Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Diets
2.3. Sample Collection and Treatment
2.4. The Determination of CAT, GSH, and SOD
2.5. RNA Analysis
2.6. Western Blot
2.7. Immunohistochemical Analysis
2.8. Immunofluorescence Analysis
2.9. Ca2+ in Mammary Gland Tissue
2.10. Statistical Analysis
3. Results
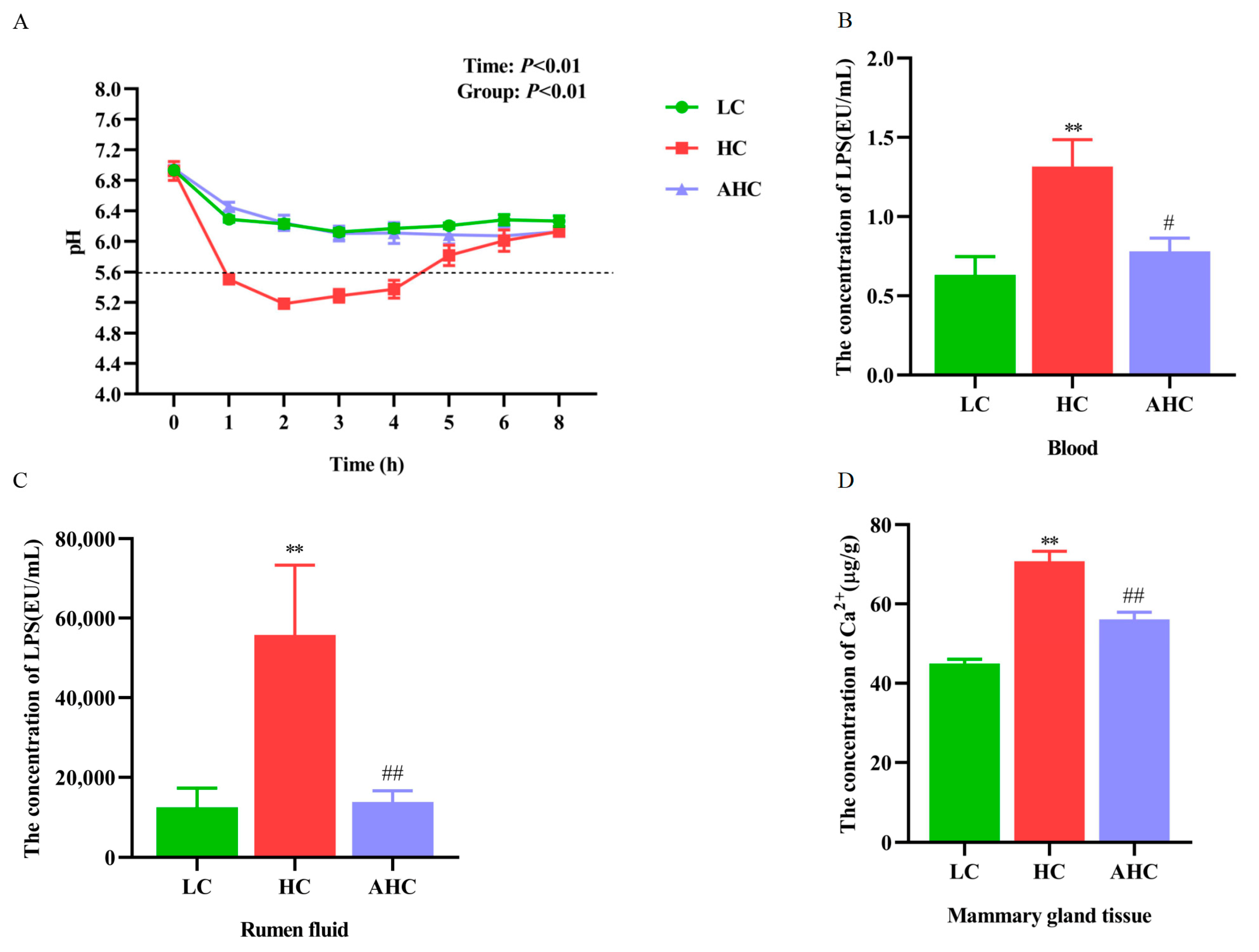
3.1. Supplemental Disodium Fumarate Effects on Ruminal pH, Ruminal Fluid, and Blood LPS Concentration, Calcium Content in Mammary Gland Tissue and Dry Matter Intake
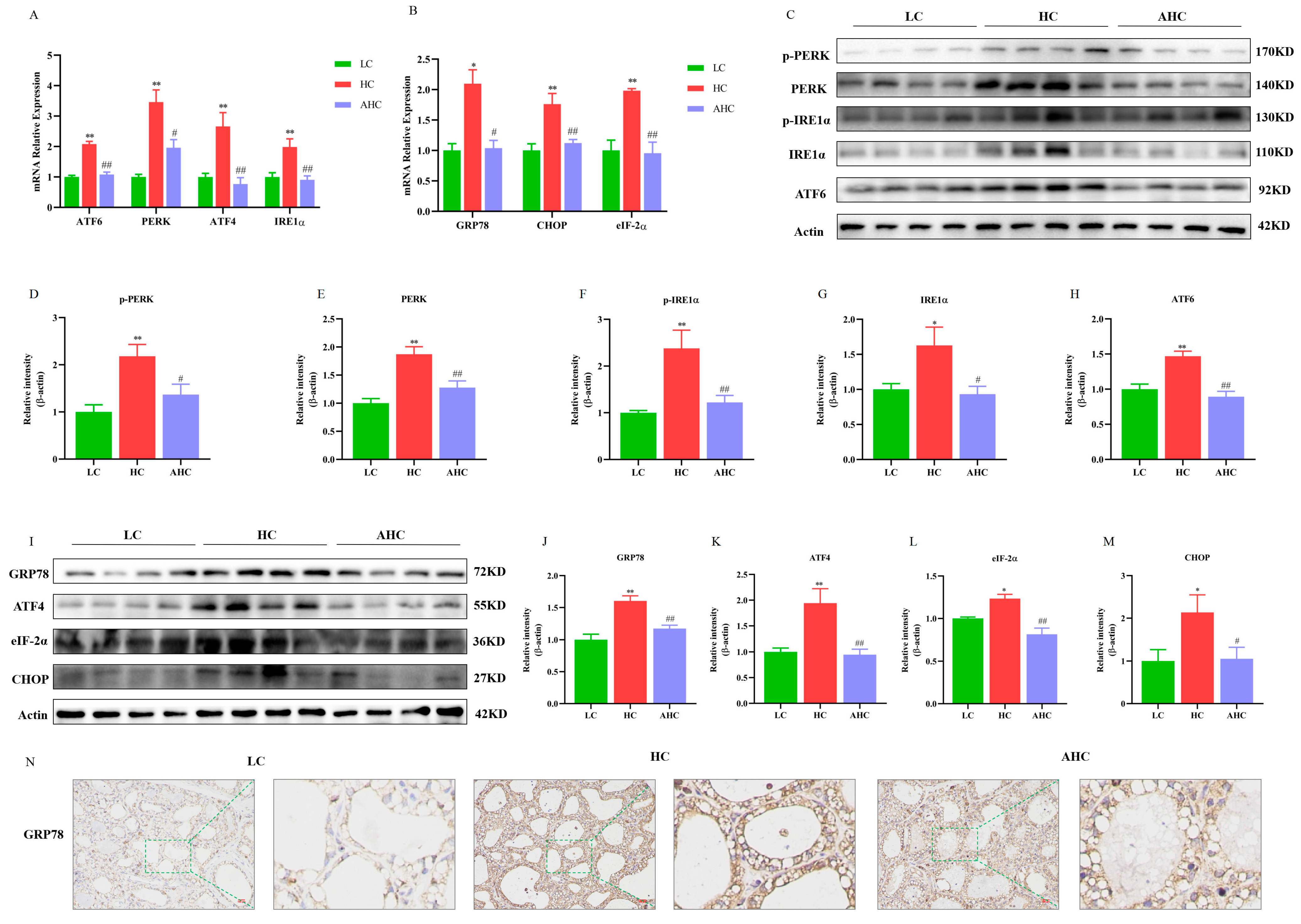
3.2. Supplemental Disodium Fumarate Effects on Endoplasmic Reticulum Stress Indicators in Mammary Gland Tissue
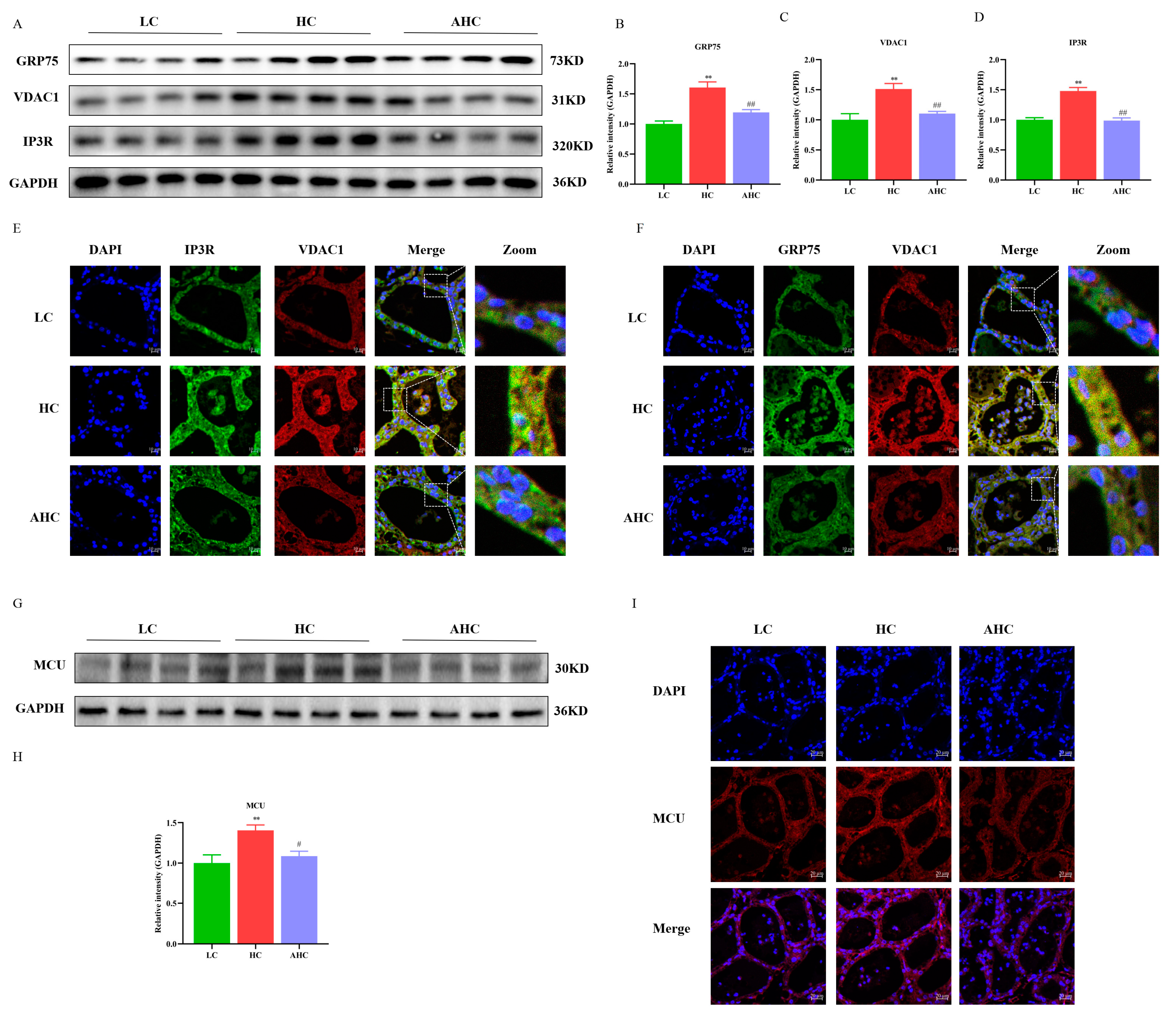
3.3. Supplemental Disodium Fumarate Effects on IP3R-VDAC1-MCU Expression in Mammary Gland Tissue
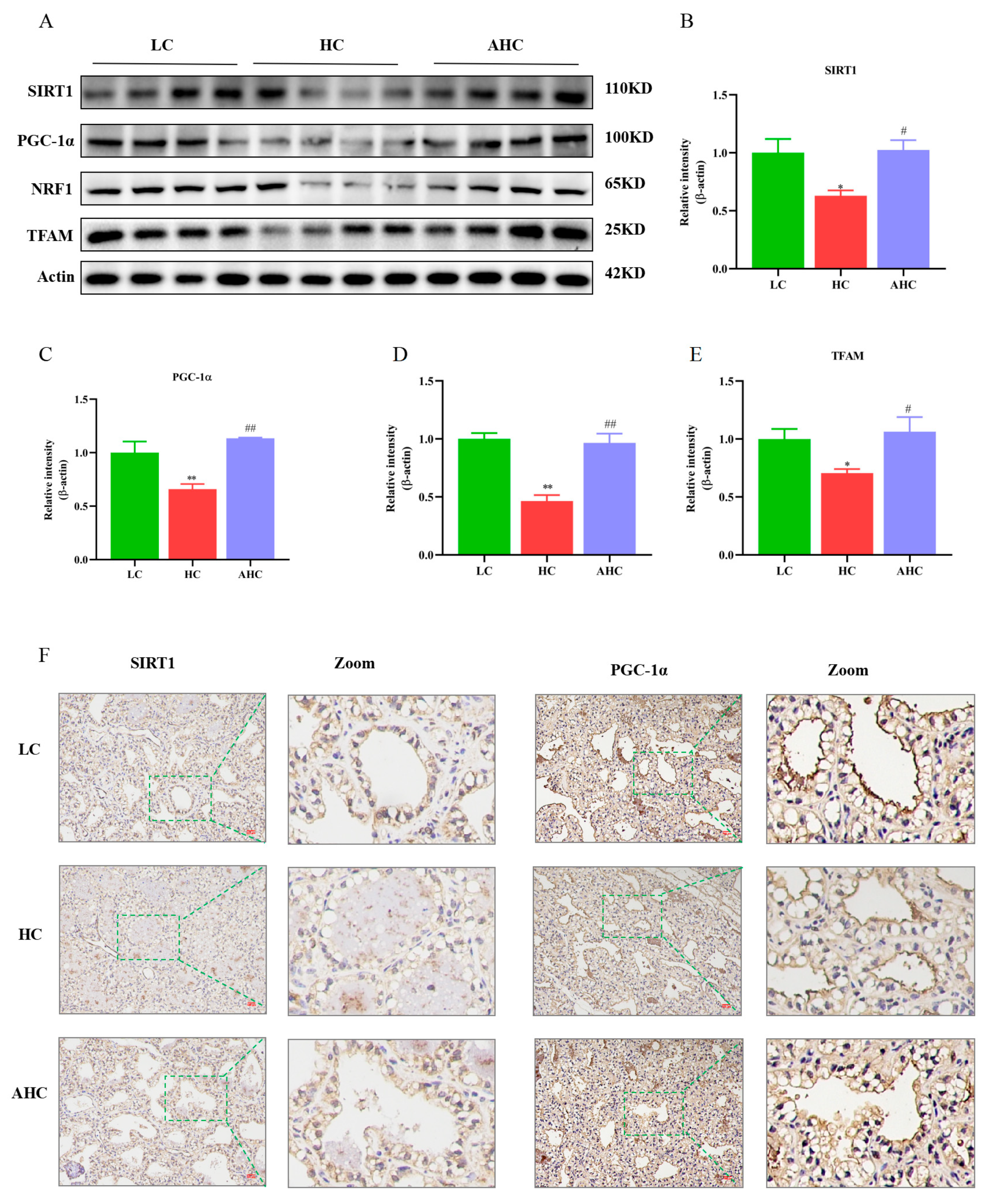
3.4. Supplemental Disodium Fumarate Effects on Mitochondrial Biogenesis in Mammary Gland Tissue
3.5. Supplemental Disodium Fumarate Effects on Mitochondrial Dynamics in Mammary Gland
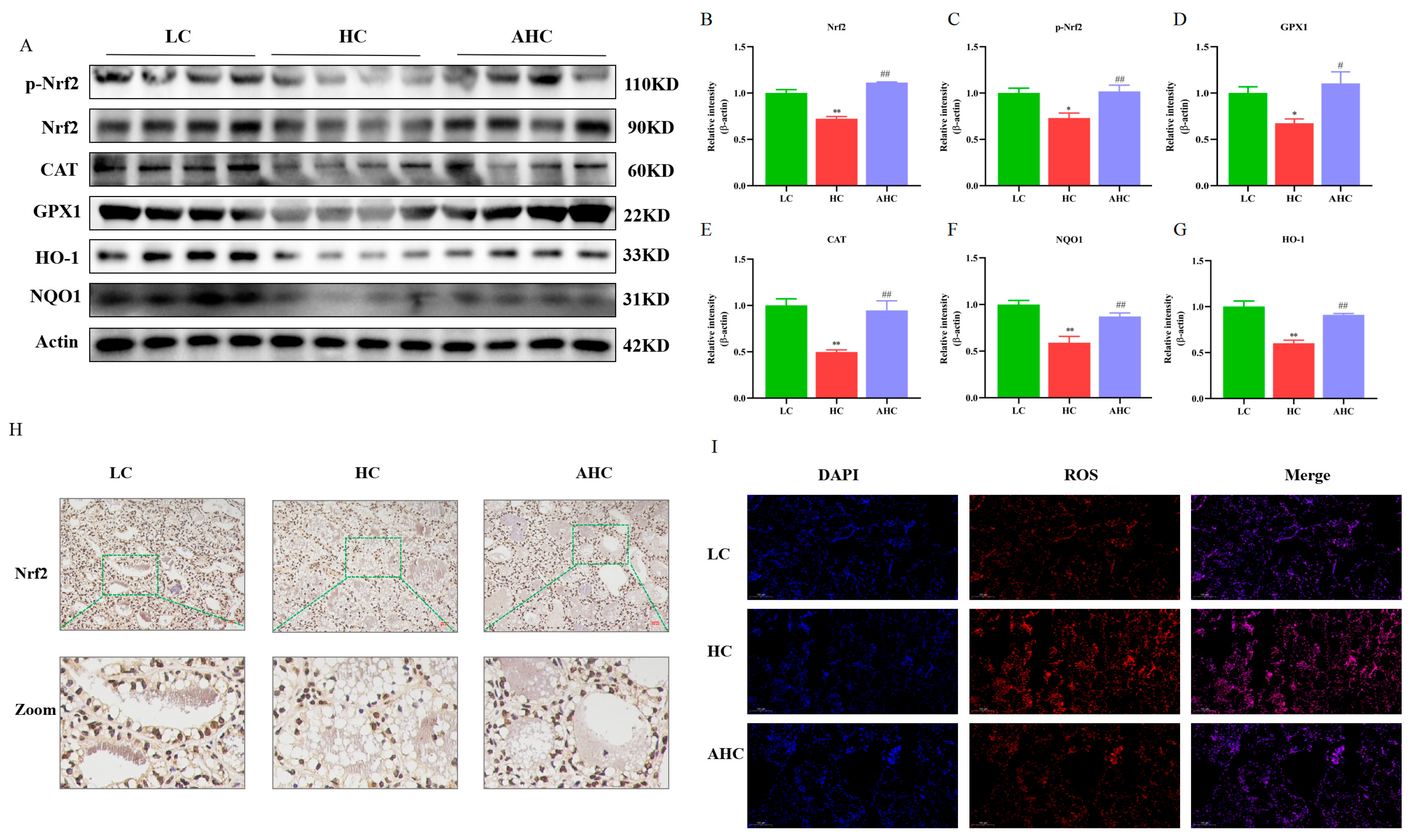
3.6. Supplemental Disodium Fumarate Effects on Oxidative Stress of Mammary Gland Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nagaraja, T.G.; Lechtenberg, K.F. Acidosis in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 2007, 23, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Kleen, J.L.; Upgang, L.; Rehage, J. Prevalence and consequences of subacute ruminal acidosis in German dairy herds. Acta Vet. Scand. 2013, 55, 48. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef]
- Koppers, A.J.; Garg, M.L.; Aitken, R.J. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic. Biol. Med. 2010, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Matsuyama, H.; Takihara, H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int. J. Urol. 2012, 19, 538–550. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. Mitochondrial Mechanisms of Neuronal Cell Death: Potential Therapeutics. Annu. Rev. Pharm. Toxicol. 2017, 57, 437–454. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Q.; Zhu, L. Could the Gut Microbiota Serve as a Therapeutic Target in Ischemic Stroke? Evid. Based Complement Altern. Med. 2021, 2021, 1391384. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Cereghetti, G.M.; Costa, V.; Scorrano, L. Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ. 2010, 17, 1785–1794. [Google Scholar] [CrossRef]
- Otera, H.; Miyata, N.; Kuge, O.; Mihara, K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J. Cell Biol. 2016, 212, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, M.; Wang, L.; Wang, Y.; Ma, N.; Xie, W.; Chang, G.; Shen, X. A high-concentrate diet provokes inflammation, endoplasmic reticulum stress, and apoptosis in mammary tissue of dairy cows through the upregulation of STIM1/ORAI1. J. Dairy Sci. 2022, 105, 3416–3429. [Google Scholar] [CrossRef]
- Memon, M.A.; Wang, Y.; Xu, T.; Ma, N.; Zhang, H.; Roy, A.C.; Aabdin, Z.U.; Shen, X. Lipopolysaccharide induces oxidative stress by triggering MAPK and Nrf2 signalling pathways in mammary glands of dairy cows fed a high-concentrate diet. Microb. Pathog. 2019, 128, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Ma, N.; Chang, G.; Shen, X. β-carotene alleviates LPS-induced inflammation through regulating STIM1/ORAI1 expression in bovine mammary epithelial cells. Int. Immunopharmacol. 2022, 113, 109377. [Google Scholar] [CrossRef]
- Kronenberg, J.; Pars, K.; Brieskorn, M.; Prajeeth, C.K.; Heckers, S.; Schwenkenbecher, P.; Skripuletz, T.; Pul, R.; Pavlou, A.; Stangel, M. Fumaric Acids Directly Influence Gene Expression of Neuroprotective Factors in Rodent Microglia. Int. J. Mol. Sci. 2019, 20, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakya, A.; Singh, G.K.; Chatterjee, S.S.; Kumar, V. Role of fumaric acid in anti-inflammatory and analgesic activities of a Fumaria indica extracts. J. Intercult. Ethnopharmacol. 2014, 3, 173–178. [Google Scholar] [CrossRef]
- Nour Omnia, A.; Shehatou George, S.G.; Rahim, M.A.; El-Awady, M.S.; Suddek, G.M. Antioxidant and anti-inflammatory effects of dimethyl fumarate in hypercholesterolemic rabbits. Egypt. J. Basic Appl. Sci. 2017, 4, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Shivanandappa, T.B.; Kumar, M.; Kushwah, A.S. Fumaric acid protect the cadmium-induced hepatotoxicity in rats: Owing to its antioxidant, anti-inflammatory action and aid in recast the liver function. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1911–1920. [Google Scholar] [CrossRef]
- Ahuja, M.; Ammal Kaidery, N.; Yang, L.; Calingasan, N.; Smirnova, N.; Gaisin, A.; Gaisina, I.N.; Gazaryan, I.; Hushpulian, D.M.; Kaddour-Djebbar, I.; et al. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J. Neurosci. 2016, 36, 6332–6351. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 1999, 82, 780–787. [Google Scholar] [CrossRef]
- Castillo, C.; Benedito, J.L.; Méndez, J.; Pereira, V.; López-Alonso, M.; Miranda, M.; Hernández, J. Organic acids as a substitute for monensin in diets for beef cattle. Anim. Feed Sci. Technol. 2004, 115, 101–116. [Google Scholar] [CrossRef]
- Wheeler William, E. Gastrointestinal Tract pH Environment and the Influence of Buffering Materials on the Performance of Ruminants. J. Anim. Sci. 1980, 51, 224–235. [Google Scholar] [CrossRef]
- Zhou, Y.W.; McSweeney, C.S.; Wang, J.K.; Liu, J.X. Effects of disodium fumarate on ruminal fermentation and microbial communities in sheep fed on high-forage diets. Animal 2012, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.D.; Ranilla, M.J. Influence of different concentrations of disodium fumarate on methane production and fermentation of concentrate feeds by rumen micro-organisms in vitro. Br. J. Nutr. 2003, 90, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.W.; Chen, Y.S.; Cheng, Y.H.; Cheng, Y.S.; Yang, C.M.; Chang, C.T. Effects of fumarate on ruminal ammonia accumulation and fiber digestion in vitro and nutrient utilization in dairy does. J. Dairy Sci. 2010, 93, 701–710. [Google Scholar] [CrossRef]
- Mao, S.Y.; Zhang, G.; Zhu, W.Y. Effect of disodium fumarate on ruminal metabolism and rumen bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. Anim. Feed Sci. Technol. 2008, 140, 293–306. [Google Scholar] [CrossRef]
- Colomer-Rocher, F.; Morand-Fehr, P.; Kirton, A.H. Standard methods and procedures for goat carcass evaluation, jointing and tissue separation. Livest. Prod. Sci. 1989, 17, 149–159. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Dai, H.; Ma, N.; Chang, G.; Aabdin, Z.U.; Shen, X. Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Anim. Biotechnol. 2022, 33, 289–296. [Google Scholar] [CrossRef]
- Gozho, G.N.; Plaizier, J.C.; Krause, D.O.; Kennedy, A.D.; Wittenberg, K.M. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef]
- Chang, G.; Liu, X.; Ma, N.; Yan, J.; Dai, H.; Roy, A.C.; Shen, X. Dietary Addition of Sodium Butyrate Contributes to Attenuated Feeding-Induced Hepatocyte Apoptosis in Dairy Goats. J. Agric. Food Chem. 2018, 66, 9995–10002. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, Y.; Xie, Z.; Zhang, Y. A High-Concentrate Diet Induced Milk Fat Decline via Glucagon-Mediated Activation of AMP-Activated Protein Kinase in Dairy Cows. Sci. Rep. 2017, 7, 44217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, N.; Abaker, J.A.; Wei, G.; Chen, H.; Shen, X.; Chang, G. A high-concentrate diet induces an inflammatory response and oxidative stress and depresses milk fat synthesis in the mammary gland of dairy cows. J. Dairy Sci. 2022, 105, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Mao, S.Y.; Long, L.M.; Zhu, W.Y. Effect of disodium fumarate on microbial abundance, ruminal fermentation and methane emission in goats under different forage: Concentrate ratios. Animal 2012, 6, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guan, N.; Ren, Y.L.; Wei, Q.J.; Tao, Y.H.; Yang, G.S.; Liu, X.Y.; Bu, D.F.; Zhang, Y.; Zhu, S.N. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018, 19, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Sun, F.; Wang, Y.; Kang, J.; Zhang, S.; Li, H. CGA restrains the apoptosis of Aβ25-35-induced hippocampal neurons. Int. J. Neurosci. 2020, 130, 700–707. [Google Scholar] [CrossRef]
- Rapizzi, E.; Pinton, P.; Szabadkai, G.; Wieckowski, M.R.; Vandecasteele, G.; Baird, G.; Tuft, R.A.; Fogarty, K.E.; Rizzuto, R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002, 159, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Mallilankaraman, K.; Doonan, P.; Cárdenas, C.; Chandramoorthy, H.C.; Müller, M.; Miller, R.; Hoffman, N.E.; Gandhirajan, R.K.; Molgó, J.; Birnbaum, M.J.; et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 2012, 151, 630–644. [Google Scholar] [CrossRef] [Green Version]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Zi, C.; Zhang, C.; Yang, Y.; Ma, J. Penehyclidine hydrochloride protects against anoxia/reoxygenation injury in cardiomyocytes through ATP-sensitive potassium channels, and the Akt/GSK-3β and Akt/mTOR signaling pathways. Cell Biol. Int. 2020, 44, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Menazza, S.; Holmström, K.M.; Parks, R.J.; Liu, J.; Sun, J.; Liu, J.; Pan, X.; Murphy, E. The ins and outs of mitochondrial calcium. Circ. Res. 2015, 116, 1810–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Yang, J.; Gao, X.; Sun, W.; Liu, S.; Han, Y.; Lu, X.; Jin, C.; Wu, S.; Cai, Y. Lanthanum chloride impairs spatial learning and memory by inducing [Ca2+]m overload, mitochondrial fission-fusion disorder and excessive mitophagy in hippocampal nerve cells of rats. Metallomics 2020, 12, 592–606. [Google Scholar] [CrossRef]
- Griparic, L.; van der Wel, N.N.; Orozco, I.J.; Peters, P.J.; van der Bliek, A.M. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 2004, 279, 18792–18798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Song, S.; Liu, X.; Zhang, M.; Xiang, W. Low MFN2 expression related to ageing in granulosa cells is associated with assisted reproductive technology outcome. Reprod Biomed Online. 2019, 38, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. Parkin-dependent mitophagy in the heart. J. Mol. Cell. Cardiol. 2016, 95, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandhok, G.; Lazarou, M.; Neumann, B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol. Rev. Camb. Philos. Soc. 2018, 93, 933–949. [Google Scholar] [CrossRef] [Green Version]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein ameliorates LPS-induced hepatocyte injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef] [PubMed]


| Items | Diets (Content) 3 | |
|---|---|---|
| LC | HC | |
| Maize | 19.28 | 46.86 |
| Soybean meal | 4.20 | 9.80 |
| Wheat bran | 2.70 | 8.10 |
| Rapeseed meal | 1.20 | 2.80 |
| Peanut vine | 35.00 | 15.00 |
| Cron stover silage | 35.00 | 15.00 |
| Limestone | 0.42 | 0.82 |
| NaCl | 0.50 | 0.50 |
| Premix 1 | 0.50 | 0.50 |
| CaHPO4 | 1.20 | 0.62 |
| Total | 100.00 | 100.00 |
| Nutrient levels 2 | ||
| CP | 9.75 | 13.53 |
| Ca | 0.60 | 0.60 |
| P | 0.40 | 0.40 |
| NDF | 40.66 | 24.41 |
| Item | Diets | p Value | ||
|---|---|---|---|---|
| LC | HC | AHC | ||
| DMI (kg/d) | 1.53 ± 0.11 | 1.39 ± 0.17 | 1.49 ± 0.15 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, M.; Zhao, X.; Huo, R.; Li, X.; Chang, G.; Shen, X. Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep. Antioxidants 2023, 12, 223. https://doi.org/10.3390/antiox12020223
Meng M, Zhao X, Huo R, Li X, Chang G, Shen X. Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep. Antioxidants. 2023; 12(2):223. https://doi.org/10.3390/antiox12020223
Chicago/Turabian StyleMeng, Meijuan, Xu Zhao, Ran Huo, Xuerui Li, Guangjun Chang, and Xiangzhen Shen. 2023. "Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep" Antioxidants 12, no. 2: 223. https://doi.org/10.3390/antiox12020223






