Stress Activated MAP Kinases and Cyclin-Dependent Kinase 5 Mediate Nuclear Translocation of Nrf2 via Hsp90α-Pin1-Dynein Motor Transport Machinery
Abstract
:1. Introduction
2. p38 Controls Glutathione Sensor Neutral Sphingomyelinase 2
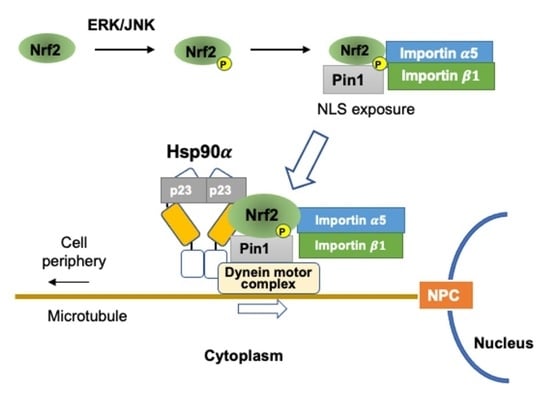
3. ERK/JNK and PPIase Pin1 Control Nrf2 Nuclear Translocation
4. PPIase and Hsp90 Cooperate in the Nuclear Transport of Signaling Molecules
5. Functional Interaction of Nrf2 with Hsp90
6. Pin1 Controls the Nuclear Translocation of Other ERK Substrates
7. Cdk5 Controls Nrf2 Nuclear Translocation through Pin1
8. Summary and Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Ishii, T.; Warabi, E.; Mann, G.E. Mechanisms underlying Nrf2 nuclear translocation by non-lethal levels of hydrogen peroxide: p38 MAPK-dependent neutral sphingomyelinase2 membrane trafficking and ceramide/PKCζ/CK2 signaling. Free Radic. Biol. Med. 2022, 191, 191–202. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.; Kwong, M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta 2000, 1517, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Chowdhry, S.; Dinkova-Kostova, A.T.; Sutherland, C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015, 43, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015, 88, 147–157. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88 Pt B, 108–146. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Wakabayashi, N.; Kensler, T.W. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat. Res. 2004, 555, 133–148. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Purdom-Dickinson, S.E.; Sheveleva, E.V.; Sun, H.; Chen, Q.M. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol. Pharmacol. 2007, 72, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Thakor, N.; Xu, E.Y.; Huang, Y.; Chen, C.; Yu, R.; Holcik, M.; Kong, A.N. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010, 38, 778–788. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Dinh, T.N.; Kappeler, K.; Tsaprailis, G.; Chen, Q.M. La autoantigen mediates oxidant induced de novo Nrf2 protein translation. Mol. Cell Proteom. 2012, 11, M111.015032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodore, M.; Kawai, Y.; Yang, J.; Kleshchenko, Y.; Reddy, S.P.; Villalta, F.; Arinze, I.J. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J. Biol. Chem. 2008, 283, 8984–8994, Erratum in J. Biol. Chem. 2008, 283, 14176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Görlich, D.; Prehn, S.; Laskey, R.A.; Hartmann, E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell 1994, 79, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Görlich, D.; Vogel, F.; Mills, A.D.; Hartmann, E.; Laskey, R.A. Distinct functions for the two importin subunits in nuclear protein import. Nature 1995, 377, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Görlich, D.; Mattaj, I.W. Nucleocytoplasmic transport. Science 1996, 271, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rexach, M.; Blobel, G. Protein import into nuclei: Association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995, 83, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Ullman, K.S.; Powers, M.A.; Forbes, D.J. Nuclear export receptors: From importin to exportin. Cell 1997, 90, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, K. Importins and exportins: How to get in and out of the nucleus. Trends Biochem. Sci. 1998, 23, 185–189, Erratum in Trends Biochem. Sci. 1998, 23, 235. [Google Scholar] [CrossRef]
- Li, W.; Jain, M.R.; Chen, C.; Yue, X.; Hebbar, V.; Zhou, R.; Kong, A.N. Nrf2 Possesses a redox-insensitive nuclear export signal overlapping with the leucine zipper motif. J. Biol. Chem. 2005, 280, 28430–28438. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yu, S.W.; Kong, A.N. Nrf2 possesses a redox-sensitive nuclear exporting signal in the Neh5 transactivation domain. J. Biol. Chem. 2006, 281, 27251–27263. [Google Scholar] [CrossRef] [Green Version]
- Kong, A.N.; Yu, R.; Chen, C.; Mandlekar, S.; Primiano, T. Signal transduction events elicited by natural products: Role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.N.; Owuor, E.; Yu, R.; Hebbar, V.; Chen, C.; Hu, R.; Mandlekar, S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab. Rev. 2001, 33, 255–271. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, M.H.; Robbins, D.J.; Boulton, T.G. ERKs, extracellular signal-regulated MAP-2 kinases. Curr. Opin. Cell Biol. 1991, 3, 1025–1032. [Google Scholar] [CrossRef]
- Ahn, N.G.; Seger, R.; Bratlien, R.L.; Krebs, E.G. Growth factor-stimulated phosphorylation cascades: Activation of growth factor-stimulated MAP kinase. Ciba Found. Symp. 1992, 164, 113–126; discussion 126–131. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Wilson, S.; Belham, C.M.; Robinson, C.J.; Scott, P.H.; Gould, G.W.; Plevin, R. Stress-activated protein kinases: Activation, regulation and function. Cell Signal. 1997, 9, 403–410. [Google Scholar] [CrossRef]
- Yu, R.; Tan, T.H.; Kong, A.N. Butylated hydroxyanisole and its metabolite tert-butylhydroquinone differentially regulate mitogen-activated protein kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. J. Biol. Chem. 1997, 272, 28962–28970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Yuan, X.; Pan, Z.; Shen, G.; Kim, J.H.; Yu, S.; Khor, T.O.; Li, W.; Ma, J.; Kong, A.N. Mechanism of action of isothiocyanates: The induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006, 5, 1918–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [Green Version]
- Zipper, L.M.; Mulcahy, R.T. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem. Biophys. Res. Commun. 2000, 278, 484–492. [Google Scholar] [CrossRef]
- Gong, P.; Hu, B.; Cederbaum, A.I. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch. Biochem. Biophys. 2004, 432, 252–260. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yen, G.C. Involvement of p38 MAPK and Nrf2 in phenolic acid-induced P-form phenol sulfotransferase expression in human hepatoma HepG2 cells. Carcinogenesis 2006, 27, 1008–1017. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Alam, J.; Wicks, C.; Stewart, D.; Gong, P.; Touchard, C.; Otterbein, S.; Choi, A.M.; Burow, M.E.; Tou, J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J. Biol. Chem. 2000, 275, 27694–27702. [Google Scholar] [CrossRef] [Green Version]
- Keum, Y.S.; Owuor, E.D.; Kim, B.R.; Hu, R.; Kong, A.N. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm. Res. 2003, 20, 1351–1356. [Google Scholar] [CrossRef]
- Ogborne, R.M.; Rushworth, S.A.; O’Connell, M.A. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler. Thromb. Vasc Biol. 2005, 25, 2100–2105. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.A.; Li, F.Y.; Leake, D.S.; Ishii, T.; Mann, G.E.; Siow, R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: Role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005, 39, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; Ogborne, R.M.; Charalambos, C.A.; O’Connell, M.A. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Papaiahgari, S.; Kleeberger, S.R.; Cho, H.Y.; Kalvakolanu, D.V.; Reddy, S.P. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J. Biol. Chem. 2004, 279, 42302–42312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pischke, S.E.; Zhou, Z.; Song, R.; Ning, W.; Alam, J.; Ryter, S.W.; Choi, A.M. Phosphatidylinositol 3-kinase/Akt pathway mediates heme oxygenase-1 regulation by lipopolysaccharide. Cell Mol. Biol. (Noisy-le-Grand) 2005, 51, 461–470. [Google Scholar]
- Choi, B.M.; Kim, S.M.; Park, T.K.; Li, G.; Hong, S.J.; Park, R.; Chung, H.T.; Kim, B.R. Piperine protects cisplatin-induced apoptosis via heme oxygenase-1 induction in auditory cells. J. Nutr. Biochem. 2007, 18, 615–622. [Google Scholar] [CrossRef]
- Manandhar, S.; Cho, J.M.; Kim, J.A.; Kensler, T.W.; Kwak, M.K. Induction of Nrf2-regulated genes by 3H-1, 2-dithiole-3-thione through the ERK signaling pathway in murine keratinocytes. Eur. J. Pharmacol. 2007, 577, 17–27. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Iles, K.E.; Liu, R.M.; Postlethwait, E.M.; Laperche, Y.; Forman, H.J. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am. J. Respir. Cell Mol. Biol. 2006, 34, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.J.; Lee, K.S.; Lee, S.; Park, J.H.; Choi, H.E.; Go, S.H.; Kwak, H.J.; Park, H.Y. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicol. Appl. Pharmacol. 2007, 223, 20–27. [Google Scholar] [CrossRef]
- Lin, A.H.; Chen, H.W.; Liu, C.T.; Tsai, C.W.; Lii, C.K. Activation of Nrf2 is required for up-regulation of the π class of glutathione S-transferase in rat primary hepatocytes with L-methionine starvation. J. Agric. Food Chem. 2012, 60, 6537–6545. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, B.J.; Marshall, Z.M.; Whorton, A.R. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003, 307, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Aburaya, M.; Tanaka, K.; Hoshino, T.; Tsutsumi, S.; Suzuki, K.; Makise, M.; Akagi, R.; Mizushima, T. Heme oxygenase-1 protects gastric mucosal cells against non-steroidal anti-inflammatory drugs. J. Biol. Chem. 2006, 281, 33422–33432. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Hannun, Y.A. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J. Biol. Chem. 1997, 272, 16281–16287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, S.; Banno, Y.; Nakashima, S.; Hayashi, K.; Yamakawa, H.; Sawada, M.; Sakai, N.; Nozawa, Y. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic PC12 cell death. J. Neurochem. 1999, 73, 675–683. [Google Scholar] [CrossRef]
- Chatterjee, S.; Han, H.; Rollins, S.; Cleveland, T. Molecular cloning, characterization, and expression of a novel human neutral sphingomyelinase. J. Biol. Chem. 1999, 274, 37407–37412. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, K.; Krut, O.; Wiegmann, K.; Kreder, D.; Micheli, M.; Schäfer, R.; Sickman, A.; Schmidt, W.E.; Schröder, J.M.; Meyer, H.E.; et al. Purification and characterization of a magnesium-dependent neutral sphingomyelinase from bovine brain. J. Biol. Chem. 2000, 275, 7641–7647. [Google Scholar] [CrossRef] [Green Version]
- Lavrentiadou, S.N.; Chan, C.; Kawcak, T.; Ravid, T.; Tsaba, A.; van der Vliet, A.; Rasooly, R.; Goldkorn, T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am. J. Respir. Cell Mol. Biol. 2001, 25, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Warabi, E. Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR). Antioxidants 2019, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Warabi, E.; Mann, G.E. Mechanisms underlying unidirectional laminar shear stress-mediated Nrf2 activation in endothelial cells: Amplification of low shear stress signaling by primary cilia. Redox Biol. 2021, 46, 102103. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Castillo, S.S.; Goldkorn, T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem. Biophys. Res. Commun. 2006, 344, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, C.J.; Truong, T.G.; Hannun, Y.A. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J. Biol. Chem. 2007, 282, 1384–1396. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Shi, S.; Liu, M.; Qin, Y.; Meng, Q.; Hua, J.; Ji, S.; Zhang, Y.; Yang, J.; Xu, J.; et al. PIN1 Maintains Redox Balance via the c-Myc/NRF2 Axis to Counteract Kras-Induced Mitochondrial Respiratory Injury in Pancreatic Cancer Cells. Cancer Res. 2019, 79, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Saeidi, S.; Kim, S.J.; Han, H.J.; Kim, S.H.; Zheng, J.; Lee, H.B.; Han, W.; Noh, D.Y.; Na, H.K.; Surh, Y.J. H-Ras induces Nrf2-Pin1 interaction: Implications for breast cancer progression. Toxicol. Appl. Pharmacol. 2020, 402, 115121. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, S.; Kim, S.J.; Guillen-Quispe, Y.N.; Jagadeesh, A.S.V.; Han, H.J.; Kim, S.H.; Zhong, X.; Piao, J.Y.; Kim, S.J.; Jeong, J.; et al. Peptidylprolyl cis-trans isomerase NIMA-interacting 1 directly binds and stabilizes Nrf2 in breast cancer. FASEB J. 2022, 36, e22068. [Google Scholar] [CrossRef]
- Göthel, S.F.; Marahiel, M.A. Peptidylprolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol. Life Sci. 1999, 55, 423–436. [Google Scholar] [CrossRef]
- Ratajczak, T.; Cluning, C.; Ward, B.K. Steroid Receptor-Associated Immunophilins: A Gateway to Steroid Signalling. Clin. Biochem Rev. 2015, 36, 31–52. [Google Scholar]
- Rostam, M.A.; Piva, T.J.; Rezaei, H.B.; Kamato, D.; Little, P.J.; Zheng, W.; Osman, N. Peptidylprolyl isomerases: Functionality and potential therapeutic targets in cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2015, 42, 117–124. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Radanyi, C.; Renoir, J.M.; Housley, P.R.; Pratt, W.B. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J. Biol. Chem. 2001, 276, 14884–14889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galigniana, M.D.; Harrell, J.M.; Murphy, P.J.; Chinkers, M.; Radanyi, C.; Renoir, J.M.; Zhang, M.; Pratt, W.B. Binding of hsp90-associated immunophilins to cytoplasmic dynein: Direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry 2002, 41, 13602–13610. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.H.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef]
- Li, J.; Soroka, J.; Buchner, J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta 2012, 1823, 624–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol Med. (Maywood) 2003, 228, 111–133. [Google Scholar] [CrossRef]
- Pratt, W.B.; Galigniana, M.D.; Harrell, J.M.; DeFranco, D.B. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004, 16, 857–872. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Echeverría, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.C.; Li, Y.; Dehm, S.M. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J. Biol. Chem. 2012, 287, 19736–19749. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; An, Y.S.; Kim, M.R.; Kim, Y.A.; Lee, J.K.; Hwang, C.S.; Chung, E.; Park, I.C.; Yi, J.Y. Heat Shock Protein 90 Regulates Subcellular Localization of Smads in Mv1Lu Cells. J. Cell Biochem. 2016, 117, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mazaira, G.I.; Piwien Pilipuk, G.; Galigniana, M.D. Corticosteroid receptors as a model for the Hsp90•immunophilin-based transport machinery. Trends Endocrinol Metab. 2021, 32, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Buchner, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2013, 36, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.H.; Prodromou, C.; Workman, P. The Hsp90 molecular chaperone: An open and shut case for treatment. Biochem. J. 2008, 410, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Synoradzki, K.; Miszta, P.; Kazlauskas, E.; Mickevičiūtė, A.; Michailovienė, V.; Matulis, D.; Filipek, S.; Bieganowski, P. Interaction of the middle domains stabilizes Hsp90α dimer in a closed conformation with high affinity for p23. Biol. Chem. 2018, 399, 337–345. [Google Scholar] [CrossRef]
- Taipale, M.; Tucker, G.; Peng, J.; Krykbaeva, I.; Lin, Z.Y.; Larsen, B.; Choi, H.; Berger, B.; Gingras, A.C.; Lindquist, S. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell 2014, 158, 434–448. [Google Scholar] [CrossRef] [Green Version]
- Rehn, A.B.; Buchner, J. p23 and Aha1. Subcell Biochem. 2015, 78, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, Z.L.; Molugu, S.K.; Herrera, N.; Ramirez, C.; Xiao, C.; Bernal, R.A. Hsp90 can accommodate the simultaneous binding of the FKBP52 and HOP proteins. Oncotarget 2011, 2, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Miao, X.; Qi, Z.; Zeng, W.; Liang, J.; Liang, Z. Hepatitis B virus X protein upregulates HSP90alpha expression via activation of c-Myc in human hepatocarcinoma cell line, HepG2. Virol. J. 2010, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, T.; Sato, N. Oligomeric forms of the 90-kDa heat shock protein. Biochem. J. 1998, 330 Pt 2, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Kobayakawa, T.; Yamada, S.; Mizuno, A.; Nemoto, T.K. Substitution of only two residues of human Hsp90alpha causes impeded dimerization of Hsp90beta. Cell Stress Chaperones. 2008, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Ngo, V.; Brickenden, A.; Liu, H.; Yeung, C.; Choy, W.Y.; Duennwald, M.L. A novel yeast model detects Nrf2 and Keap1 interactions with Hsp90. Dis. Model Mech. 2022, 15, dmm.049258. [Google Scholar] [CrossRef]
- Jia, Z.; Dong, A.; Che, H.; Zhang, Y. 17-DMAG Protects Against Hypoxia-/Reoxygenation-Induced Cell Injury in HT22 Cells Through Akt/Nrf2/HO-1 Pathway. DNA Cell Biol. 2017, 36, 95–102. [Google Scholar] [CrossRef]
- Lazaro, I.; Oguiza, A.; Recio, C.; Lopez-Sanz, L.; Bernal, S.; Egido, J.; Gomez-Guerrero, C. Interplay between HSP90 and Nrf2 pathways in diabetes-associated atherosclerosis. Clin. Investig. Arterioscler. 2017, 29, 51–59. [Google Scholar] [CrossRef]
- Baird, L.; Suzuki, T.; Takahashi, Y.; Hishinuma, E.; Saigusa, D.; Yamamoto, M. Geldanamycin-Derived HSP90 Inhibitors Are Synthetic Lethal with NRF2. Mol. Cell Biol. 2020, 40, e00377-20. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle 2013, 12, 3154–3158. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Locasale, J.W.; Swanson, K.D.; Sharfi, H.; Heffron, G.J.; Amador-Noguez, D.; Christofk, H.R.; Wagner, G.; Rabinowitz, J.D.; Asara, J.M.; et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 2010, 329, 1492–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhang, J.; Manley, J.L. Turning on a fuel switch of cancer: hnRNP proteins regulate alternative splicing of pyruvate kinase mRNA. Cancer Res. 2010, 70, 8977–8980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayley, J.P.; Devilee, P. The Warburg effect in 2012. Curr. Opin. Oncol. 2012, 24, 62–67. [Google Scholar] [CrossRef]
- Tamada, M.; Suematsu, M.; Saya, H. Pyruvate kinase M2: Multiple faces for conferring benefits on cancer cells. Clin. Cancer Res. 2012, 18, 5554–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipp, F.V. Cancer metabolism meets systems biology: Pyruvate kinase isoform PKM2 is a metabolic master regulator. J. Carcinog. 2013, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Ojo, D.; Yan, J.; Tang, D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015, 356, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zheng, Y.; Xia, Y.; Ji, H.; Chen, X.; Guo, F.; Lyssiotis, C.A.; Aldape, K.; Cantley, L.C.; Lu, Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012, 14, 1295–1304, Erratum in Nat. Cell Biol. 2013, 15, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef]
- Kaelin, W.G. Proline hydroxylation and gene expression. Annu. Rev. Biochem. 2005, 74, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007, 2007, cm8. [Google Scholar] [CrossRef]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef] [Green Version]
- Kallio, P.J.; Okamoto, K.; O’Brien, S.; Carrero, P.; Makino, Y.; Tanaka, H.; Poellinger, L. Signal transduction in hypoxic cells: Inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998, 17, 6573–6586. [Google Scholar] [CrossRef]
- Jalouli, M.; Déry, M.A.; Lafleur, V.N.; Lamalice, L.; Zhou, X.Z.; Lu, K.P.; Richard, D.E. The prolyl isomerase Pin1 regulates hypoxia-inducible transcription factor (HIF) activity. Cell Signal. 2014, 26, 1649–1656. [Google Scholar] [CrossRef]
- Han, H.J.; Saeidi, S.; Kim, S.J.; Piao, J.Y.; Lim, S.; Guillen-Quispe, Y.N.; Choi, B.Y.; Surh, Y.J. Alternative regulation of HIF-1α stability through Phosphorylation on Ser451. Biochem. Biophys. Res. Commun. 2021, 545, 150–156. [Google Scholar] [CrossRef]
- Tang, X.; Chang, C.; Hao, M.; Chen, M.; Woodley, D.T.; Schönthal, A.H.; Li, W. Heat shock protein-90alpha (Hsp90α) stabilizes hypoxia-inducible factor-1α (HIF-1α) in support of spermatogenesis and tumorigenesis. Cancer Gene Ther. 2021, 28, 1058–1070, Erratum in Cancer Gene Ther. 2021, 28, 1071–1072. [Google Scholar] [CrossRef]
- Depping, R.; Steinhoff, A.; Schindler, S.G.; Friedrich, B.; Fagerlund, R.; Metzen, E.; Hartmann, E.; Köhler, M. Nuclear translocation of hypoxia-inducible factors (HIFs): Involvement of the classical importin alpha/beta pathway. Biochim. Biophys. Acta 2008, 1783, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chachami, G.; Paraskeva, E.; Mingot, J.M.; Braliou, G.G.; Görlich, D.; Simos, G. Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem. Biophys. Res. Commun. 2009, 390, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Depping, R.; Jelkmann, W.; Kosyna, F.K. Nuclear-cytoplasmatic shuttling of proteins in control of cellular oxygen sensing. J. Mol. Med. 2015, 93, 599–608. [Google Scholar] [CrossRef]
- Mylonis, I.; Chachami, G.; Paraskeva, E.; Simos, G. Atypical CRM1-dependent nuclear export signal mediates regulation of hypoxia-inducible factor-1alpha by MAPK. J. Biol. Chem. 2008, 283, 27620–27627. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Blasco, D.; Santofimia-Castaño, P.; Gonzalez, A.; Almeida, A.; Bolaños, J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Hanson, J.M.; Chu, W.A.; Johnson, J.A. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J. Biol. Chem. 2001, 276, 20011–20016. [Google Scholar] [CrossRef] [Green Version]
- Strocchi, P.; Pession, A.; Dozza, B. Up-regulation of cDK5/p35 by oxidative stress in human neuroblastoma IMR-32 cells. J. Cell Biochem. 2003, 88, 758–765. [Google Scholar] [CrossRef]
- Sang, Y.; Li, Y.; Zhang, Y.; Alvarez, A.A.; Yu, B.; Zhang, W.; Hu, B.; Cheng, S.Y.; Feng, H. CDK5-dependent phosphorylation and nuclear translocation of TRIM59 promotes macroH2A1 ubiquitination and tumorigenicity. Nat. Commun. 2019, 10, 4013. [Google Scholar] [CrossRef] [Green Version]
- Pozo, K.; Bibb, J.A. The Emerging Role of Cdk5 in Cancer. Trends Cancer. 2016, 2, 606–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, J.; Song, Y.S.; Li, Y.; Jia, Y.H.; Zhao, H.D. Cdk5 links with DNA damage response and cancer. Mol. Cancer. 2017, 16, 60. [Google Scholar] [CrossRef] [Green Version]
- Oner, M.; Lin, E.; Chen, M.C.; Hsu, F.N.; Shazzad Hossain Prince, G.M.; Chiu, K.Y.; Teng, C.J.; Yang, T.Y.; Wang, H.Y.; Yue, C.H.; et al. Future Aspects of CDK5 in Prostate Cancer: From Pathogenesis to Therapeutic Implications. Int. J. Mol. Sci. 2019, 20, 3881. [Google Scholar] [CrossRef] [Green Version]
- Do, P.A.; Lee, C.H. The Role of CDK5 in Tumours and Tumour Microenvironments. Cancers 2020, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sicinski, P. A kinase of many talents: Non-neuronal functions of CDK5 in development and disease. Open Biol. 2020, 10, 190287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vartholomaiou, E.; Madon-Simon, M.; Hagmann, S.; Mühlebach, G.; Wurst, W.; Floss, T.; Picard, D. Cytosolic Hsp90α and its mitochondrial isoform Trap1 are differentially required in a breast cancer model. Oncotarget 2017, 8, 17428–17442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barginear, M.F.; Van Poznak, C.; Rosen, N.; Modi, S.; Hudis, C.A.; Budman, D.R. The heat shock protein 90 chaperone complex: An evolving therapeutic target. Curr. Cancer Drug Targets 2008, 8, 522–532. [Google Scholar] [CrossRef]
- Barrott, J.J.; Haystead, T.A. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, R.; Yuen, J.; Yuen, M.F.; Lai, C.L.; Lee, T.K.; Man, K.; Poon, R.T.; Fan, S.T.; Wong, C.M.; Ng, I.O.; et al. PIN1 overexpression and beta-catenin gene mutations are distinct oncogenic events in human hepatocellular carcinoma. Oncogene 2004, 23, 4182–4186. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Wang, L.; Xu, J.; Jiang, P.; Wang, W.; Huang, Y.; Lv, M.; Liu, S. Knockdown of the prolyl isomerase Pin1 inhibits Hep-2 cell growth, migration, and invasion by targeting the β-catenin signaling pathway. Biochem. Cell Biol. 2018, 96, 734–741. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Shi, F.; Wang, J. Pin1 modulates chemo-resistance by up-regulating FoxM1 and the involvements of Wnt/β-catenin signaling pathway in cervical cancer. Mol. Cell Biochem. 2016, 413, 179–187. [Google Scholar] [CrossRef]
- Xu, G.G.; Etzkorn, F.A. Pin1 as an anticancer drug target. Drug News Perspect. 2009, 22, 399–407. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Lu, K.P. The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target. Nat. Rev. Cancer 2016, 16, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xue, X.; Chen, Y.; Zheng, N.; Wang, J. Targeting prolyl isomerase Pin1 as a promising strategy to overcome resistance to cancer therapies. Pharmacol. Res. 2022, 184, 106456. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kozono, S.; Kats, L.; Nechama, M.; Li, W.; Guarnerio, J.; Luo, M.; You, M.H.; Yao, Y.; Kondo, A.; et al. Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer. Nat. Med. 2015, 21, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Y.; Liang, Z.M.; Li, N.N.; Liu, Y.; Zhu, Y.; Liao, D.; Zhou, X.Z.; Lu, K.P.; Yao, Y.; et al. Targeting Pin1 by All-Trans Retinoic Acid (ATRA) Overcomes Tamoxifen Resistance in Breast Cancer via Multifactorial Mechanisms. Front. Cell Dev. Biol. 2019, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, C.; Pinch, B.J.; Koikawa, K.; Zaidman, D.; Poon, E.; Manz, T.D.; Nabet, B.; He, S.; Resnick, E.; Rogel, A.; et al. Sulfopin is a covalent inhibitor of Pin1 that blocks Myc-driven tumors in vivo. Nat. Chem Biol. 2021, 17, 954–963. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Mu, C.; Li, M.; Li, K.; Li, S.; Wu, W.; Du, L.; Zhang, X.; Li, C.; et al. Pancreatic tumor eradication via selective Pin1 inhibition in cancer-associated fibroblasts and T lymphocytes engagement. Nat. Commun. 2022, 13, 4308. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, M.Y.; Lim, S.C.; Hien, T.T.; Kim, J.W.; Ahn, S.G.; Yoon, J.H.; Kim, S.K.; Choi, H.S.; Kang, K.W. Role of Pin1 in neointima formation: Down-regulation of Nrf2-dependent heme oxygenase-1 expression by Pin1. Free Radic. Biol. Med. 2010, 48, 1644–1653. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Mouawad, C.A.; Mrad, M.F.; Al-Hariri, M.; Soussi, H.; Hamade, E.; Alam, J.; Habib, A. Role of nitric oxide and CCAAT/enhancer-binding protein transcription factor in statin-dependent induction of heme oxygenase-1 in mouse macrophages. PLoS ONE. 2013, 8, e64092. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Pollet, M.; Krutmann, J.; Haarmann-Stemmann, T. Commentary: Usage of Mitogen-Activated Protein Kinase Small Molecule Inhibitors: More Than Just Inhibition! Front. Pharmacol. 2018, 9, 935. [Google Scholar] [CrossRef]
- Korashy, H.M.; Anwar-Mohamed, A.; Soshilov, A.A.; Denison, M.S.; El-Kadi, A.O. The p38 MAPK inhibitor SB203580 induces cytochrome P450 1A1 gene expression in murine and human hepatoma cell lines through ligand-dependent aryl hydrocarbon receptor activation. Chem Res. Toxicol. 2011, 24, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Mandlekar, S.; Lei, W.; Fahl, W.E.; Tan, T.H.; Kong, A.N. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J. Biol. Chem. 2000, 275, 2322–2327. [Google Scholar] [CrossRef] [Green Version]
- Nioi, P.; Hayes, J.D. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004, 555, 149–171. [Google Scholar] [CrossRef]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [Green Version]
- Gharavi, N.; El-Kadi, A.O. tert-Butylhydroquinone is a novel aryl hydrocarbon receptor ligand. Drug Metab. Dispos. 2005, 33, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Köhle, C.; Bock, K.W. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007, 73, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Biernat, J.; Drewes, G.; Gustke, N.; Trinczek, B.; Mandelkow, E. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging. 1995, 16, 355–362; discussion 362–363. [Google Scholar] [CrossRef]
- Johnson, G.V.; Stoothoff, W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004, 117, 5721–5729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, U.; Utton, M.; Gallo, J.M.; Miller, C.C. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 1996, 109 Pt 6, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Lovestone, S.; Hartley, C.L.; Pearce, J.; Anderton, B.H. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: The effects on the organization and stability of microtubules. Neuroscience 1996, 73, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Wulf, G.; Zhou, X.Z.; Davies, P.; Lu, K.P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399, 784–788. [Google Scholar] [CrossRef]
- Min, S.H.; Cho, J.S.; Oh, J.H.; Shim, S.B.; Hwang, D.Y.; Lee, S.H.; Jee, S.W.; Lim, H.J.; Kim, M.Y.; Sheen, Y.Y.; et al. Tau and GSK3beta dephosphorylations are required for regulating Pin1 phosphorylation. Neurochem. Res. 2005, 30, 955–961. [Google Scholar] [CrossRef]
- Ma, S.L.; Pastorino, L.; Zhou, X.Z.; Lu, K.P. Prolyl isomerase Pin1 promotes amyloid precursor protein (APP) turnover by inhibiting glycogen synthase kinase-3β (GSK3β) activity: Novel mechanism for Pin1 to protect against Alzheimer disease. J. Biol. Chem. 2012, 287, 6969–6973. [Google Scholar] [CrossRef] [Green Version]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Driver, J.A.; Zhou, X.Z.; Lu, K.P. Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1850, 2069–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef]



| Cells | Activators | MAPK Dependence | References |
|---|---|---|---|
| Human hepatoma HepG2 | Sodium arsenite and mercury chloride | JNK-dependent ARE reporter gene and HO-1 expression | [35] |
| Pyrrolidine dithiocarbamate | ERK and p38 inhibitors PD98059 and SB202190 reduced about 50% in γ-glutamylcystein synthetase expression | [36] | |
| Diallyl sulfide | ERK- and p38- dependent Nrf2 nuclear translocation and HO-1 expression | [37] | |
| Gallic acid | p38 inhibitor reduced ARE-dependent P-form of phenol sulfotransferase expression | [38] | |
| Human hepatocyte | Quercetin | p38- and ERK-dependent Nrf2 activation and HO-1 expression | [39] |
| Human mammary epithelia MCF-7 | Cadmium chloride | p38-dependent but ERK-independent HO-1 expression | [40] |
| Human HeLa | Phenethyl isothiocyanate | JNK-dependent ARE-reporter gene expression | [41] |
| Human monocytic THP-1 | α-Lipoic acid | p38 inhibitor significantly reduced Nrf2 dependent HO-1 expression | [42] |
| Human aortic smooth muscle | Oxidized low-density lipoprotein | ERK, p38, and JNK inhibitors respectively reduced HO-1 expression and Nrf2 nuclear translocation | [43] |
| Human prostate carcinoma PC-3 | Phenethyl isothiocyanate | ERK and JNK phosphorylate Nrf2 and induce nuclear translocation of Nrf2 | [34] |
| Human monocyte | Curcumin | p38 inhibitor but not ERK inhibitor reduced ARE-dependent GCLM and HO-1 mRNA expression | [44] |
| Mouse alveolar epithelial C10 | Hyperoxia | Hyperoxia activates NADPH oxidase, which results in ERK-dependent Nrf2 activation | [45] |
| Mouse macrophage RAW 264.7 | Lipopolysaccharide | p38 inhibitor significantly reduced Nrf2 dependent HO-1 expression | [46] |
| Mouse cochlear | Piperine | JNK inhibitor significantly reduced ARE-reporter gene expression and HO-1 expression | [47] |
| Mouse keratinocyte | 3H-1,2-dithiole-3-thione | ERK inhibitor but not p38 inhibitor suppressed Nrf2 nuclear translocation and ARE-reporter gene expression | [48] |
| Rat epithelial L2 | 4-hydroxynonenal | ERK- and p38-dependent EPRE-mediated γ-glutamyl transpeptidase expression | [49] |
| Rat vascular smooth muscle | 15d-PGJ2 | p38 inhibitor abolished Nrf2 dependent HO-1 expression | [50] |
| Rat primary hepatocytes | Methionine restriction | ERK-dependent Nrf2 nuclear translocation and GSH-S-transferase π expression | [51] |
| Rat kidney epithelial NRK-52E | Curcumin | p38 inhibitor reduced about 50% of HO-1 expression, but ERK and JNK inhibitors did not suppress HO-1 expression | [52] |
| Bovine aortic endothelial | Spermine NONOate (NO donor) | p38 and ERK inhibitors SB203580 and PD98059 respectively reduce HO-1 expression | [53] |
| Guinea pig gastric mucosal | Indomethacin | p38 inhibitor significantly reduced Nrf2 nuclear accumulation and HO-1 expression | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, T.; Warabi, E.; Mann, G.E. Stress Activated MAP Kinases and Cyclin-Dependent Kinase 5 Mediate Nuclear Translocation of Nrf2 via Hsp90α-Pin1-Dynein Motor Transport Machinery. Antioxidants 2023, 12, 274. https://doi.org/10.3390/antiox12020274
Ishii T, Warabi E, Mann GE. Stress Activated MAP Kinases and Cyclin-Dependent Kinase 5 Mediate Nuclear Translocation of Nrf2 via Hsp90α-Pin1-Dynein Motor Transport Machinery. Antioxidants. 2023; 12(2):274. https://doi.org/10.3390/antiox12020274
Chicago/Turabian StyleIshii, Tetsuro, Eiji Warabi, and Giovanni E. Mann. 2023. "Stress Activated MAP Kinases and Cyclin-Dependent Kinase 5 Mediate Nuclear Translocation of Nrf2 via Hsp90α-Pin1-Dynein Motor Transport Machinery" Antioxidants 12, no. 2: 274. https://doi.org/10.3390/antiox12020274
APA StyleIshii, T., Warabi, E., & Mann, G. E. (2023). Stress Activated MAP Kinases and Cyclin-Dependent Kinase 5 Mediate Nuclear Translocation of Nrf2 via Hsp90α-Pin1-Dynein Motor Transport Machinery. Antioxidants, 12(2), 274. https://doi.org/10.3390/antiox12020274