Abstract
The Mediterranean diet (MD) has beneficial effects on human health, which is evidenced by the observation of lower incidence rates of chronic diseases in Mediterranean countries. The MD dietary pattern is rich in antioxidants, such as melatonin, which is a hormone produced mainly by the pineal gland and controls several circadian rhythms. Additionally, melatonin is found in foods, such as fruit and vegetables. The purpose of this systematic review was to assess the melatonin content in Mediterranean foods and to evaluate the influence of the MD on melatonin levels in both humans and model organisms. A comprehensive search was conducted in four databases (PubMed, Scopus, Cochrane Library and Web of Science) and data were extracted. A total of 31 records were chosen. MD-related foods, such as tomatoes, olive oil, red wine, beer, nuts, and vegetables, showed high melatonin contents. The consumption of specific MD foods increases melatonin levels and improves the antioxidant status in plasma.
1. Introduction
The Mediterranean diet (MD) is a nutritional pattern with widely known health properties [1]. Traditionally, the MD is consumed in geographic areas where olives (Olea europea L.) and grapes (Vitis vinifera L.) are cultivated, and olive oil and wine are regularly produced and consumed [2,3]. Other features are associated with the benefits of the MD. The MD has been declared as part of the intangible heritage of humanity by UNESCO, but including other habits and factors of the Mediterranean countries, the weather and moderate exercise practice [4]. In terms of the nutritional pattern, the MD is balanced in calories and macro- and micronutrient intake. As essential components, the MD includes whole grains, fruits, vegetables, legumes, nuts, yogurt, fish, and white meat [5,6]. Minerals, vitamins, and other bioactive compounds are included in different foods of the MD, such as fruits, vegetables, and olive oil, among others [6,7,8]. The MD dietary habits are correlated with a lower incidence of cancer [9,10], cardiovascular [11,12] and neurodegenerative diseases [13,14,15], when compared to industrialized countries’ dietary patterns [16].
From a phytochemical point of view, the MD is rich in phenylpropanoids, isoprenoids, and alkaloids [6,17], compounds with high antioxidant activity. In fact, the antioxidant properties of the bioactive compounds included in the MD are some of the major contributors to the health properties of the MD [18]. For example, polyphenols have been widely studied in terms of the antioxidant properties of the MD [19,20,21,22]. On the other hand, melatonin has been recently characterized as a phytochemical element in MD foods, which increases the MD’s health potential [23,24].
Melatonin is a hormone that is biosynthesized from tryptophan in four well-defined intracellular steps [25]. Melatonin synthesis is regulated by the light/dark cycle and the enzymes involved in the process are expressed during the night, because the light inhibits their expression [26]. It is involved in different plant functions, such as plant growth regulation, delaying flowering delay, photosynthetic system protection, oxidative stress damage protection, and biotic and abiotic stresses [27,28,29]. Melatonin was first discovered as a secretory product of the pineal gland. It is the main chronobiotic hormone that regulates the circadian rhythms [30] and seasonal changes in vertebrate physiology via its daily nocturnal increase in blood [31,32]. However, subsequent studies showed that melatonin is present in bacteria, unicellular eukaryotic organisms, invertebrates and vertebrates, algae, plants, and fungi, and is found in various edibles, such as vegetables, fruit, herbs, and seeds [33,34]. In mammals, melatonin is synthesized in many tissues and organs [35,36,37,38,39,40], and melatonin shows remarkable functional versatility, exhibiting antioxidant [41], oncostatic [42], antiaging [43], and immunomodulatory [44] effects. The antioxidant functions of melatonin have been widely described. Melatonin is a free radical scavenger; it directly scavenges reactive oxygen species (ROS) and nitrogen-based species with even more effectivity than vitamin E [45,46,47,48,49]. Additionally, melatonin induces the activity of antioxidant enzymes, such as glutathione reductase and peroxidase [50,51].
The MD is a nutritional pattern rich in many antioxidants and bioactive compounds; therefore, the identification of melatonin in edibles and the multiple functions of melatonin are an interesting perspective. The aim of this systematic review was to assess the melatonin content in Mediterranean foods and to evaluate the influence of the MD on melatonin levels in both humans and model organisms.
2. Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) [52,53]. This review was registered on the International Prospective Register of Systematic Reviews (PROSPERO, registration No. CRD42022332235).
2.1. Searching Strategy
A comprehensive search was conducted in four databases (PubMed, Scopus, Web of Science and Cochrane Library), searching all years of records up until February 2022. The language restriction was English and Spanish. The search terms were categorized into the following three key concepts: study population, MD (MD-related foods and intervention) and melatonin levels. The specific terms used were “adult” OR “middle-aged” OR “young” OR “m?n” OR “wom?n” OR “food” OR “plant” AND “mediterranean diet” OR “Olive Oil” OR “grape” OR “tomato” OR “nuts” OR “legume” OR “cereal” AND “melatonin”, respectively. In addition, the reference lists of the elected articles were manually searched for relevant publications. To choose which MD-related foods were specifically included in the research algorithm, foods previously described to have melatonin content were used, based on the work of Meng et al. [54]. In regard to the MD, the foods were selected according to Schwingshackl et al. [6].
2.2. Selection Criteria
The published studies in this review were required to adhere to the following criteria: (1) they must be original research; (2) human adult or animal studies or edible food studies; (3) MD or MD-related food interventions or analyses; (4) must include melatonin measurements.
2.3. Data Extraction and Reliability
The PRISMA recommendations were followed [52,53]. First, in order to identify relevant articles, the titles were screened and abstracts were analyzed. Then, the selected articles were fully reviewed for eligibility by two independent researchers.
3. Results
3.1. Search and Selection of Studies
The searching and selection strategies are detailed in Figure 1. A total of 999 and 7 records were identified through database searching and the search of the reference lists of the retrieved articles, respectively. Duplicates were removed, leaving a set of 617 records, from which 36 records were screened. Finally, 5 records were excluded for not following the selection criteria and 31 records were selected. The records were divided into the following two different categories: foods from the MD with melatonin [55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] and influence of the MD on melatonin levels [60,72,79,80,81,82,83,84,85,86].
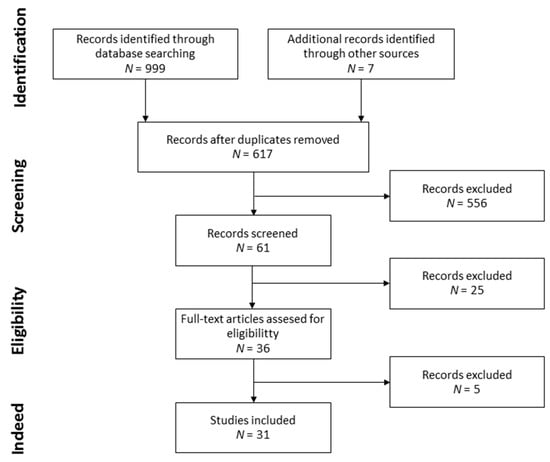
Figure 1.
Flow diagram of record selection.
3.2. Melatonin in Mediterranean Diet Foods
3.2.1. Melatonin Content in Fruit and Vegetables
Melatonin is present in different MD-associated foods and drinks, with grapes and olive oil being the first two products of the MD in which melatonin was detected (Table 1). Wine is a grape by-product drink associated with the nutritional pattern of the MD and is rich in phytochemicals, such as polyphenols and melatonin. Red wines are richer in melatonin (7.44–0.24 ng/mL) compared to white wines (3.93–0.16 ng/mL), which is similar to grapes [55,56,58]. However, Rodriguez-Naranjo et al. showed that the melatonin content of different wines from Spain was between 423.01 and 74.14 ng/mL, with no differences being observed between white and red wines [57]. It is important to consider that different factors, such as environmental factors, agricultural practices, vintage, and wine-making procedures, may influence the melatonin levels in wine. For example, the melatonin content was different among different brands that produce the same wine [56]. Additionally, Albana grape-derived wine showed a lower content of melatonin compared to the Albana grape itself (the melatonin level of which is 1.2 ng/g), supporting the hypothesis that part of melatonin is lost during the wine-making process [55]. Beer is other typical beverage in the MD; however, the melatonin content in beer was lower when compared to wines [60].

Table 1.
Melatonin content in grapes, wine, beer, and oils of the MD.
Olive oil is a by-product of olive that is known worldwide for its anti-inflammatory and antioxidant properties, while virgin olive oil is the main oil used in the MD. De la Puerta et al. measured the melatonin content of different virgin olive oils and reported values that ranged between 71 and 119 pg/mL, with the highest amount of melatonin being detected in virgin olive oils produced via the cold process. Additionally, the refinement process reduces the melatonin content in olive oil, even though refined olive oil showed a higher content of melatonin than sunflower oil [61].
The highest melatonin content was detected in fruit and vegetables (Table 2), but the melatonin quantity in the same fruit varies depending on the harvest conditions, such as the time of day, year, maturity, and variety. For example, tomato is one of most consumed fruit in the MD and its melatonin content has been widely studied, with wide differences being observed between varieties. Stürtz et al. studied different tomato varieties, including Lycopersicon esculentum from Spain, and the highest content of melatonin was detected in the Raf and Bond varieties, with 50 and 25 ng/g, respectively [62]. It is important to highlight that Dubbels et al. [64] and Pape et al. [63] measured the melatonin content of other varieties of Lycopersicon esculentum grown in Germany, showing a 10–100-times lower content when compared with that described by Stürtz et al. Pape et al. measured melatonin content using ELISA and HPLC-PD and different organic solvents to extract melatonin, and the melatonin content was between 0.5984 and 1.0685 ng/g [63], which was similar to the findings of Dubbles et al. [64]. Despite the fact that Pape et al. [63] and Stürtz et al. [62] used the same tomato species (L. esculentum) and HPLC to measure the melatonin content, the solvents for the extraction and the harvest processes were different. Additionally, Stürtz et al. studied the melatonin content in L. esculentum and found that it differed within the same tomato variety because of the different harvests [62].

Table 2.
Melatonin content in fruit and vegetables.
In regard to Solanum lycopersicum, the melatonin content found by all authors [66,67,68] was lower compared to that of Lycopersicum esculentum determined by Stürtz et al. [62]. However, Spanish Solanum lycopersicum [68] showed the highest melatonin content, when compared to the same species grown in other countries [66,67].
3.2.2. Melatonin Content in Nuts, Legumes, and Animal-Derived Products
Different types of nuts, including almonds, pistachios, nuts, and chestnuts, which grow in Mediterranean countries, are included in the nutritional pattern of the MD and have approximately 1 ng/g of melatonin (Table 3). The nut variety influences melatonin levels in nuts, with different varieties of almonds (Prunus dulcis L.) and pistachios (Pistacia vera L.) showing melatonin content values between 600–2000 pg/g and 1000–12,000 pg/g, respectively [73,74]. Verde et al. measured melatonin in almonds and pistachios by HPLC-FLD after extraction with a combination of organic solvents and found a similar quantity of melatonin to Paroni et al., who measured melatonin content by LC-MS/MS after SPE extraction in both types of nuts. However, Bronte DOP pistachio and cv. Palo almond showed higher contents of melatonin when compared to other varieties. These differences are greater when comparing pistachio varieties [74]. It is important to highlight that Oladi et al. determined the melatonin content in four different varieties of pistachio from Iran, which was 1000–10,000-times higher than that of other nuts and pistachio varieties [75]. In contrast, Manchester et al. measured the melatonin content in almonds derived from another species referred to as Prunus amygdalus [76], showing 10-times higher levels compared to that of Prunus Dulcis. Additionally, it is important to consider that nuts can be eaten raw or roasted, and the processing of foods influences melatonin bioavailability. In fact, it was reported that roasting decreased melatonin content in most nuts, while this was increased in roasted peanuts [73].

Table 3.
Melatonin content in nuts and seeds.
Legumes and grains are one of the main components of the MD and are very rich in melatonin, in contrast with animal-derived products, such as meat, fish and eggs, in which melatonin content is very low (Table 4). Lentils showed the highest amount of melatonin, which was doubled in sprouts, compared to raw lentils [80]. However, comparison with other studies cannot be made, because there are no multiple records regarding the melatonin content in the same legume.

Table 4.
Melatonin content in meat, fish, egg, and oils.
3.3. Melatonin Levels and Mediterranean Diet
Despite the fact that different foods of the MD contain melatonin, the influence of their intake is not clear. All the records that show data of melatonin levels after the intake of the MD are shown in Table 5. The consumption of melatonin-rich foods, such as fruit and legumes, increased melatonin levels in humans and animal models. However, the available data are not comparable, because different foods were used in all the records of the different study populations.

Table 5.
Characteristics of the interventional studies.
For example, rats fed with 3 g of walnuts (Juglans regia L.), which contain about 10.5 ng of melatonin, showed increased serum melatonin concentrations from 11.5 to 38.0 pg/mL, and their serum total antioxidant capacity (TAS) also increased [72]. In contrast, in humans, the serum melatonin concentration was significantly higher 1 h after the intake of 100 mL of red wine [82]. In addition, it has been reported that the moderate consumption of beer (330 mL and 660 mL for women and men, respectively) increased both melatonin and TAS of human serum after 45 min of beer ingestion [60].
4. Discussion
The MD is demonstrated to have benefits for human health, because of the nutritious quality of its foods. The MD nutritional pattern includes a high consumption of fruit and vegetables, mono and polyunsaturated fats, and proteins, within products rich in micronutrients, with antioxidant and immunomodulatory effects. The melatonin content in edibles has been widely studied; however, the existing evidence for some products is not conclusive. The factors that influence the differences in melatonin content include melatonin extraction and measurement techniques, species or variety of the food, growing and harvest conditions and food processing; however, their level of influence remains unknown.
There are different protocols for melatonin extraction and quantification. Organic solvents, in combination with different purification steps, have been predominantly used for melatonin extraction [87]. Additionally, the organic solvents and their proportion used are important for melatonin extraction, with the most commonly used solvents being methanol and chloroform. In regard to melatonin quantification, techniques such as radioimmunoassay, ELISA, HPLC and mass spectrometry were used. Radioimmunoassay and ELISA are less specific techniques than HPLC for measurements in plant material because there could be cross-reactivity with other plant metabolites. HPLC techniques are highly sensitive, accurate and versatile and can be used with very different biological matrices, and HPLC coupled with fluorescence detection is the most used technique in the records studied. Mass spectrometry has been also used in various records due to its high sensitivity, but mass spectrometry techniques usually have problems with plant matrices. Some authors validated their methodology and performed studies under diverse conditions and following distinct techniques [55,56,58,73,74,75]. The results among all the records showed differences in melatonin quantity depending on the methodology followed, but the variability shown by different methodologies does not explain the variabilities found by different authors for the same product.
In contrast, the level of influence of other factors on melatonin content is not clear. There is ample research about the post-harvest exogenous melatonin effects in fruits and vegetables [88,89,90], and some studies focus on the environmental effects on melatonin production in fruits and vegetables. For example, the growing conditions play a crucial role in the melatonin content of foods. For example, Tan et al. reported that sunlight exposure is a conditioning factor, because sunlight stimulates melatonin synthesis in plants [91]. Additionally, there is evidence that suggests that Mediterranean plants, which grow under high sunlight exposure, have higher melatonin content, compared to the same species living under lower sunlight exposure in other locations. In addition, it is not clear whether the ripening process modulates the melatonin content in fruit. For example, the melatonin amount in grape berries decreases with ripening [92], but other studies have reported constant melatonin content during ripening [93]. In addition, melatonin is described to have a circadian rhythm in fruit, showing increased levels during the day; therefore, the moment of the harvest would determine the melatonin content [94].
There is extensive evidence in relation to the effects of melatonin supplementation [95]. On the contrary, little has been reported about the effects of melatonin consumption via foods with high melatonin content. The consumption of foods rich in melatonin increased melatonin levels in blood; however, the mechanism has not yet been described. The increase in melatonin in blood could be due to a retention in the fall of melatonin content during the day or to an increase in melatonin synthesis. Additionally, it is important to consider that melatonin is a hormone that follows a circadian rhythm in humans and animals. Therefore, the time when melatonin is ingested, and the time of the measurement are crucial factors. Only one of the human studies carried out an intervention with melatonin-rich foods before bed time and they found an increase in blood melatonin and an improvement in sleep quality after tomato consumption [81]. Nevertheless, the effects on sleep quality could be due to other compounds in the tomato. The remaining human studies monitored the intake of food after overnight fasting and found an increase om melatonin and its derivatives. An improvement of sleep quality was not evaluated by the authors, since melatonin is rapidly (30–50 min) degraded by different cytochromes [96], so an early intake of melatonin probably would not have an effect on sleep quality.
The antioxidant effects of intrinsic food melatonin consumption have been studied in three different articles included in this review [60,72,79], and the authors associated an increase in the antioxidant capacity with the melatonin intake within the foods being evaluated, including fruits, nuts and beer. For example, Maldonado et al. 2013 suggested that higher TAS was associated with higher melatonin concentration in beer [60]. However, other components of beer have not been evaluated and this includes the work of Reiter et al. 2005 and Sae-Teaw et al. 2013, when they associated nut consumption with TAS [72]. Serum antioxidant capacity was measured with two different protocols by Sae-Teaw et al. in 2013 after the consumption of different fruits, and the increase in the antioxidant capacity of serum correlated with melatonin increase in serum [79]. Melatonin has been broadly established as a potent antioxidant molecule [45,46,47,48,49,50,51], so the increases in TAS and antioxidant capacity after nut, beer and fruit intake could be associated with melatonin, but other food components could also affect TAS, such as polyphenols, which are abundant in beer [97,98], nuts [99,100,101] and fruits [102,103], also increase TAS [104,105].
In regard to animal studies, only one [83] performed a long-term study, in which an oil extracted from nuts resulted in an increase in blood melatonin levels, an effect that was enhanced when this oil was combined with unsaturated fatty acids. In contrast, all the other studies reported a one-time intervention, in which an increase in melatonin was detected after the intake of foods with high melatonin content. However, the model animals used were different in all the records; therefore, a measurement of the effect could not be determined. Additionally, the studies did not analyze the same food; therefore, there is only a single piece of evidence for each food item studied.
Despite all the data analyzed in this review about the increase in melatonin levels after the intake of melatonin-rich products, it is important to highlight that an increase in melatonin is not consistent with the melatonin content of the foods. Therefore, the effects of the consumption of certain products are not only due to their melatonin content, but rather the product’s matrix and other components may play a role in melatonin metabolism that enhances the effect of melatonin intake.
In conclusion, the melatonin content in foods is not a constant parameter; however, tomato, wine, beer, nuts, and olive oil are MD foods with melatonin and specially, specific varieties of these foods have high contents of melatonin. The punctual or periodical consumption of these foods could contribute to increased blood melatonin levels and could have an impact on sleep quality and antioxidant status.
Author Contributions
E.G.-C., J.R.C., M.D.M.-A. and M.d.C.M.-L. contributed to the collection, interpretation, and writing of the manuscript. S.M.-d.l.P. critically reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Andalusian Government (2021/CTS-1074). Elena Grao-Cruces has the benefit of a doctoral fellowship (BES-2012-056104) from the Andalusian Government. Maria C. Millan-Linares acknowledges the financial grant US-1381492 supported by the Andalusian Plan for Research, Development, and Innovation 2020 from the European Regional Development Fund (ERDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analysis. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef]
- Hansen, J.M. Agriculture in the Prehistoric Aegean: Data versus Speculation. Am. J. Archaeol. 1988, 92, 39–52. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Hershey, M.S.; Zape, I.; Trichopoulou, A. Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. Am. Soc. Nutr. Sci. 2001, 131, 3065S–3073S. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2019, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary Micronutrient and Mineral Intake in the Mediterranean Healthy Eating, Ageing, and Lifestyle (MEAL) Study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef]
- Rozanska, A.; Russo, M.; Caccopña, F.; Salafia, F.; Polkowska, Z.; Dugo, P.; Modello, L. Concentration of Potentially Bioactive Compounds in Italian Extra Virgin Olive Oils from Various Sources by Using LC-MS and Multivariate Data Analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Cao, T.; Lu, X.; Zhang, T.; Luo, B.; Li, Z. Mediterranean Diet Patterns in Relation to Lung Cancer Risk: A Meta-Analysis. Front. Nutr. 2022, 11, 844382. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer 2011, 109, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Marventao, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Qin, L.Q.; Dong, J.Y. Mediterranean Diet and Mortality in People with Cardiovascular Disease: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 19, 2623. [Google Scholar] [CrossRef]
- Fu, J.; Tan, L.J.; Lee, J.E.; Shin, S. Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 946361. [Google Scholar] [CrossRef] [PubMed]
- Ballarini, T.; Melo van Lent, D.; Brunner, J.; Schroder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; et al. Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology 2021, 96, e2920–e2932. [Google Scholar] [CrossRef] [PubMed]
- Strikwerda, A.J.; Dommershuijsen, L.J.; Ikram, M.K.; Voortman, T. Diet Quality and Risk of Parkison’s Disease: The Rotterdam Study. Nutrients 2021, 13, 3970. [Google Scholar] [CrossRef]
- Menotti, A.; Puddu, P.E.; Catasta, G. Dietary Habits, Cardiovascular And Other Causes Of Death In A Practically Extinct Cohort Of Middle-Aged Men Followed-Up For 61 Years. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Davinelli, S.; Accardi, G.; Aiello, A.; Caruso, C.; Duro, G.; Ligotti, M.E.; Pojero, F.; Scapagnini, G.; Candore, G. Healthy ageing and Mediterranean diet: A focus on hormetic phytochemicals. Mech. Ageing Dev. 2021, 200, 111592. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Khan, S.A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols COntained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Sarapis, K.; George, E.S.; Marx, W.; Mayr, H.L.; Willcox, J.; Esmaili, T.; Powell, K.L.; Folasire, O.S.; Lohning, A.E.; Garg, M.; et al. Extra virgin olive oil high in polyphenols improves antioxidant status in adults: A double-blind randomized controlled, cross-over study (OLIVAUS). Eur. J. Nutr. 2022, 61, 1073–1086. [Google Scholar] [CrossRef]
- Sarkhosh-Khorasani, S.; Sangsefidi, Z.S.; Hosseinzadeh, M. The effect of grape products containing polyphenols on oxidative stress: A systematic review and meta-analysis of randomized clinical trials. Nutr. J. 2021, 20, 25. [Google Scholar] [CrossRef]
- Herbello-hermelo, P.; Lamas, J.P.; Lores, M.; Dominguez-Gonzalez, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Polyphenol bioavailability in nuts and sedes by an in vitro dialyzability approach. Food Chem. 2018, 254, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing For Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef]
- Iritri, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar]
- Iritri, M.; Varoni, E.M. Melatonin in Mediterranean diet, a new perspective. J. Sci. Food Agric. 2015, 95, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, W.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schomerus, C.; Korf, H.W. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–378. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Murch, S.J.; Erland, L.A.E. A Systematic Review of Melatonin in Plants: An Example of Evolution of literature. Front. Plant Sci. 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, L.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Pfeffer, M.; von Gall, C.; Wicht, H.; Korf, H.W. The Role of the Melatoninergic System in Circadian and Seasonal Rhythms-Insights From Different Mouse Strains. Front. Physiol. 2022, 13, 883637. [Google Scholar] [CrossRef] [PubMed]
- Harderland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Harderland, R.; Manchester, L.C.; Rosales-Corral, S.; Coto-Montes, A.; Boga, J.A.; Reiter, R.J. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J. Pineal. Res. 2012, 53, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef] [PubMed]
- Harderland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin- A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, J.P.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Calvo, J.R.; Gonzalez-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Shi, L.; Li, N.; Bo, L.; Xu, Z. Melatonin and Hypothalamic-Pituitary-Gonadal Axis. Curr. Med. Chem. 2013, 20, 2017–2031. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Targhazeh, N.; Reiter, R.J.; Rahimi, M.; Qujeq, D.; Yousefi, T.; Shahavi, M.H.; Mir, S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie 2022, 200, 44–59. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Q.; Tian, M.; Gao, L. Mechanisms of melatonin in anti-aging and its regulation effects in radiation-induced premature senescence. Radiat. Med. Prot. 2021, 2, 33–37. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wang, X.; Wiu, J.; Zhu, Y.; Liang, T.; Gao, B.; Wu, Z.; Lian, C.; Peng, Y.; Liang, A.; et al. Melatonin Rescued Reactive Oxygen Species-Impaired Osteogenesis of human Bone Marrow Mesenchymal Stem Cells in the Presence of Tumor Necrosis Factor-Alpha. Stem Cells Int. 2019, 2019, 6403967. [Google Scholar] [CrossRef]
- Huang, C.C.; Lai, C.J.; Tsai, M.H.; Wu, Y.C.; Chen, K.T.; Jou, M.J.; Fu, P.I.; Wu, C.H.; Wei, I.H. Effects of melatonin on the nitric oxide system and protein nitration in the hypobarix hypoxic rat hippocampus. BMC Neurosci. 2015, 16, 61. [Google Scholar] [CrossRef]
- Fardid, R.; Salajegheh, A.; Mosleh-Shirazi, M.A.; Sharifzadeh, S.; Okhovat, M.A.; Najafi, M.; Rezaeyan, A.; Abaszadeh, A. Melatonin Ameliorates The Production of COX-2, Inos, and The Formation of 8-OHdG in Non-Targeted Lung Tissue after Pelvic Irradiation. Cell J. 2017, 19, 324–331. [Google Scholar]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Kim, M.O. Neurological Enhancement Effects of Melatonin against Brain Injury-induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Sadeghi, E.; Agah, S.; Nachvak, S.M.; Fazelian, S.; Moradi, F.; Persad, E.; Heshmati, J. Effect of melatonin supplementation on oxidative stress parameters: A systematic review and meta-analysis. Pharmacol. Res. 2020, 161, 105210. [Google Scholar] [CrossRef]
- Abadi, S.H.M.H.; Shirazi, A.; Alizadeh, A.M.; Changizi, V.; Najafi, M.; Khalighfard, S.; Nosrati, H. The Effect of Melatonin on Superoxide Dismutase and Glutathione Peroxidase Activity, and Malondialdehyde Levels in the Targeted and the Non-targeted Lung and Heart Tissues after Irradiation in Xenograft Mice Colon Cancer. Curr. Mol. Pharmacol. 2018, 11, 326–335. [Google Scholar] [CrossRef]
- Taysi, S.; Ucuncu, H.; Elmastas, M.; Aktan, b.; Buyukokuroglu, M.E. Effect of melatonin on lipid peroxidation, glutathione and glutathione-dependent enzyme activities in experimental otitis media with effusion in guinea pigs. J. Pineal Res. 2005, 39, 283–286. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, K.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Bourton, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BJM 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Viegas, O.; Esteves, C.; Rocha, J.; Melo, A.; Ferreira, I.M.P.L.V.O. Simultaneous determination of melatonin and trans-resveratrol in wine by dispersive liquid-liquid microextraction followed by HPLC-FLD. Food Chem. 2021, 339, 128091. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is ynthesized by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A new bioactive compound in wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- De la Puerta, C.; Carrascosa-Salmoral, M.P.; Garcia-Luna, P.P.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. Melatonin is a phytochemical in olive oil. Food Chem. 2007, 104, 609–612. [Google Scholar] [CrossRef]
- Stürtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Pape, C.; Lüning, K. Quantification of melatonin in phototrophic organisms. J. Pineal Res. 2006, 41, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high-performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Reinholds, I.; Pugajeva, I.; Radenkovs, V.; Rjabova, J.; Bartkevics, V. Development and Validation of New Ultra-High-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry method for Determination of Melatonin in Fruits. J. Chromatogr. Sci. 2016, 54, 977–984. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Manwiwattanakul, G.; Loypimai, P.; Kerr, W.L. Melatonin and its derivative contents in tropical fruits and fruit tablets. J. Food Compos. Anal. 2021, 103, 104109. [Google Scholar] [CrossRef]
- Okazaki, M.; Ezura, H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J. Pineal Res. 2009, 46, 338–343. [Google Scholar] [CrossRef]
- Riga, P.; Medina, S.; Garcia-Flores, L.A. Melatonin content of pepper and tomato fruits: Effects of cultivar and solar radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Verde, A.; Minguez, J.M.; Leao-Martins, J.M.; Gago-Martinez, A.; Gallardo, M. Melatonin content in walnuts and other commercial nuts. Influence of cultivar, ripening and processing (roasting). J. Food Compos. Anal. 2022, 105, 104180. [Google Scholar] [CrossRef]
- Paroni, R.; Dei Cas, M.; Rizzo, J.; Ghidoni, R.; Montagna, M.T.; Rubino, F.M.; Iritri, M. Bioactive phytochemicals of tree nuts. Determination of the melatonin and sphingolipid content in almonds and pistachios. J. Food Compos. Anal. 2019, 82, 103227. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Sangsopha, J.; Johns, N.P.; Johns, J.; Moongngarm, A. Dietary sources of melatonin and benefits from production of high melatonin pasteurized milk. J. Food Sci. Technol. 2020, 57, 2026–2037. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martin-Cabrejas, M.A. Bioavailability of Melatonin from Lentil Sprouts and Its Role in the Plasmatic Antioxidant Status in Rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef]
- Yang, T.H.; Chen, Y.C.; Ou, T.H.; Chien, Y.W. Dietary supplement of tomato can accelerate urinary aMT6s level and improve sleep quality in obese postmenopausal women. Clin. Nutr. 2020, 39, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Paroni, R.; Antognetti, J.; Lodi, G.; Sardella, A.; Carrassi, A.; Iritri, M. Effect of red wine intake on serum and salivary melatonin levels: A randomized, placebo-controlled clinical trial. Molecules 2018, 23, 2474. [Google Scholar] [CrossRef] [PubMed]
- Johns, N.P.; Johns, J.; Porasuphatana, S.; Plaimee, P.; Sae-Teaw, M. Dietary Intake of Melatonin from Tropical Fruit Altered Urinary Excretion of 6-sulfatoxymelatonin in Healthy Volunteers. J. Agric. Food Chem. 2013, 61, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, M.J.; Arola, L.; Blade, C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.P.; Lamarque, A.L.; Comba, A.; Berra, M.A.; Silva, R.A.; Labuckas, D.O.; Das, U.N.; Eynard, A.R.; Pasqualini, M.E. Synergistic anti-tumor effects of melatonin and PUFAs from walnuts in a murine mammary adenocarcinoma model. Nutrition 2015, 31, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodriguez-Rodriguez, P.; Lopez de Pablo, A.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Melatonin-Measurement Methods and the Factors Modifying the Results. A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 1916. [Google Scholar] [CrossRef]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Bal, E. Effect of melatonin treatments on biochemical quality and postharvest life of nectarines. J. Food Meas. Charact. 2021, 15, 288–295. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Mascio, P.D.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N 1-acetyl-N 2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance of phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, R.; Fico, G.; Iritri, M. The presence of melatonin in grapevine (Vitis vinifera L.) Berry tissues. J. Pineal Res. 2001, 51, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Hall, B.A.; Le, C.H.; Saxena, P.K. Changes in the levels of indoleamine phytochemicals during veraison and ripening of wine grapes. J. Pineal Res. 2010, 49, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, H.E.; Gonzalez, C.V.; Wunderlin, D.A.; Silva, M.F. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: Evidence of its antioxidant role in fruits. J. Pineal Res. 2011, 31, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements Among US Adults, 1999-2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, G.; Tan, D.X.; Li, F.; Ma, X. A novel enzyme-dependent melatonin metabolite in humans. J. Pineal Res. 2013, 54, 100–106. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef]
- Habschied, K.; Loncaric, A.; Mastanjevic, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Bisignano, C.; Gugliandolo, E.; Carughi, A.; Esposito, E.; Mandalari, G.; Cuzzocrea, S. The Anti-Inflammatory and Antioxidant Potential of Pistachios (Pistacia vera L.) In Vitro and In Vivo. Nutrients 2017, 9, 915. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative Effects and Mechanism of Study of Bioactive Peptides from Defatted Walnut (Junglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef]
- Hong, M.Y.; Groven, S.; Marx, A.; Rasmussen, C.; Beidler, J. Anti-Inflammatory, Antioxidant, and hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats. Molecules 2018, 23, 3126. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Tikhonova, N.G.; Tendinik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Partkinson Disease-Like Disturbances in Mice. Molecules 2020, 25, 5339. [Google Scholar] [CrossRef] [PubMed]
- Legua, P.; Modica, G.; Porras, I.; Conesa, A.; Continella, A. Bioactive compounds, antioxidant activity and fruit quality evaluation of eleven blood orange cultivars. J. Sci. Food Agric. 2022, 102, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, M.; Clark, C.C.T.; Bahrami, A.; Teymoori, F.; Movahed, M.; Sohrab, G.; Hejazi, E. Dietary intake of polyphenols and total antioxidant capacity and risk of prostate cancer: A case-control study in Iranian men. Eur. J. Cancer Care 2021, 30, e13364. [Google Scholar] [CrossRef] [PubMed]
- Rickards, L.; Lynn, A.; Harrop, D.; Barker, M.E.; Russel, M.; Ranchordas, M.K. Effect of Polyphenol-Rich Foods, juices, and Concentrates on Recovery from Exercise Induced Muscle Damage: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).