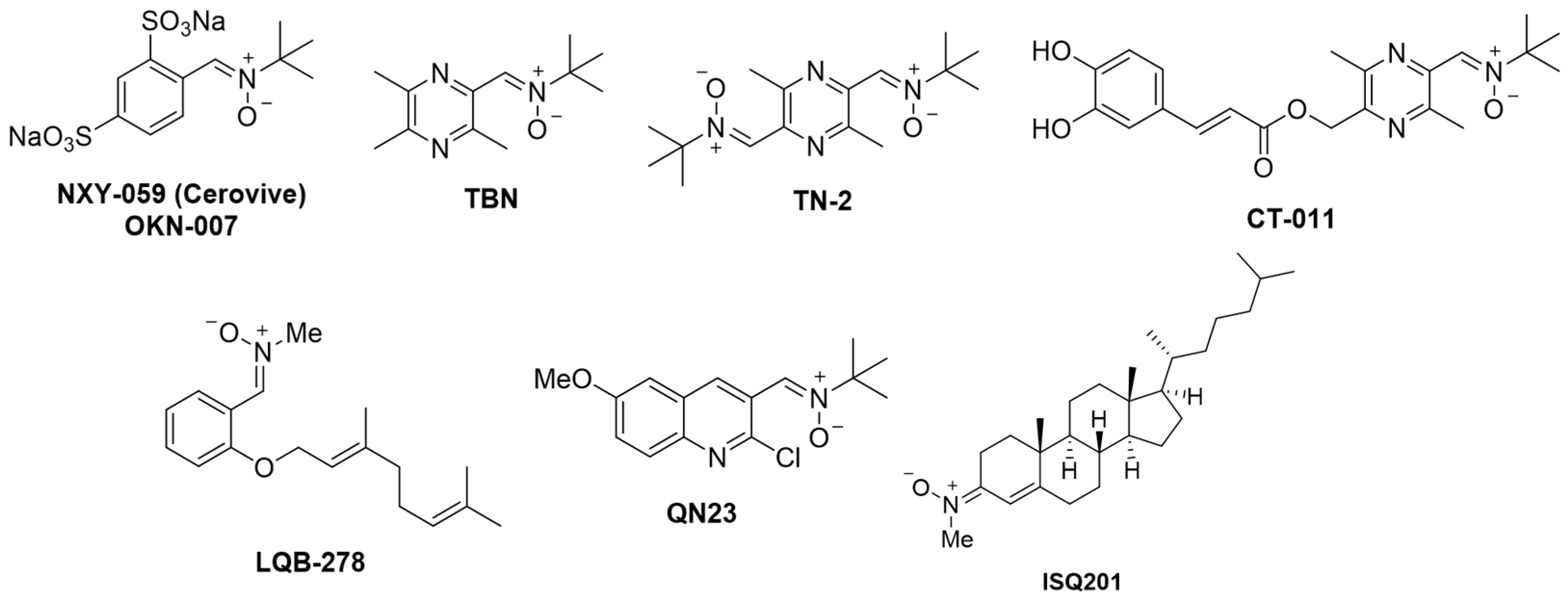
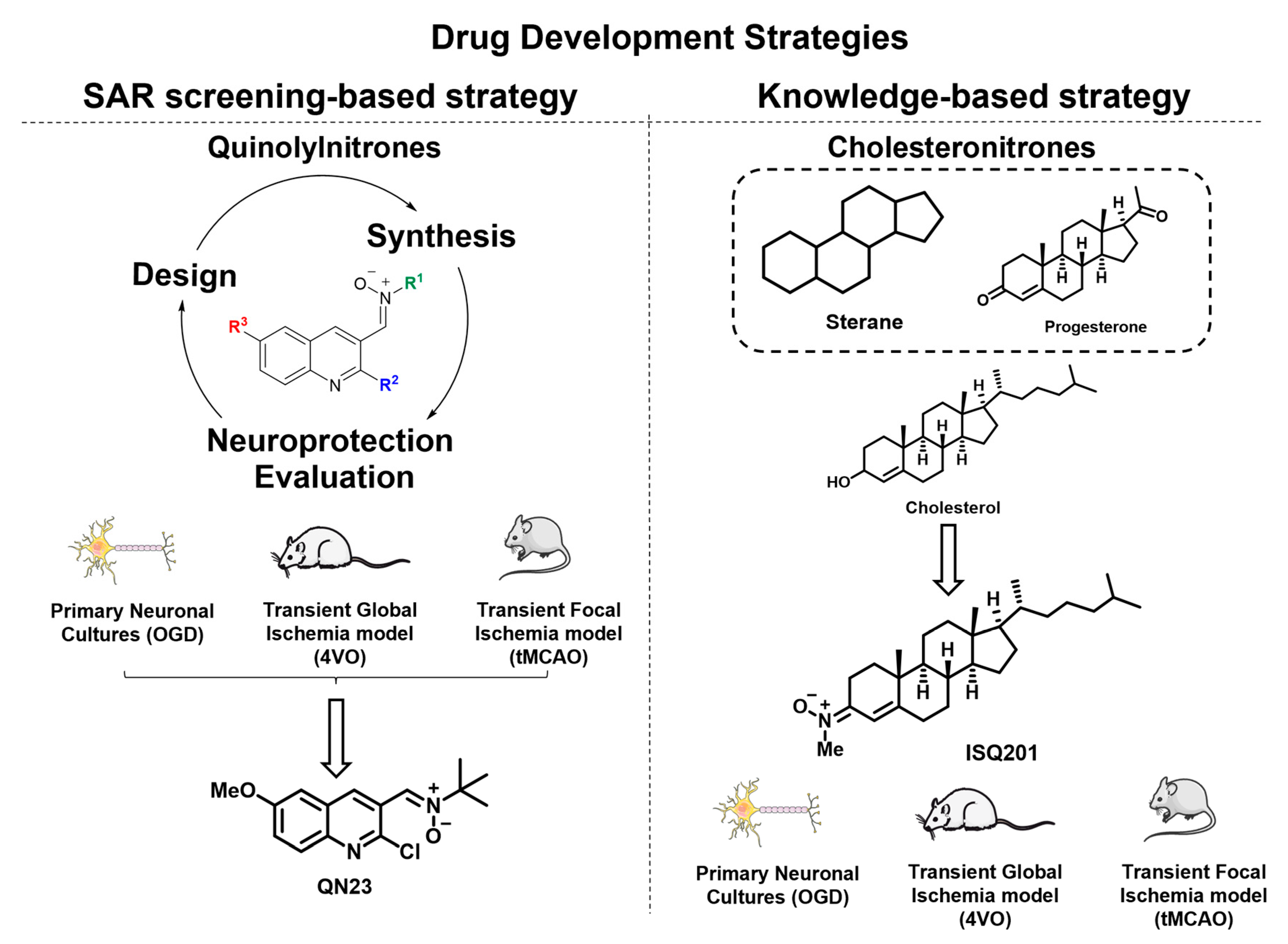
2.1. Quinolylnitrones as a Result of a Screening Program
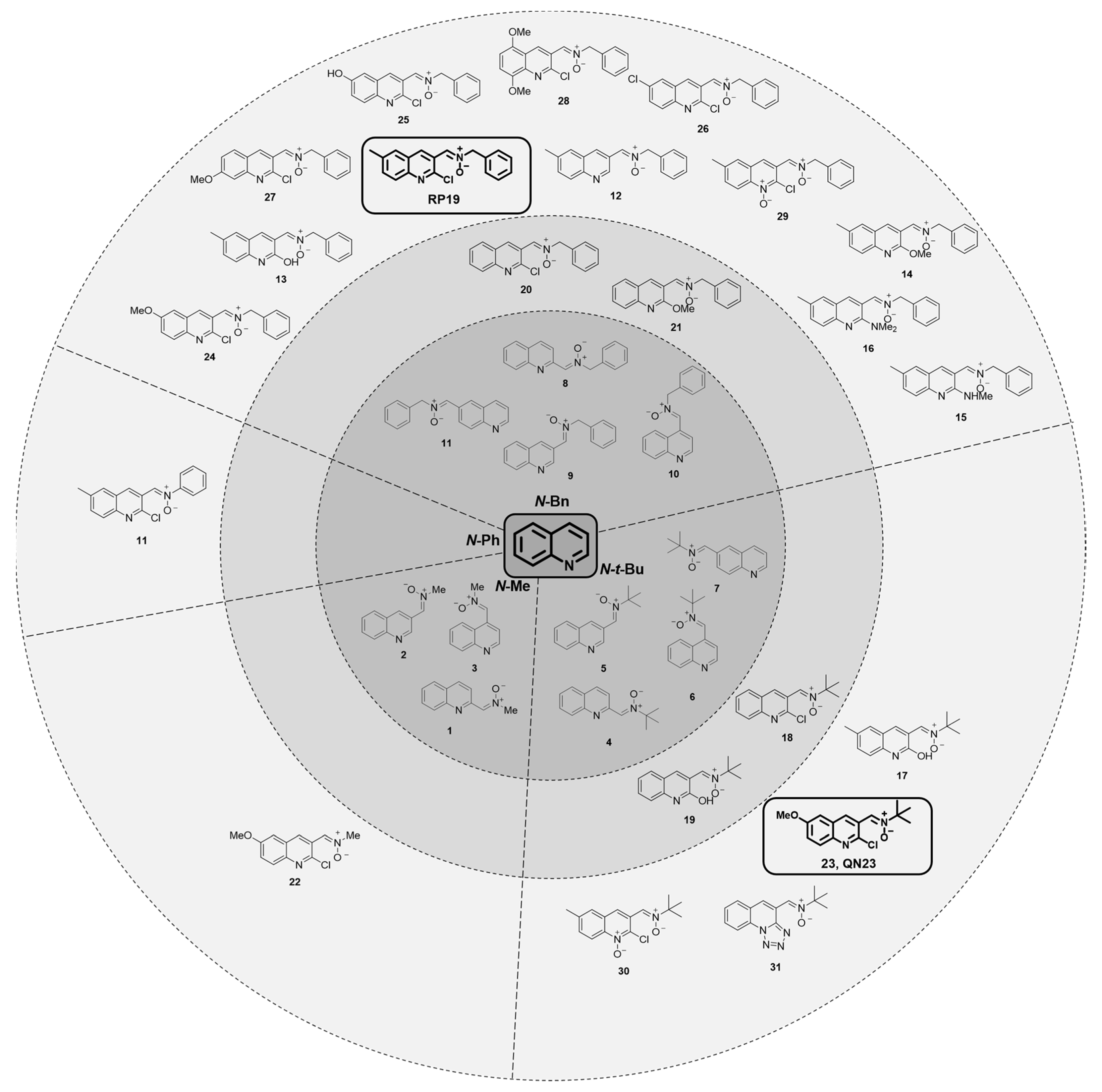
2.1.1. Biological Characterization of RP19: A First-Hit Quinolylnitrone
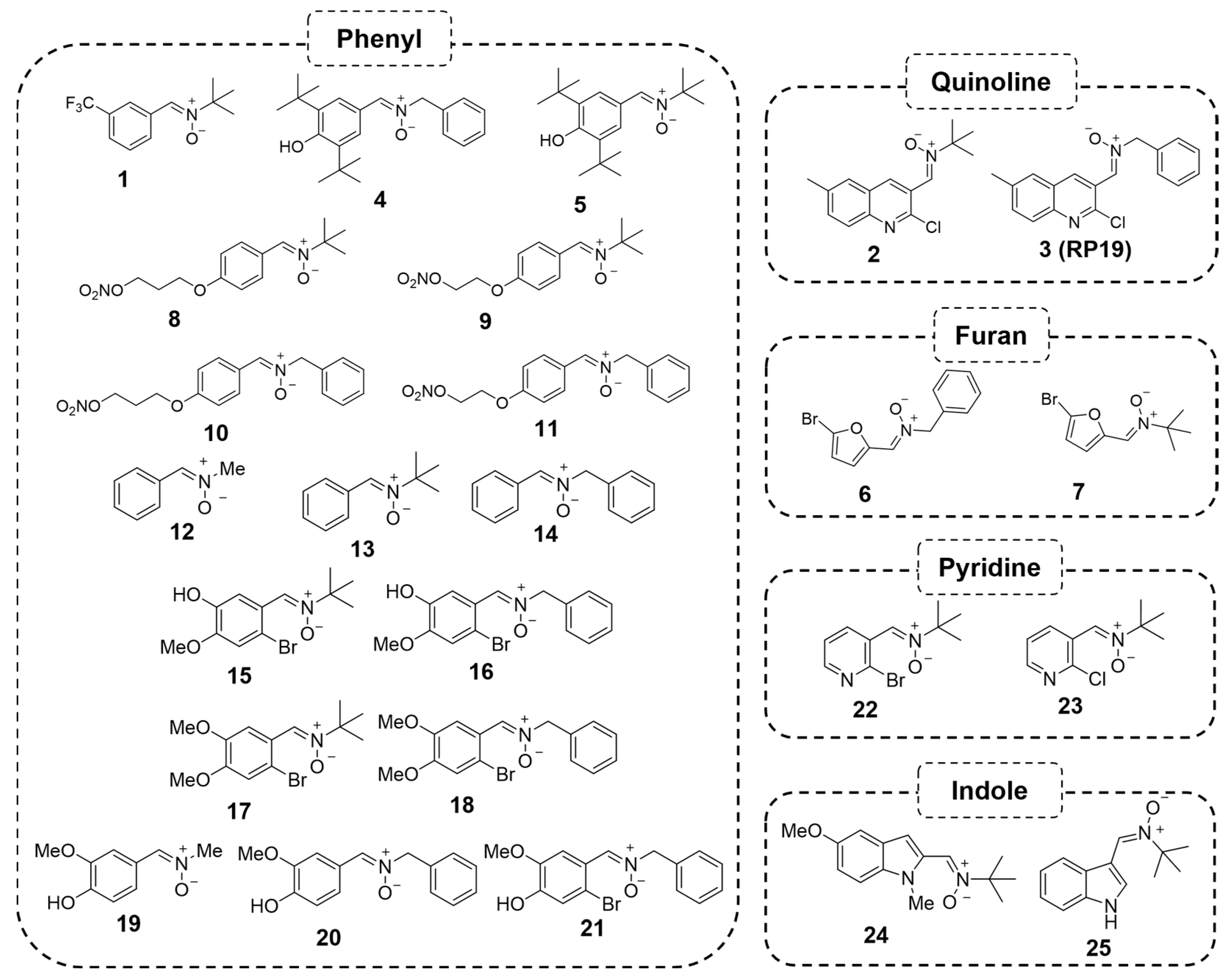
2.1.2. Structure–Activity Relationship Study: The Identification of QN23
2.1.3. Future Perspectives: New Quinolyl Derivatives on the Way
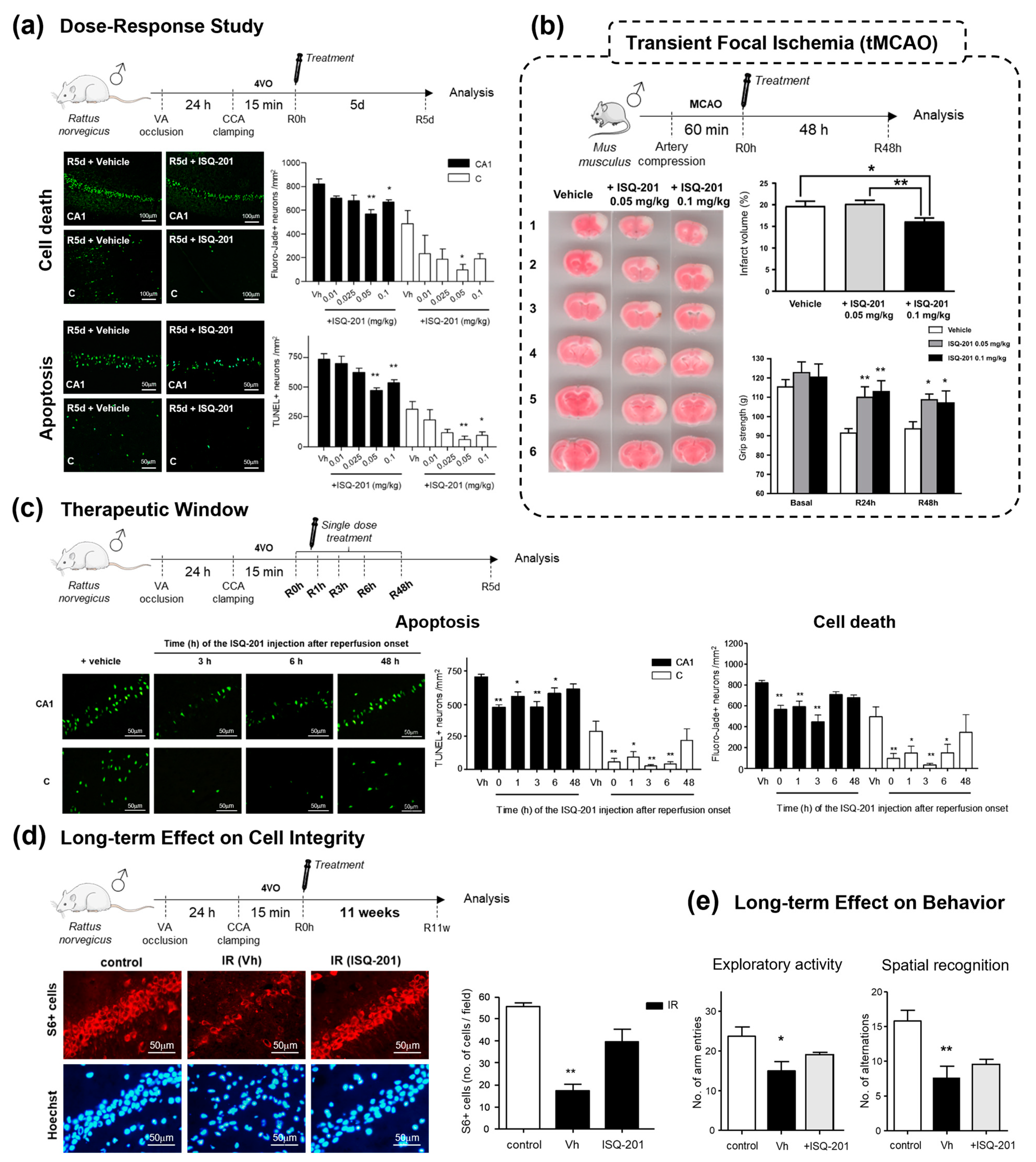
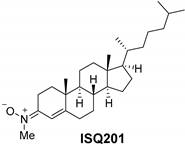
2.2. Nitrone Attachment to Steroids: Cholesterol-Derived Nitrone ISQ201
3. Discussion
4. Summary and Outlook
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hurford, R.; Sekhar, A.; Hughes, T.A.T.; Muir, K.W. Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 2020, 20, 304–316. [Google Scholar] [CrossRef]
- Comerota, A.J. Pharmacologic and pharmacomechanical thrombolysis for acute deep vein thrombosis: Focus on ATTRACT CME. Methodist Debakey Cardiovasc. J. 2018, 20, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chomova, M.; Zitnanova, I. Look into brain energy crisis and membrane pathophysiology in ischemia and reperfusion. Stress 2016, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Peso, A.; Chioua, M.; Frezza, V.; Martínez-Alonso, E.; Marco-Contelles, J.; Alcázar, A. Nitrones, old fellows for new therapies in ischemic stroke. In Neuroprotective Therapy for Stroke and Ischemic Disease; Lapchak, P., Zhang, J., Eds.; Springer In-ternational Publishing AG: Cham, Switzerland, 2017; pp. 251–283. [Google Scholar]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef]
- Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, X. Edaravone offers neuroprotection for acute diabetic stroke patients. Ir. J. Med. Sci. 2016, 185, 819–824. [Google Scholar] [CrossRef]
- Lee, X.-R.; Xiang, G.-L. Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin. Neurol. Neurosurg. 2018, 167, 157–161. [Google Scholar] [CrossRef]
- Sharma, P.; Sinha, M.; Shukla, R.; Garg, R.K.; Verma, R.; Singh, M.K. A randomized controlled clinical trial to compare the safety and efficacy of edaravone in acute ischemic stroke. Ann. Indian Acad. Neurol. 2011, 14, 103–106. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Huang, L.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A promising therapeutic agent for ischemic stroke. CNS Neurol. Disord.—Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef]
- Zhang, J.; Bhuiyan, M.I.H.; Zhang, T.; Karimy, J.K.; Wu, Z.; Fiesler, V.M.; Zhang, J.; Huang, H.; Hasan, M.N.; Skrzypiec, A.E.; et al. Modulation of brain cation-Cl(-) cotransport via the SPAK kinase inhibitor ZT-1a. Nat. Commun. 2020, 11, 78. [Google Scholar] [CrossRef]
- Hu, H.-J.; Song, M. Disrupted Ionic Homeostasis in Ischemic Stroke and New Therapeutic Targets. J. Stroke Cerebrovasc. Dis. 2017, 12, 2706–2719. [Google Scholar] [CrossRef] [PubMed]
- Melanis, K.; Stefanou, M.-I.; Themistoklis, K.M.; Papasilekas, T. mTOR pathway—A potential therapeutic target in stroke. Ther. Adv. Neurol. Disord. 2023, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Shivam, K.; Khan, H.; Kaur, A.; Dua, K.; Singh, S.; Singh, T.G. Emerging targets for modulation of immune response and inflammation in stroke. Neurochem. Res. 2023, 48, 1663–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xin, J.; Chen, J.; Sun, C.; Huo, B.; Zhang, W.; Liu, X. Scientific landscape of oxidative stress in stroke: From a bib-liometric analysis to an in-depth review. Neurochem. Res. 2023, 48, 3327–3348. [Google Scholar] [CrossRef]
- Novelli, G.P.; Angiolini, P.; Tani, R.; Consales, G.; Bordi, L. Phenyl-T-Butyl-Nitrone is active against traumatic shock in rats. Free. Radic. Res. Commun. 1986, 1, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A. Nitrones as therapeutics in age-related diseases. Aging Cell 2006, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Shuaib, A.; Ashwood, T.; Wasiewski, W.; Alderfer, V.; et al. NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II Trials. Stroke 2008, 39, 1751–1758. [Google Scholar] [CrossRef]
- Savitz, S.I. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: A need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp. Neurol. 2007, 205, 20–25. [Google Scholar] [CrossRef]
- Bath, P.M.; Gray, L.J.; Bath, A.J.; Buchan, A.; Miyata, T.; Green, A.R. Effects of NXY-059 in experimental stroke: An individual animal meta-analysis. Br. J. Pharmacol. 2009, 157, 1157–1171. [Google Scholar] [CrossRef]
- Rosselin, M.; Poeggeler, B.; Durand, G. Nitrone derivatives as therapeutics: From chemical modification to specific-targeting. Curr. Top. Med. Chem. 2017, 17, 2006–2022. [Google Scholar] [CrossRef]
- Socrier, L.; Rosselin, M.; Gomez Giraldo, A.M.; Chantemargue, B.; Di Meo, F.; Trouillas, P.; Durand, G.; Morandat, S. Nitrone-Trolox conjugate as an inhibitor of lipid oxidation: Towards synergistic antioxidant effects. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1489–1501. [Google Scholar] [CrossRef]
- Murphy, M.P.; Echtay, K.S.; Blaikie, F.H.; Asin-Cayuela, J.; Cocheme, H.M.; Green, K.; Buckingham, J.A.; Taylor, E.R.; Hurrell, F.; Hughes, G.; et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: Studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J. Biol. Chem. 2003, 278, 48534–48545. [Google Scholar] [CrossRef]
- Robertson, L.; Hartley, R.C. Synthesis of N-arylpyridinium salts bearing a nitrone spin trap as potential mitochondria-targeted antioxidants. Tetrahedron 2009, 5, 5284–5292. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Rockenbauer, A.; Zweier, J.L.; Durand, G.; Villamena, F.A. Lipophilic beta-cyclodextrin cyclic-nitrone conjugate: Synthesis and spin trapping studies. J. Org. Chem. 2009, 74, 5369–5380. [Google Scholar] [CrossRef]
- Ortial, S.; Morandat, S.; Bortolato, M.; Roux, B.; Polidori, A.; Pucci, B.; Durand, G. PBN derived amphiphilic spin-traps. II/Study of their antioxidant properties in biomimetic membranes. Colloids Surfaces B Biointerfaces 2014, 113, 384–393. [Google Scholar] [CrossRef]
- Ning, X.; Temming, R.P.; Dommerholt, J.; Guo, J.; Ania, D.B.; Debets, M.F.; Wolfert, M.A.; Boons, G.J.; van Delft, F.L. Protein modification by strain-promoted alkyne-nitrone cycloaddition. Angew. Chem. Int. Ed. Engl. 2010, 49, 3065–3068. [Google Scholar] [CrossRef]
- MacKenzie, D.A.; Sherratt, A.R.; Chigrinova, M.; Kell, A.J.; Pezacki, J.P. Bioorthogonal labelling of living bacteria using unnatural amino acids containing nitrones and a nitrone derivative of vancomycin. Chem. Commun. 2015, 51, 12501–12504. [Google Scholar] [CrossRef]
- Bilodeau, D.A.; Margison, K.D.; Serhan, M.; Pezacki, J.P. Bioorthogonal reactions utilizing nitrones as versatile dipoles in cycloaddition reactions. Chem. Rev. 2021, 121, 6699–6717. [Google Scholar] [CrossRef]
- Zalles, M.; Smith, N.; Saunders, D.; Lerner, M.; Fung, K.M.; Battiste, J.; Towner, R.A. A tale of two multi-focal therapies for glioblastoma: An antibody targeting ELTD1 and nitrone-based OKN-007. J. Cell Mol. Med. 2022, 26, 570–582. [Google Scholar] [CrossRef]
- Towner, R.A.; Saunders, D.; Lerner, M.; Silasi Mansat, R.; Yuan, T.; Barber, D.; Faakye, J.; Nyul-Toth, A.; Csiszar, A.; Meerveld, B.G.-V.; et al. Temporary opening of the blood-brain barrier with the nitrone compound OKN-007. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 363–373. [Google Scholar]
- Sun, Y.; Jiang, J.; Zhang, Z.; Yu, P.; Wang, L.; Xu, C.; Liu, W.; Wang, Y. Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. Bioorg. Med. Chem. 2008, 16, 8868–8874. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Wu, L.; Zhou, X.; Gu, J.; Li, C.; Liu, W.; Long, C.; Yang, X.; Shan, L.; et al. Neuroprotective effect and mechanism of action of tetramethylpyrazine nitrone for ischemic stroke therapy. Neuromol. Med. 2018, 20, 97–111. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, K.; Wang, Y.; Zhang, Z.; Liu, Y.; Hou, Q.; Yang, X.; Hoi, M.P.M. Evaluation of therapeutic effects of tetra-methylpyrazine nitrone in Alzheimer’s disease mouse model and proteomics analysis. Front. Pharmacol. 2023, 14, 1082602. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, S.; Zheng, C.; Wang, F.; Luo, Y.; Wu, L.; Cao, J.; Guo, B.; Yu, P.; Zhang, G.; et al. Tetramethylpyrazine nitrone improves motor dysfunction and pathological manifestations by activating the PGC-1α/Nrf2/HO-1 pathway in ALS mice. Neuropharmacology 2021, 182, 108380. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, G.; Zhang, Z.; Yu, P.; Zhong, H.; Du, J.; Wang, Y. Novel multi-functional nitrones for treatment of ischemic stroke. Bioorg. Med. Chem. 2012, 20, 3939–3945. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tan, G.; Cao, J.; Zhang, G.; Yi, P.; Yu, P.; Sun, Y.; Zhang, Z.; Wang, Y. Design, synthesis, and biological evaluation of novel tetramethylpyrazine derivatives as potential neuroprotective agents. Chem. Pharm. Bull. 2017, 65, 56–65. [Google Scholar] [CrossRef]
- Thimoteo, R.R.C.; Costa, D.S.S.; Martino, T.; Barcellos, J.C.F.; Coelho, M.G.P.; Costa, P.R.R.; Sabino, K.C.C.; Simao, T.; Dias, A.G.; Justo, G. Synthesis and biological evaluation of cyclic analogues from nitrone LQB-278: A new potential antileukemia compound. Anticancer. Res. 2021, 41, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Sucunza, D.; Soriano, E.; Hadjipavloulitina, D.; Alcázar, A.; Ayuso, I.; Oset-Gasque, M.J.; González, M.P.; Monjas, L.; Rodríguez-Franco, M.I.; et al. α-Aryl-N-alkyl Nitrones, as potential agents for stroke treatment: Synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective, and brain–blood barrier permeability properties. J. Med. Chem. 2012, 55, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Lesteage, P. Chemical and pharmacological aspects of heteroaryl-nitrones. Curr. Med. Chem. 2000, 7, 1255–1267. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Martínez-Alonso, E.; Chioua, M.; Escobar-Peso, A.; Gonzalo-Gobernado, R.; Montaner, J.; Marco-Contelles, J.; Alcázar, A. Quinolinyl nitrone RP19 induces neuroprotection after transient brain ischemia. ACS Chem. Neurosci. 2017, 8, 2202–2213. [Google Scholar] [CrossRef]
- Chioua, M.; Salgado-Ramos, M.; Diez-Iriepa, D.; Escobar-Peso, A.; Iriepa, I.; Hadjipavlou-Litina, D.; Martínez-Alonso, E.; Alcázar, A.; Marco-Contelles, J. Novel quinolylnitrones combining neuroprotective and antioxidant properties. ACS Chem. Neurosci. 2019, 10, 2703–2706. [Google Scholar] [CrossRef]
- Chioua, M.; Martínez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; et al. New quinolylnitrones for stroke therapy: Antioxidant and neu-roprotective (Z)-N-tert-Butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine oxide as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef]
- Martínez-Alonso, E.; Escobar-Peso, A.; Aliena-Valero, A.; Torregrosa, G.; Chioua, M.; Fernández-Serra, R.; González-Nieto, D.; Ouahid, Y.; Salom, J.B.; Masjuan, J.; et al. Preclinical characterization of antioxidant quinolyl nitrone QN23 as a new candidate for the treatment of ischemic stroke. Antioxidants 2022, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Escobar-Peso, A.; Fernández, I.; Alcázar, A.; Marco-Contelles, J. Improving the efficacy of quinolylnitrones for ischemic stroke therapy, QN4 and QN15 as new neuroprotective agents after oxygen-glucose depriva-tion/reoxygenation-induced neuronal injury. Pharmaceuticals 2022, 15, 1363. [Google Scholar] [CrossRef]
- Stein, D.G. Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues Clin. Neurosci. 2011, 13, 352–359. [Google Scholar] [CrossRef]
- Guennoun, R. Progesterone in the brain: Hormone, neurosteroid and neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- Vahidinia, Z.; Karimian, M.; Joghataei, M.T. Neurosteroids and their receptors in ischemic stroke: From molecular mecha-nisms to therapeutic opportunities. Pharmacol. Res. 2020, 160, 105163. [Google Scholar] [CrossRef]
- Weintraub, P.M.; Tiernan, P.L. Steroidal nitrones. J. Org. Chem. 1974, 39, 1061–1065. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Day, M.J.; Hesse, R.H.; Pechet, M.M. A new rearrangement of ketonic nitrones; a convenient alternative to the Beckmann rearrangement. J. Chem. Soc. Perkin Trans. 1975, 1764–1767. [Google Scholar] [CrossRef]
- Hardy, M.; Ouari, O.; Charles, L.; Finet, J.-P.; Iacazio, G.; Monnier, V.; Rockenbauer, A.; Tordo, P. Synthesis and spin-trapping behavior of 5-ChEPMPO, a cholesteryl ester analogue of the spin trap DEPMPO. J. Org. Chem. 2005, 70, 10426–10433. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Chioua, M.; Martínez-Alonso, E.; Soriano, E.; Montaner, J.; Masjuán, J.; Hadjipavlou-Litina, D.J.; Marco-Contelles, J.; Alcázar, A. CholesteroNitrones for Stroke. J. Med. Chem. 2015, 58, 6704–6709. [Google Scholar] [CrossRef]
- Martínez-Alonso, E.; Escobar-Peso, A.; Ayuso, M.I.; Gonzalo-Gobernado, R.; Chioua, M.; Montoya, J.J.; Montaner, J.; Fernández, I.; Marco-Contelles, J.; Alcázar, A. Characterization of a cholesteronitrone (ISQ-201), a novel drug candidate for the treatment of ischemic stroke. Antioxidants 2020, 9, 291. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Clemens, J.A. Rodent Models of Global Cerebral Ischemia. Curr. Protoc. Neurosci. 2000, 12, 9.5.1–9.5.25. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Savitz, S.I.; Baron, J.C.; Fisher, M.; STAIR X Consortium. Stroke treatment academic industry roundtable X: Brain cytoprotection therapies in the reperfusion era. Stroke 2019, 50, 1026–1031. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: Implication for their lipid-peroxidation inhibition. J. Phys. Chem. B 2012, 116, 1309–1318. [Google Scholar] [CrossRef]
- Chamorro, Á.; Lo, E.H.; Renú, A.; van Leyden, K.; Lyden, D.P. The future of neuroprotection in stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Wobst, H.J. Opportunities and challenges in phenotypic screening for neurodegenerative disease research. J. Med. Chem. 2020, 63, 1823–1840. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.J.; Zunino, G.; Bixby, J.L.; Lemmon, V.P. Phenotypic screening with primary neurons to identify drug targets for regeneration and degeneration. Mol. Cell. Neurosci. 2017, 80, 161–169. [Google Scholar] [CrossRef] [PubMed][Green Version]


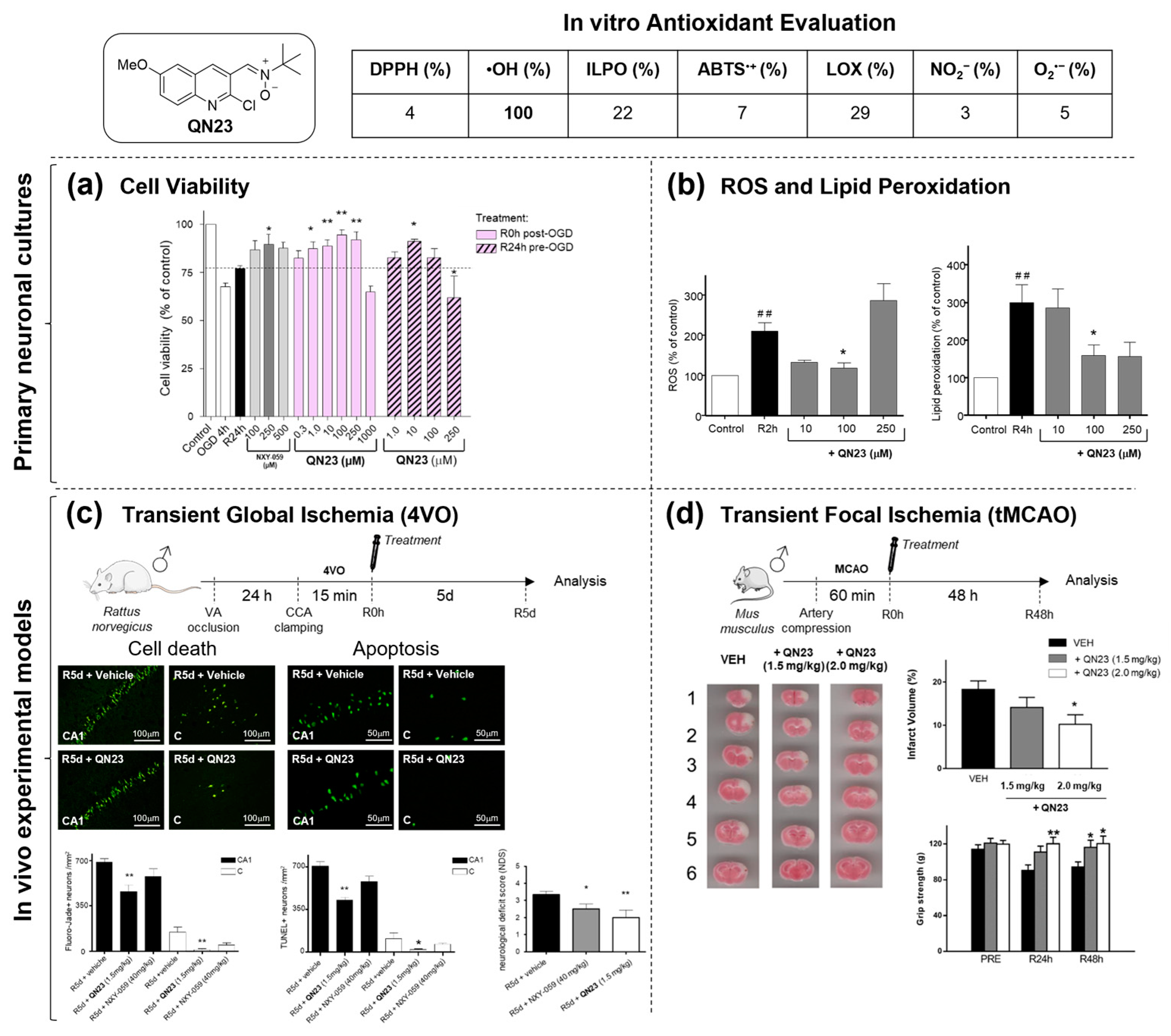
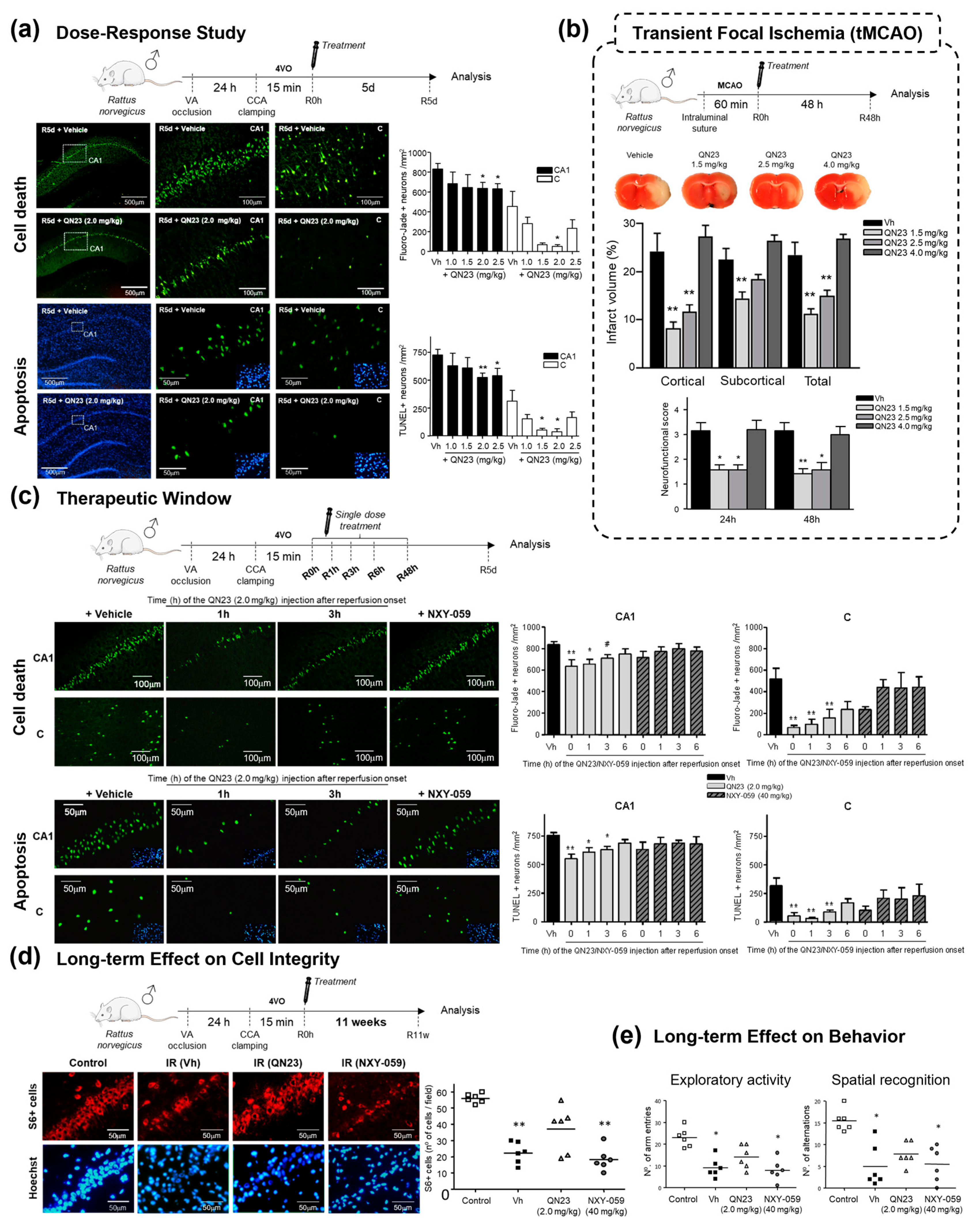


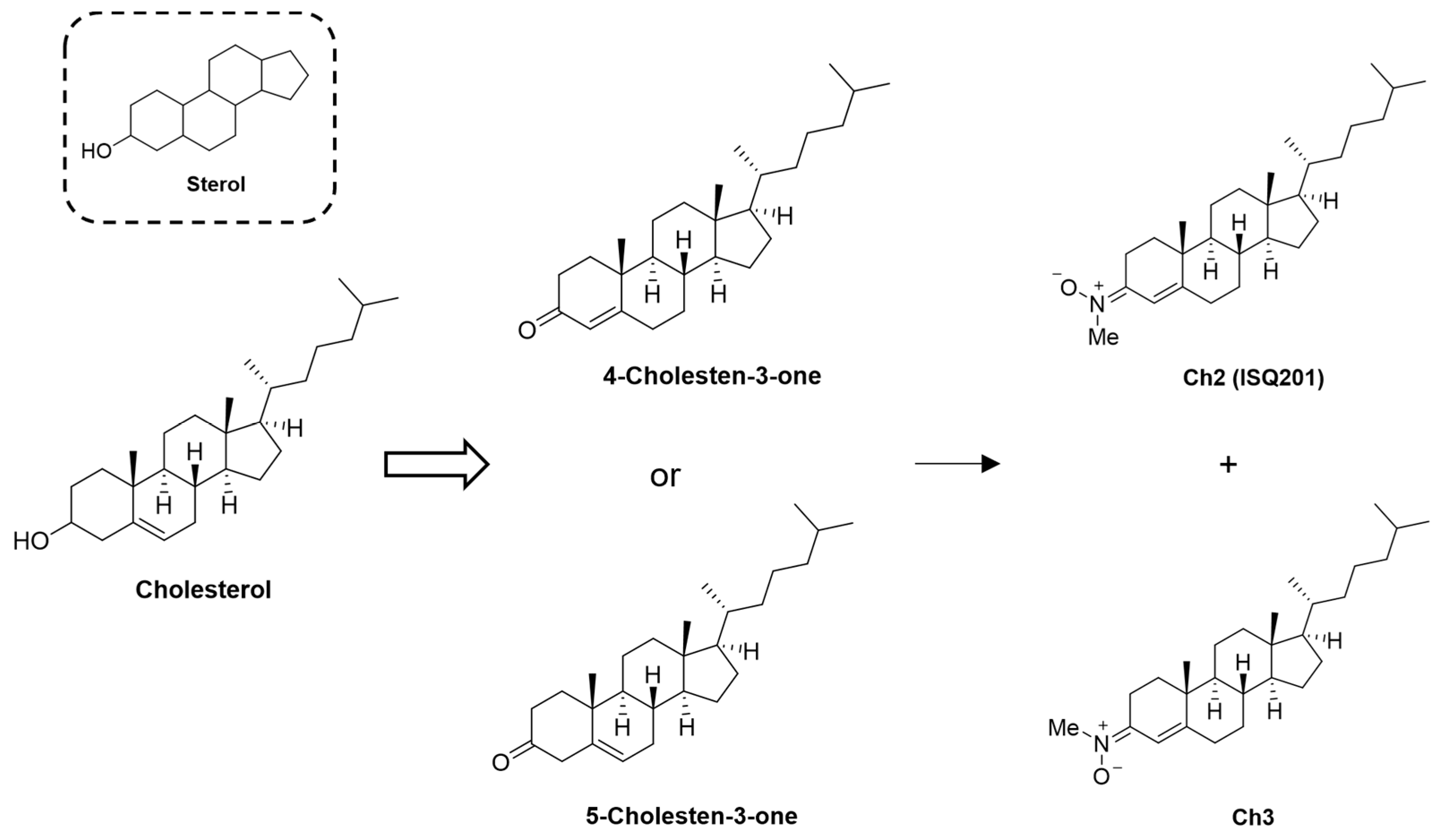
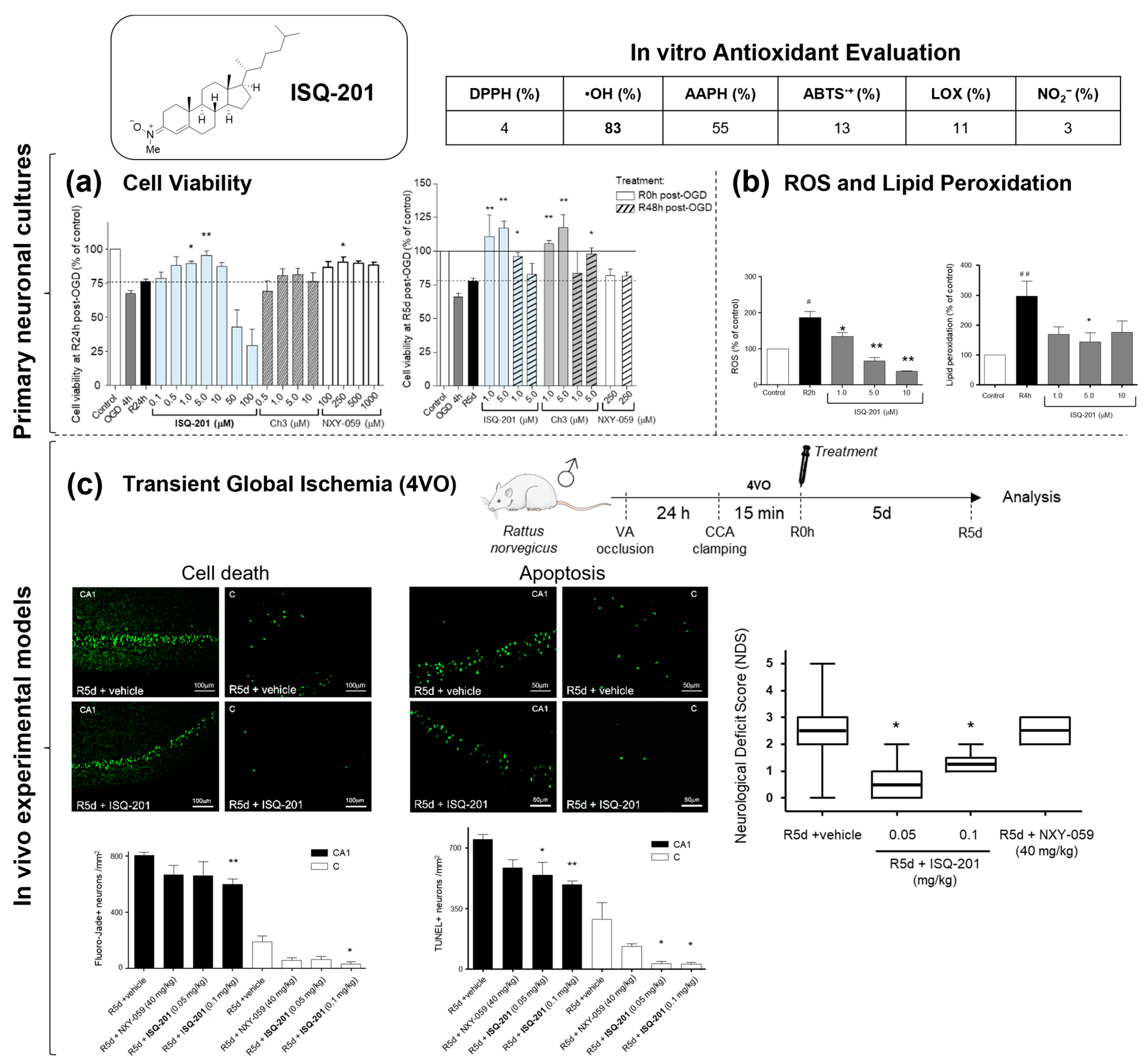
 |  | |
| Structural Family | Quinolylnitrones | Cholesteronitrones |
| Development strategy | SAR-screening-based strategy | Knowledge-based strategy |
| Screening | Yes | No |
| SAR | Yes | No |
| Maximal effective concentration in cellular OGD model | 100 µM | 5 µM |
| Effective dose (range) in transient cerebral ischemia | 1.5–2.5 mg/kg | 0.05–0.1 mg/kg |
| Therapeutic window | Up to 3 h after reperfusion onset | Up to 6 h after reperfusion onset |
| Elimination half-life (dose) | 0.16 h (2.0 mg/kg) | 1.66 h (0.07 mg/kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Peso, A.; Martínez-Alonso, E.; Masjuan, J.; Alcázar, A. Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke. Antioxidants 2023, 12, 2102. https://doi.org/10.3390/antiox12122102
Escobar-Peso A, Martínez-Alonso E, Masjuan J, Alcázar A. Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke. Antioxidants. 2023; 12(12):2102. https://doi.org/10.3390/antiox12122102
Chicago/Turabian StyleEscobar-Peso, Alejandro, Emma Martínez-Alonso, Jaime Masjuan, and Alberto Alcázar. 2023. "Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke" Antioxidants 12, no. 12: 2102. https://doi.org/10.3390/antiox12122102
APA StyleEscobar-Peso, A., Martínez-Alonso, E., Masjuan, J., & Alcázar, A. (2023). Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke. Antioxidants, 12(12), 2102. https://doi.org/10.3390/antiox12122102







