Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultivation
2.2. Purification of Glucansucrase
2.3. Effect of Temperature, pH and Metal Ions on the Activity of Purified Glucansucrase
2.4. Synthesis of EPS In Vitro
2.5. Determination of Chemical Composition
2.6. Molecular Mass Distribution
2.7. Monosaccharide Composition Analysis
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy Analysis
2.9. X-ray Diffraction (XRD) Analysis
2.10. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.11. Scanning Electron Microscopy (SEM) and Atomic Force Micrograph (AFM) Analysis
2.12. Thermal Analysis
2.13. Water Solubility Index (WSI) and Water Holding Capacity (WHC) Analysis
2.14. Emulsification Activity (EA) Analysis
2.15. Antioxidant Activity Analysis
3. Results
3.1. Purification and Characterisation of Glucansucrase
3.2. Analysis of the EPS Produced by Ln. pseudomesenteroides Glucansucrase
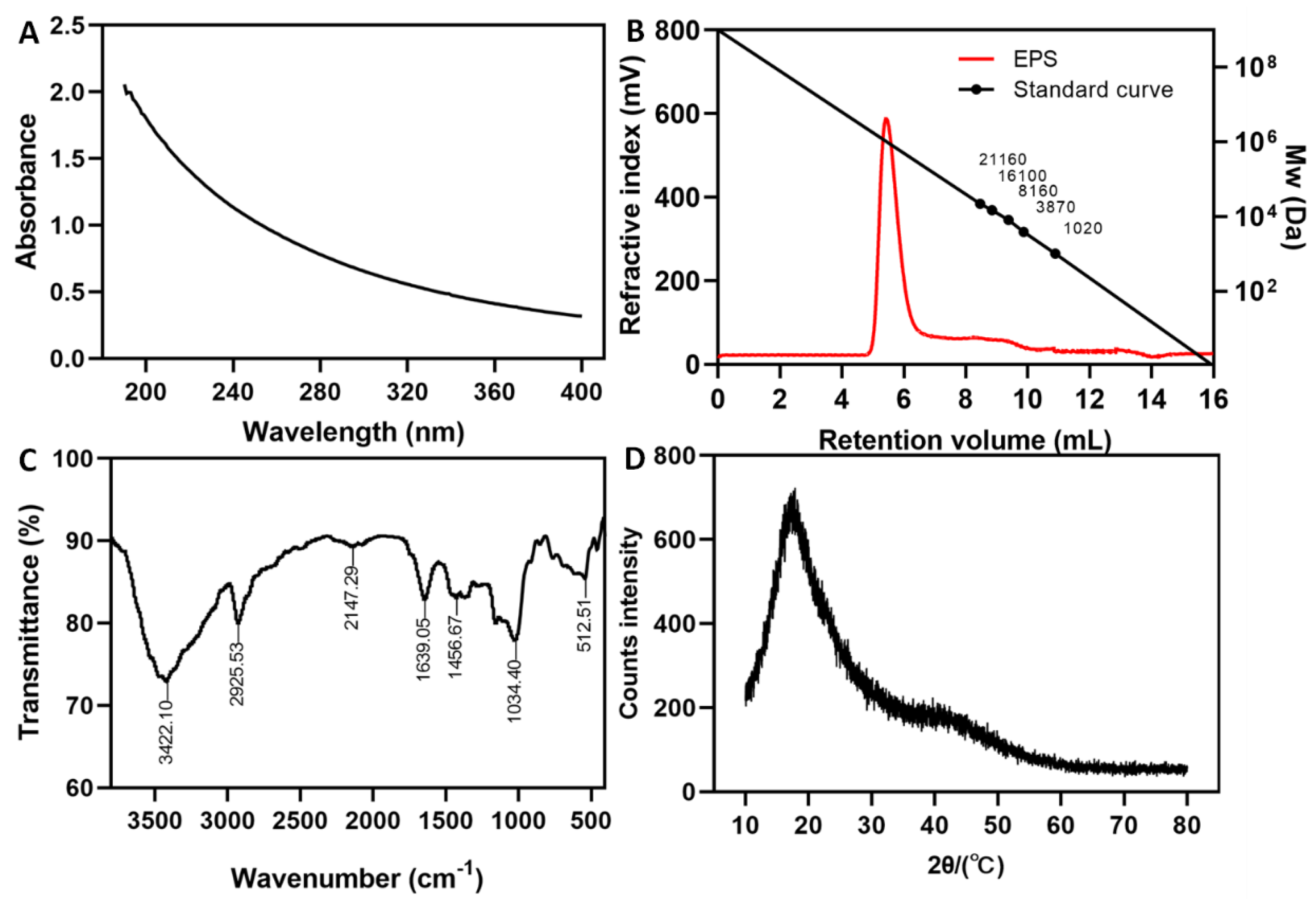
3.3. Molecular Mass and Monosaccharide Composition Analysis of EPS
3.4. FT-IR and XRD Analysis of EPS
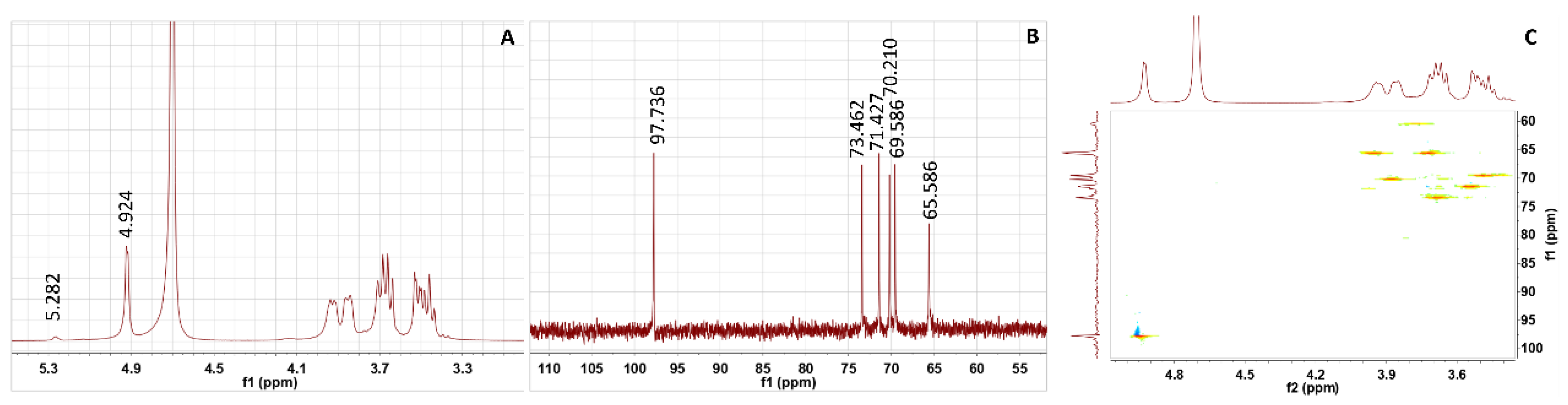
3.5. NMR Analysis of EPS
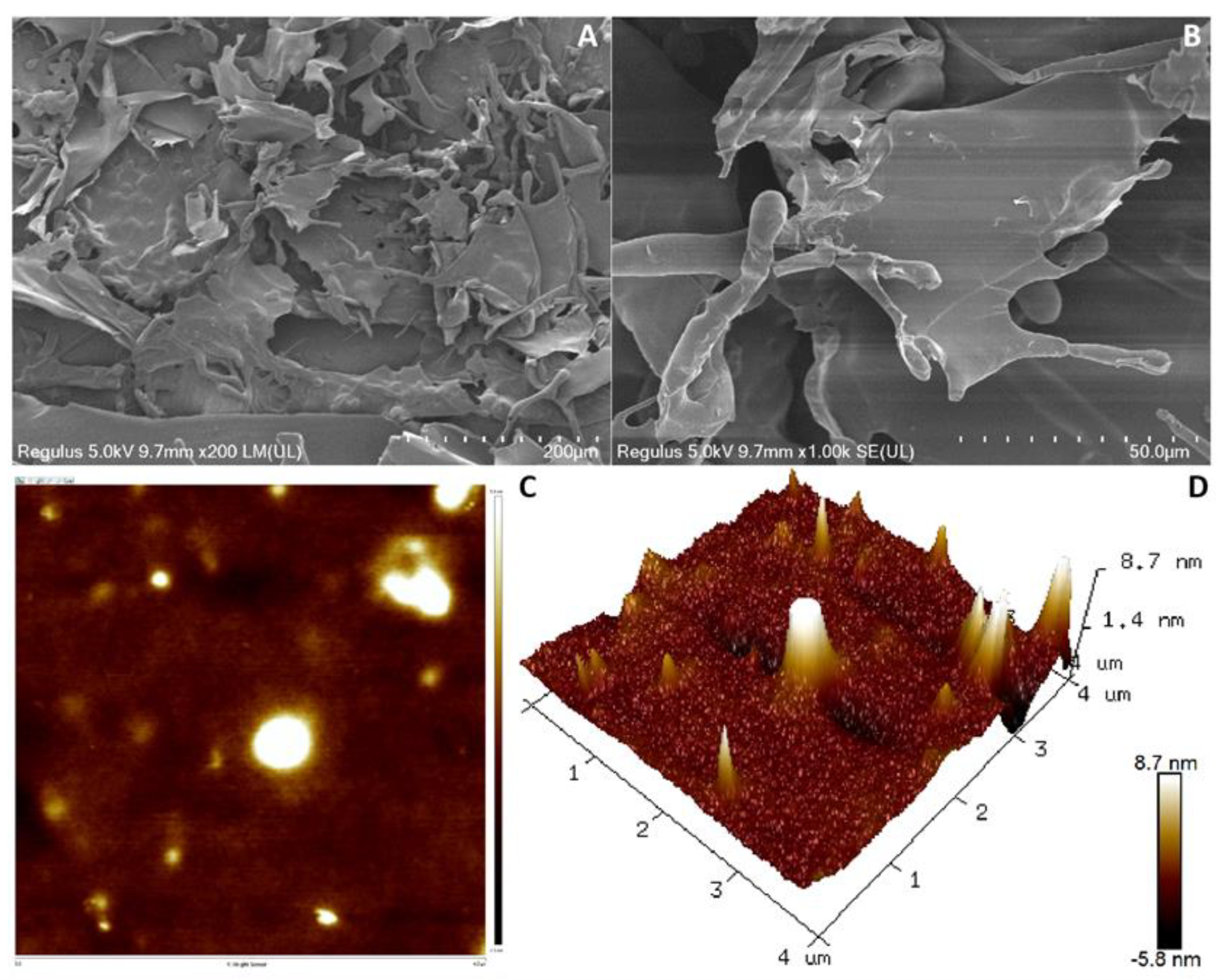
3.6. SEM and AFM Analysis of Dextran
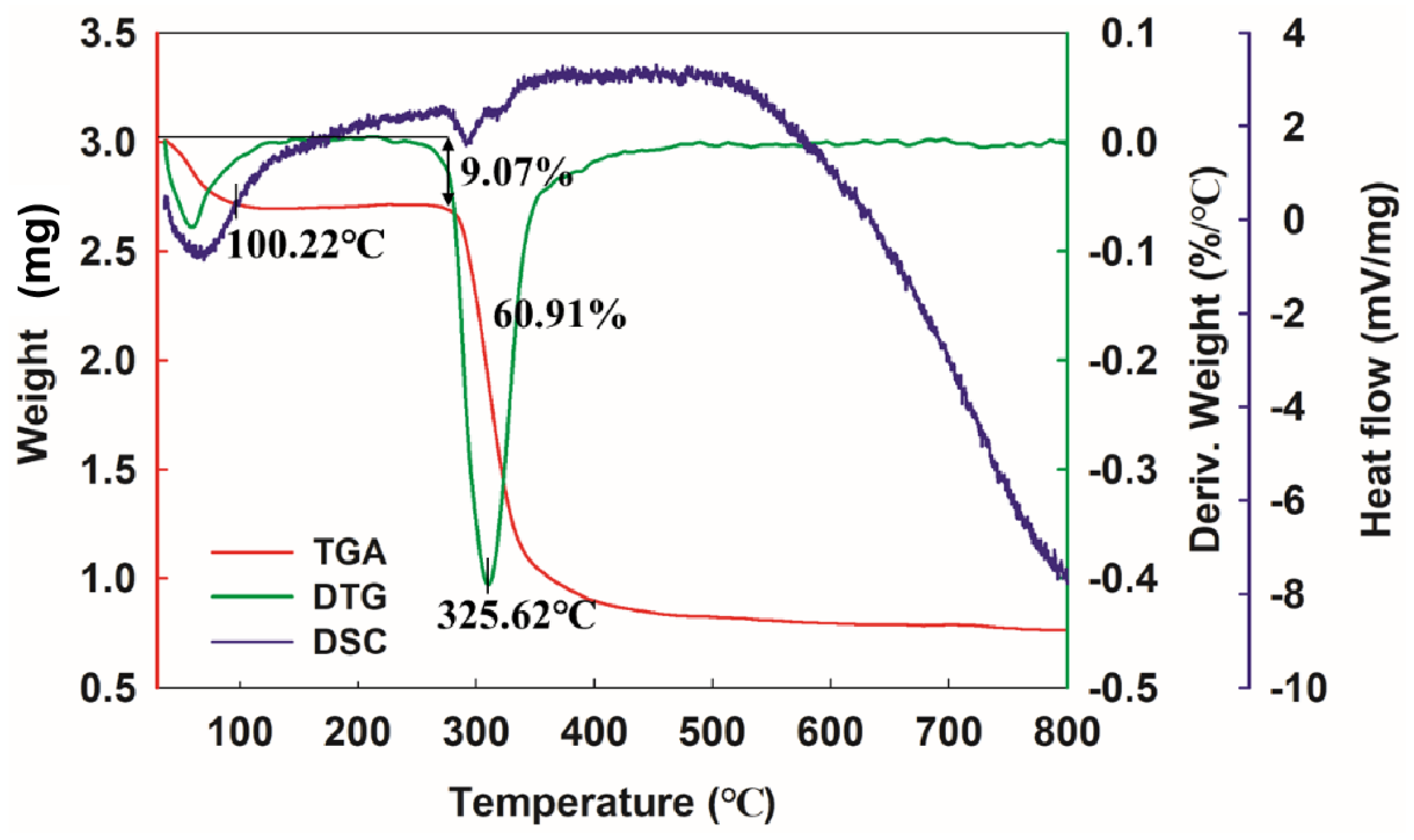
3.7. Thermal Analysis of Dextran
3.8. WSI and WHC Analysis of Dextran
3.9. EA Analysis of Dextran
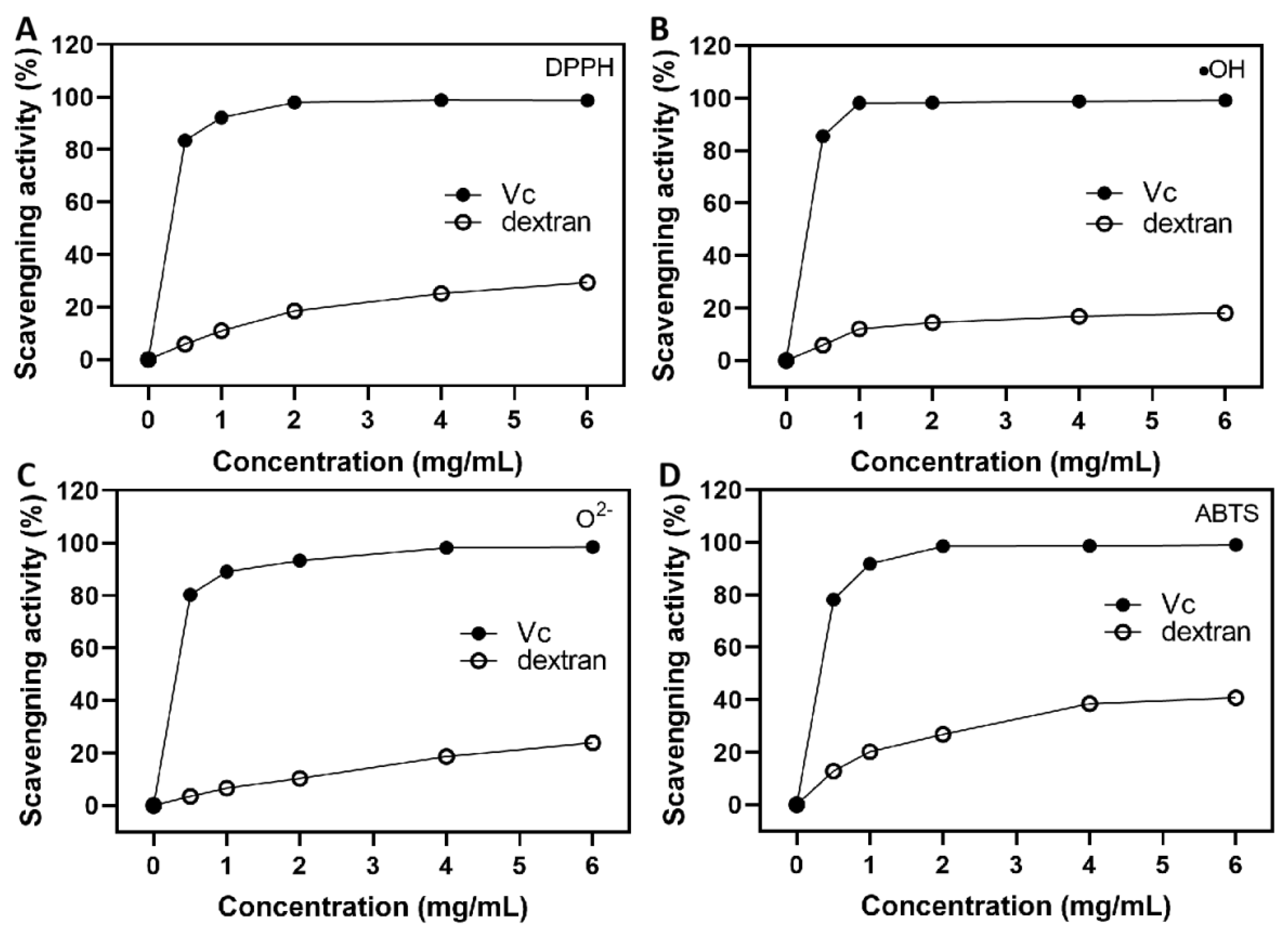
3.10. Antioxidant Activity Analysis of Dextran
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luan, D.; Ji, Y.; Peng, L.; Liu, Q.; Zeng, N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohyd. Polym. 2021, 261, 117863. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, M.; He, Y.; Zhai, C.; Li, C. A review on the production, structure, bioactivities and applications of Tremella polysaccharides. Int. J. Immunopath. Ph. 2021, 35, 205873842110005. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Lee, C.M.; Lee, S.H.; Baik, S.H. Evaluation of probiotic properties of Pediococcus acidilactici M76 producing functional exopolysaccharides and its lactic acid fermentation of black raspberry extract. Microorganisms 2021, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Niu, F.J.; Xie, Y.X.; Xie, S.M.; Wan, X.H. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The anti-cancer effects and mechanisms of lactic acid bacteria exopolysaccharides in vitro: A review. Carbohyd. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Madhuri, K.; Prabhakar, K. Microbial exopolysaccharides: Biosynthesis and potential applications. Orient. J. Chem. 2014, 30, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Calzini, M.A.; Malico, A.A.; Mitchler, M.M.; Williams, G.J. Protein engineering for natural product biosynthesis and synthetic biology applications. Protein Eng. Des. Sel. 2021, 34, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Delbianco, M. Synthetic polysaccharides. Recent Trends Carbohydr. Chem. 2020, 1, 333–371. [Google Scholar]
- Rana, S.; Upadhyay, L. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Dols, M.; Remaud-Simeon, M.; Monsan, P.F. Dextransucrase production by Leuconostoc mesenteroides NRRL B1299 comparison with L. mesenteroides NRRL B512F. Enzyme Microb. Tech. 1997, 20, 523–530. [Google Scholar] [CrossRef]
- Monchois, V.; Remaud-Simeon, M.; Russell, R.; Monsan, P.; Willemot, R.M. Characterization of Leuconostoc mesenteroides NRRL B-512F dextransucrase (DSRS) and identification of amino-acid residues playing a key role in enzyme activity. Appl. Microbiol. Biot. 1997, 48, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qian, Z.; Ge, J.; Du, R. Glucansucrase produced by lactic acid bacteria: Structure, properties and applications. Fermentation 2022, 8, 629. [Google Scholar] [CrossRef]
- Paul, F.; Auriol, D.; Oriol, E.; Monsan, P. Production and purification of dextransucrase from Leuconostoc mesenteroides, NRRL B 512 (F). Ann. N. Y. Acad. Sci. 2010, 434, 267–270. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohyd. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 420–428. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Springerplus 1980, 89, 449–454. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xu, R.; Shen, Q.; Ding, X.; Gao, W.; Li, P. Chemical characterization and antioxidant activity of an exopolysaccharide fraction isolated from Bifidobacterium animalis RH. Eur. Food Res. Technol. 2011, 232, 231–240. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, L.; Peng, L.; Ahmed, Z.; Ping, X.; Bai, X. Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohyd. Polym. 2010, 82, 895–903. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohyd. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Jiang, J.; Du, R.; Guo, S.; Ping, W.; Ling, H.; Ge, J. Purification and characterization of an exopolysaccharide from Leuconostoc lactis L2. Int. J. Biol. Macromol. 2019, 139, 1224–1231. [Google Scholar] [CrossRef]
- Jiang, J.; Guo, S.; Ping, W.; Zhao, D.; Ge, J. Optimization production of exopolysaccharide from Leuconostoc lactis L2 and its partial characterization. Int. J. Biol. Macromol. 2020, 159, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol. 2020, 161, 1181–1188. [Google Scholar] [CrossRef]
- Du, R.; Zhao, F.; Pan, L.; Ye, H.; Xiao, H.; Zhou, Z. Optimization and purification of glucansucrase produced by Leuconostoc mesenteroides DRP2-19 isolated from Chinese Sauerkraut. Prep. Biochem. Biotech. 2018, 48, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Goyal, A. Isolation, purification and functional characterization of glucansucrase from probiotic Lactobacillus plantarum DM5. J. Sci. Food Agric. 2014, 94, 1715–1724. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Goyal, A. Purification and characterization of an extracellular dextransucrase from Pediococcus pentosaceus isolated from the soil of north east India. Food Technol. Biotech. 2011, 49, 297–303. [Google Scholar]
- Song, L.; Song, M.; Jiang, B.; Xu, T.; Cui, S.W.; Zhang, T. Leuconostoc citreum SK24.002 glucansucrase: Biochemical characterisation and de novo synthesis of α-glucan. Int. J. Biol. Macromol. 2016, 91, 123–131. [Google Scholar] [CrossRef]
- López-Munguía, A.; Pelenc, V.; Remaud, M.; Biton, J.; Michel, J.M.; Lang, C.; Paul, F.; Monsan, P. Production and purification of alternansucrase, a glucosyltransferase from Leuconostoc mesenteroides NRRL B-1355, for the synthesis of oligoalternans. Enzyme Microb. Tech. 1993, 15, 77–85. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Wang, Y.; Zhao, B.; Zhou, Z. Determination of glucansucrase encoding gene in Leuconostoc mesenteroides. Int. J. Biol. Macromol. 2019, 137, 761–766. [Google Scholar] [CrossRef]
- Majumder, A.; Mangtani, A.; Goyal, A. Purification, identification and functional characterization of glucansucrase from Leuconostoc dextranicum NRRL B-1146. Curr. Trends Biotechnol. Pharm. 2008, 2, 493–505. [Google Scholar]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ge, J. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, R.; Qiao, X.; Zhao, B.; Zhou, Z.; Han, Y. Optimization and characterization of exopolysaccharides with a highly branched structure extracted from Leuconostoc citreum B-2. Int. J. Biol. Macromol. 2020, 142, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Du, R.; Du, F.; Han, Y.; Xiao, H.; Zhou, Z. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, X.; Min, W.; Yang, Z. Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int. J. Biol. Macromol. 2018, 115, 985–993. [Google Scholar] [CrossRef]
- Du, R.; Xing, H.; Yang, Y.; Jiang, H.; Zhou, Z.; Han, Y. Optimization, purification and structural characterization of a dextran produced by L. mesenteroides isolated from Chinese sauerkraut. Carbohyd. Polym. 2017, 174, 409–416. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Y.; Tian, J.; Zhou, J.; Li, W. Influences of drying methods on the structural, physicochemical and antioxidant properties of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 157, 220–231. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Pan, W.; Shen, X.; He, Y.; Yin, H.; Zhou, K.; Zou, L.; Chen, S.; Liu, S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2019, 121, 342–349. [Google Scholar] [CrossRef]
- Grinev, V.S.; Tregubova, K.V.; Anis’Kov, A.A.; Sigida, E.N.; Yegorenkova, I.V. Isolation, structure, and potential biotechnological applications of the exopolysaccharide from Paenibacillus polymyxa 92. Carbohyd. Polym. 2020, 232, 115780. [Google Scholar] [CrossRef]
- Sran, K.S.; Bisht, B.; Mayilraj, S.; Choudhury, A.R. Structural characterization and antioxidant potential of a novel anionic exopolysaccharide produced by marine Microbacterium aurantiacum FSW-25. Int. J. Biol. Macromol. 2019, 131, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, K.; Chandirika, J.U.; Mathimani, T.; Vinothkanna, A.; Rajaram, R.; Annadurai, G. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohyd. Polym. 2020, 227, 115369. [Google Scholar]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S. Purification, partial structural characterization and health benefits of exopolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process Biochem. 2020, 99, 79–86. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Chiva, R.; Celador-Lera, L.; Velázquez, E.; Prieto, A.; Dueñas, M.T.; Tamame, M.; López, P. Lactic acid bacteria isolated from fermented doughs in spain produce dextrans and riboflavin. Foods 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Govindarajan, R.K.; Yin, H. Characterization and biotechnological functional activities of exopolysaccharides produced by Lysinibacillus fusiformis KMNTT-10. J. Polym. Environ. 2021, 29, 1742–1751. [Google Scholar] [CrossRef]
- Liu, J.S.; Zeng, Y.X.; Bi, S.Y.; Zhou, J.W.; Jia, A.Q. Characterization and chemical modification of PLN-1, an exopolysaccharide from Phomopsis liquidambari NJUSTb1. Carbohyd. Polym. 2021, 253, 117197. [Google Scholar] [CrossRef]
- Matsuzaki, C.; Takagaki, C.; Tomabechi, Y.; Forsberg, L.S.; Heiss, C.; Azadi, P.; Matsumoto, K.; Katoh, T.; Hosomi, K.; Kunisawa, J. Structural characterization of the immunostimulatory exopolysaccharide produced by Leuconostoc mesenteroides strain NTM048. Carbohyd. Res. 2017, 448, 95–102. [Google Scholar] [CrossRef]
- Kang, H.K.; Kimura, A.; Kim, D. Bioengineering of Leuconostoc mesenteroides glucansucrases that gives selected bond formation for glucan synthesis and/or acceptor-product synthesis. J. Agr. Food Chem. 2011, 59, 4148–4155. [Google Scholar] [CrossRef]
- Bejar, W.; Gabriel, V.; Amari, M.; Morel, S.; Mezghani, M.; Maguin, E.; Fontagné-Faucher, C.; Bejar, S.; Chouayekh, H. Characterization of glucansucrase and dextran from Weissella sp. TN610 with potential as safe food additives. Int. J. Biol. Macromol. 2013, 52, 125–132. [Google Scholar] [CrossRef]
- Kralj, S.; Geel-Schutten, G.V.; Rahaoui, H.; Leer, R.J.; Faber, E.J.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Molecular characterization of a novel glucosyltransferase from Lactobacillus reuteri strain 121 synthesizing a unique, highly branched glucan with α-(1→4) and α-(1→6) glucosidic bonds. Appl. Environ. Microb. 2002, 68, 4283. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Liang, Q.; Zhang, F.; Xu, X.; Hua, W.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895. [Google Scholar] [CrossRef] [PubMed]
- Kanamarlapudi, S.; Muddada, S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. BioMed. Res. Int. 2017, 5, 4201809. [Google Scholar]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Niu, G. Preparation, partial characterization and biological activity of exopolysaccharidesproduced from Lactobacillus fermentum S1. J Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; Vieira, R.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Saravanan, C.; Shetty, P.K.H. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int. J. Biol. Macromol. 2016, 90, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathogenesis 2019, 132, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, S.; Zhang, X.; Zeng, X.; Li, D. Capsular and slime-polysaccharide production by Lactobacillus rhamnosus JAAS8 isolated from Chinese sauerkraut: Potential application in fermented milk products. J. Biosci. Bioeng. 2010, 110, 53–57. [Google Scholar] [CrossRef]
- Govarthanan, M.; Kamala-Kannan, S.; Selvankumar, T.; Mythili, R.; Srinivasan, P.; Kim, H. Effect of blue light on growth and exopolysaccharides production in phototrophic Rhodobacter sp. BT18 isolated from brackish water. Int. J. Biol. Macromol. 2019, 131, 74–80. [Google Scholar] [CrossRef]

| Hydrocarbons/Oil | EA (%) | |
|---|---|---|
| E24 | E48 | |
| Diesel | 56.26 ± 1.79 | 57.50 ± 0.34 |
| Gasoline | 82.11 ± 0.52 | 81.67 ± 1.20 |
| Hexane | 53.82 ± 2.41 | 55.93 ± 1.67 |
| Benzene | 62.77 ± 0.09 | 60.22 ± 1.05 |
| Soybean oil | 85.53 ± 1.37 | 83.81 ± 0.56 |
| Sunflower oil | 70.67 ± 2.05 | 67.52 ± 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, R.; Yu, L.; Sun, M.; Ye, G.; Yang, Y.; Zhou, B.; Qian, Z.; Ling, H.; Ge, J. Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications. Antioxidants 2023, 12, 275. https://doi.org/10.3390/antiox12020275
Du R, Yu L, Sun M, Ye G, Yang Y, Zhou B, Qian Z, Ling H, Ge J. Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications. Antioxidants. 2023; 12(2):275. https://doi.org/10.3390/antiox12020275
Chicago/Turabian StyleDu, Renpeng, Liansheng Yu, Meng Sun, Guangbin Ye, Yi Yang, Bosen Zhou, Zhigang Qian, Hongzhi Ling, and Jingping Ge. 2023. "Characterization of Dextran Biosynthesized by Glucansucrase from Leuconostoc pseudomesenteroides and Their Potential Biotechnological Applications" Antioxidants 12, no. 2: 275. https://doi.org/10.3390/antiox12020275






