Oxidative Stress, Antioxidants and Hypertension
Abstract
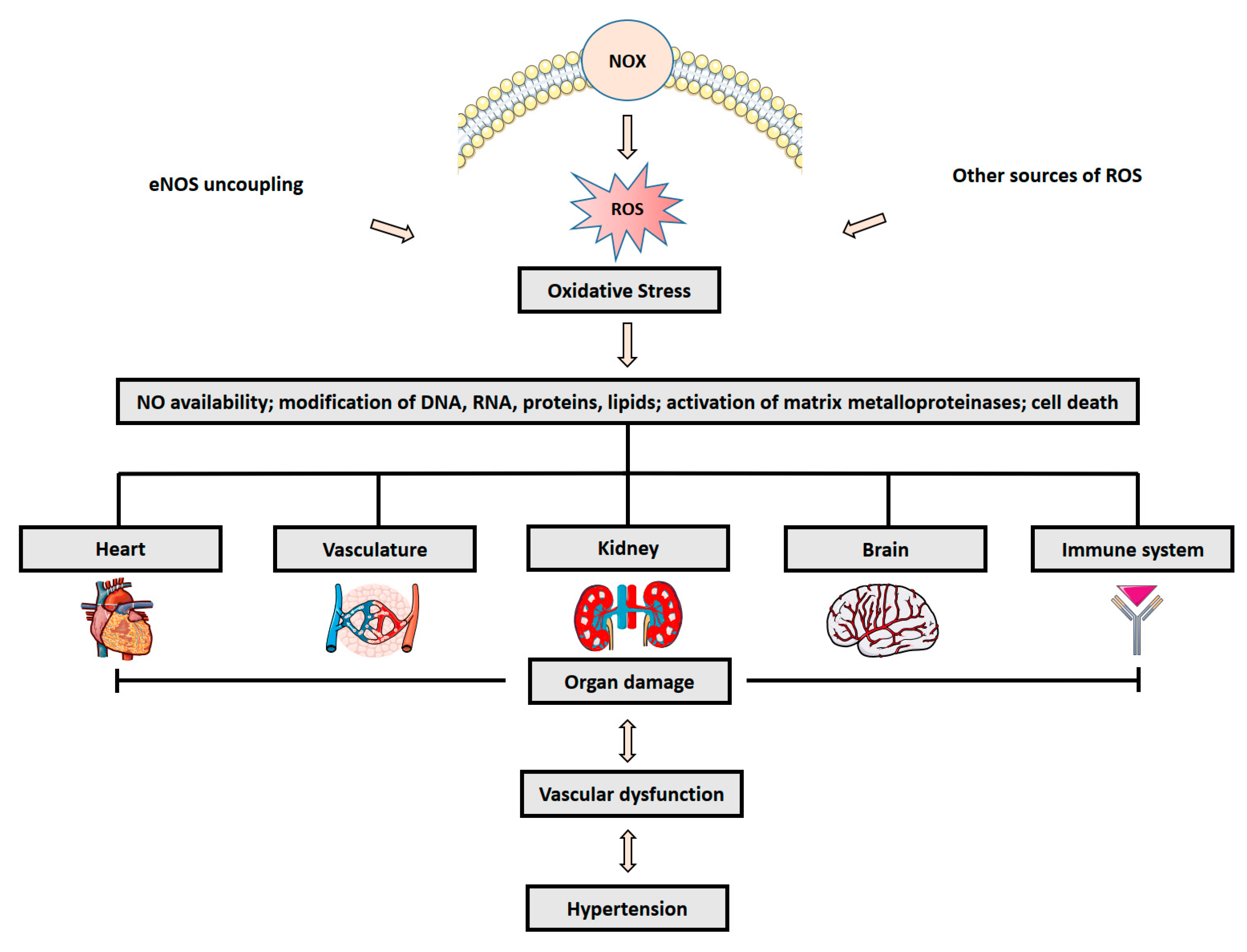
:1. Introduction
2. Role of Oxidative Stress in Hypertension
3. Detection and Biomarkers of ROS
4. Mechanism of Antioxidants and Potential Therapeutic Strategy in Hypertension
4.1. Vitamins
4.2. Polyphenols
4.3. α-Lipoic Acid
4.4. N-Acetylcysteine
4.5. Coenzyme Q10
4.6. Superoxide Dismutase
4.7. Antioxidants and Hypertension
5. Open Questions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Mowry, F.E.; Biancardi, V.C. Neuroinflammation in hypertension: The renin-angiotensin system versus pro-resolution pathways. Pharmacol. Res. 2019, 144, 279–291. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Dugani, S.; Gaziano, T.A. 25 by 25: Achieving Global Reduction in Cardiovascular Mortality. Curr. Cardiol. Rep. 2016, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [Green Version]
- Li, E.C.K.; Heran, B.S.; Wright, J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014, 2014, CD009096. [Google Scholar] [CrossRef]
- Oger, E.; Kerbrat, S.; Nowak, E.; Paillard, F.; Scarabin, P.; Happe, A. Effectiveness of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on total and cardiovascular mortality and morbidity in primary prevention: A nationwide study based on French Health Insurance Data (SNDS). J. Clin. Hypertens. 2022, 24, 438–448. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef]
- Mercier, K.; Smith, H.; Biederman, J. Renin-angiotensin-aldosterone system inhibition: Overview of the therapeutic use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and direct renin inhibitors. Prim. Care 2014, 41, 765–778. [Google Scholar] [CrossRef]
- de Mello, W.C. Local Renin Angiotensin Aldosterone Systems and Cardiovascular Diseases. Med. Clin. N. Am. 2017, 101, 117–127. [Google Scholar] [CrossRef]
- Rueckschloss, U.; Duerrschmidt, N.; Morawietz, H. NADPH Oxidase in Endothelial Cells: Impact on Atherosclerosis. Antioxidants Redox Signal. 2003, 5, 171–180. [Google Scholar] [CrossRef]
- Hofmann, A.; Brunssen, C.; Morawietz, H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vasc. Pharmacol. 2018, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Jiménez-Altayó, F.; Campuzano, V. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis and Progression of Genetic Diseases of the Connective Tissue. Antioxidants 2020, 9, 1013. [Google Scholar] [CrossRef]
- Wigner, P.; Dziedzic, A.; Synowiec, E.; Miller, E.; Bijak, M.; Saluk-Bijak, J. Variation of genes encoding nitric oxide synthases and antioxidant enzymes as potential risks of multiple sclerosis development: A preliminary study. Sci. Rep. 2022, 12, 10603. [Google Scholar] [CrossRef]
- Krakowian, D.; Skiba, D.; Kudelski, A.; Pilawa, B.; Ramos, P.; Adamczyk, J.; Pawłowska-Góral, K. Application of EPR spectroscopy to the examination of pro-oxidant activity of coffee. Food Chem. 2014, 151, 110–119. [Google Scholar] [CrossRef]
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.D.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017, 174, 1784–1796. [Google Scholar] [CrossRef] [Green Version]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef]
- Muller, G.; Morawietz, H. NAD(P)H Oxidase and Endothelial Dysfunction. Horm. Metab. Res. 2009, 41, 152–158. [Google Scholar] [CrossRef]
- Hsieh, H.-J.; Liu, C.-A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [Green Version]
- Pan, S. Molecular Mechanisms Responsible for the Atheroprotective Effects of Laminar Shear Stress. Antioxid. Redox Signal. 2009, 11, 1669–1682. [Google Scholar] [CrossRef] [Green Version]
- Harrison, D.G.; Widder, J.; Grumbach, I.; Chen, W.; Weber, M.; Searles, C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J. Intern. Med. 2006, 259, 351–363. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef]
- Giebe, S.; Cockcroft, N.; Hewitt, K.; Brux, M.; Hofmann, A.; Morawietz, H.; Brunssen, C. Cigarette smoke extract counteracts atheroprotective effects of high laminar flow on endothelial function. Redox Biol. 2017, 12, 776–786. [Google Scholar] [CrossRef]
- Giebe, S.; Hofmann, A.; Brux, M.; Lowe, F.; Breheny, D.; Morawietz, H.; Brunssen, C. Comparative study of the effects of cigarette smoke versus next generation tobacco and nicotine product extracts on endothelial function. Redox Biol. 2021, 47, 102150. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Morawietz, H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid. Redox Signal. 2009, 11, 1711–1731. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS Uncoupling in Cardiovascular Diseases—The Role of Oxidative Stress and Inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Briones, A.M.; Touyz, R.M. Oxidative Stress and Hypertension: Current Concepts. Curr. Hypertens. Rep. 2010, 12, 135–142. [Google Scholar] [CrossRef]
- Ambrosino, P.; Bachetti, T.; D’Anna, S.E.; Galloway, B.; Bianco, A.; D’Agnano, V.; Papa, A.; Motta, A.; Perrotta, F.; Maniscalco, M. Mechanisms and Clinical Implications of Endothelial Dysfunction in Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 136. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Callahan, T.S.; Epstein, S.E. Effect of antihypertensive treatment on endothelium-dependent vascular relaxation in patients with essential hypertension. J. Am. Coll. Cardiol. 1993, 21, 1145–1151. [Google Scholar] [CrossRef]
- Souza-Barbosa, L.A.; Ferreira-Melo, S.E.; Ubaid-Girioli, S.; Nogueira, E.A.; Yugar-Toledo, J.C.; Moreno, H., Jr. Endothelial vascular function in hypertensive patients after renin-angiotensin system blockade. J. Clin. Hypertens. 2006, 8, 803–811. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Salvetti, A. Vitamin C Improves Endothelium-Dependent Vasodilation by Restoring Nitric Oxide Activity in Essential Hypertension. Circulation 1998, 97, 2222–2229. [Google Scholar] [CrossRef] [Green Version]
- Dillon, G.A.; Greaney, J.L.; Shank, S.; Leuenberger, U.A.; Alexander, L.M. AHA/ACC-defined stage 1 hypertensive adults do not display cutaneous microvascular endothelial dysfunction. Am. J. Physiol. Circ. Physiol. 2020, 319, H539–H546. [Google Scholar] [CrossRef]
- Kakabadze, K.; Megreladze, I.; Khvichia, N.; Mitagvaria, N.; Kipiani, N.; Dumbadze, M.; Sanikidze, T. Some Aspects of Role of Nitric Oxide in the Mechanisms of Hypertension (Experimental Study). Cardiol. Res. 2021, 12, 16–24. [Google Scholar] [CrossRef]
- Tanito, M.; Nakamura, H.; Kwon, Y.-W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced Oxidative Stress and Impaired Thioredoxin Expression in Spontaneously Hypertensive Rats. Antioxid. Redox Signal. 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bächler, J.P. Relationship between oxidative stress and essential hypertension. Hypertens. Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Morawietz, H. Endothelial NADPH oxidases: Friends or foes? Basic Res. Cardiol. 2011, 106, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Brandes, R.P.; Weissmann, N.; Schröder, K. NADPH oxidases in cardiovascular disease. Free. Radic. Biol. Med. 2010, 49, 687–706. [Google Scholar] [CrossRef]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef] [Green Version]
- Starkov, A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Viel, E.C.; Benkirane, K.; Javeshghani, D.; Touyz, R.M.; Schiffrin, E.L. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am. J. Physiol. Circ. Physiol. 2008, 295, H281–H288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandes, R.P.; Weissmann, N.; Schröder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free. Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Schröder, K. NADPH Oxidases in Redox Regulation of Cell Adhesion and Migration. Antioxid. Redox Signal. 2014, 20, 2043–2058. [Google Scholar] [CrossRef]
- Takeya, R.; Ueno, N.; Kami, K.; Taura, M.; Kohjima, M.; Izaki, T.; Nunoi, H.; Sumimoto, H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003, 278, 25234–25246. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M.; Briones, A.M.; Sedeek, M.; Burger, D.; Montezano, A.C. NOX Isoforms and Reactive Oxygen Species in Vascular Health. Mol. Interv. 2011, 11, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueckschloss, U.; Galle, J.; Holtz, J.; Zerkowski, H.R.; Morawietz, H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: Antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 2001, 104, 1767–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabeebaccus, A.A.; Reumiller, C.M.; Shen, J.; Zoccarato, A.; Santos, C.X.; Shah, A.M. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc. Res. 2023, 118, 3305–3319. [Google Scholar] [CrossRef]
- Morawietz, H. Cardiovascular protection by Nox4. Cardiovasc. Res. 2018, 114, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Langbein, H.; Brunssen, C.; Hofmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016, 37, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, S170–S180. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.; Clempus, R.; Lassègue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 Overexpression Potentiates Angiotensin II-Induced Hypertension and Vascular Smooth Muscle Hypertrophy in Transgenic Mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.E.; Alom-Ruiz, S.P.; Wang, M.; Zhang, M.; Walker, S.; Yu, B.; Brewer, A.; Shah, A.M. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res. Cardiol. 2011, 106, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Rueckschloss, U.; Quinn, M.T.; Holtz, J.; Morawietz, H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: Protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1845–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-Induced Production of Mitochondrial Superoxide in Angiotensin II-Mediated Endothelial Oxidative Stress and Hypertension. Antioxidants Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doughan, A.; Harrison, D.; Dikalov, S. Molecular Mechanisms of Angiotensin II-Mediated Mitochondrial Dysfunction: Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.B.; Trevelin, S.C.; Richards, D.A.; Santos, C.X.; Sawyer, G.; Markovinovic, A.; Zhang, X.; Zhang, M.; Brewer, A.C.; Yin, X.; et al. Fibroblast Nox2 (NADPH Oxidase-2) Regulates ANG II (Angiotensin II)–Induced Vascular Remodeling and Hypertension via Paracrine Signaling to Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 2021, 41, 698–710. [Google Scholar] [CrossRef]
- Michihara, A.; Oda, A.; Mido, M. High Expression Levels of NADPH Oxidase 3 in the Cerebrum of Ten-Week-Old Stroke-Prone Spontaneously Hypertensive Rats. Biol. Pharm. Bull. 2016, 39, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Li, K.; Yu, Y.; Huang, H.; Yu, Y.; Wang, Z.; Yan, J.; Pu, Y.; Li, Z.; Li, D.; et al. Genome-wide association study identifies loci and candidate genes for non-idiopathic pulmonary hypertension in Eastern Chinese Han population. BMC Pulm. Med. 2018, 18, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byon, C.H.; Heath, J.M.; Chen, Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016, 9, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowley, A.W., Jr.; Yang, C.; Zheleznova, N.N.; Staruschenko, A.; Kurth, T.; Rein, L.; Kumar, V.; Sadovnikov, K.; Dayton, A.; Hoffman, M.; et al. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2016, 67, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Kurth, T.; Zheleznova, N.N.; Yang, C.; Cowley, A.W., Jr. NOX4/H2O2/mTORC1 Pathway in Salt-Induced Hypertension and Kidney Injury. Hypertension 2020, 76, 133–143. [Google Scholar] [CrossRef]
- Montezano, A.C.; Tsiropoulou, S.; Dulak-Lis, M.; Harvey, A.; Camargo, L.D.L.; Touyz, R.M. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr. Opin. Nephrol. Hypertens. 2015, 24, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Elbatreek, M.H.; Sadegh, S.; Anastasi, E.; Guney, E.; Nogales, C.; Kacprowski, T.; Hassan, A.A.; Teubner, A.; Huang, P.-H.; Hsu, C.-Y.; et al. NOX5-induced uncoupling of endothelial NO synthase is a causal mechanism and theragnostic target of an age-related hypertension endotype. PLoS Biol. 2020, 18, e3000885. [Google Scholar] [CrossRef]
- Martínez-Revelles, S.; García-Redondo, A.B.; Avendaño, M.S.; Varona, S.; Palao, T.; Orriols, M.; Roque, F.R.; Fortuño, A.; Touyz, R.M.; Martínez-González, J.; et al. Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of p38MAPK. Antioxid. Redox Signal. 2017, 27, 379–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, M.; Wilcox, C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Wang, W.; Li, Y.-L.; Schultz, H.D.; Liu, D.; Cornish, K.G.; Zucker, I.H. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2271–H2279. [Google Scholar] [CrossRef]
- Chan, S.H.; Wu, K.L.; Chang, A.Y.; Tai, M.-H.; Chan, J.Y. Oxidative Impairment of Mitochondrial Electron Transport Chain Complexes in Rostral Ventrolateral Medulla Contributes to Neurogenic Hypertension. Hypertension 2009, 53, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, J.-C.; Yu, H.T.; Lim, B.J.; Koh, M.J.; Lee, J.; Chang, D.-Y.; Choi, Y.S.; Lee, S.-H.; Kang, S.-M.; Jang, Y.; et al. Immunosenescent CD8 + T Cells and C-X-C Chemokine Receptor Type 3 Chemokines Are Increased in Human Hypertension. Hypertension 2013, 62, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abais-Battad, J.M.; Lund, H.; Dasinger, J.H.; Fehrenbach, D.J.; Cowley, A.W., Jr.; Mattson, D.L. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free. Radic. Biol. Med. 2020, 146, 333–339. [Google Scholar] [CrossRef]
- Malinouski, M.; Zhou, Y.; Belousov, V.V.; Hatfield, D.L.; Gladyshev, V.N. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE 2011, 6, e14564. [Google Scholar] [CrossRef] [Green Version]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef] [Green Version]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Liu, K.; Shi, X.; Swartz, H. Detection of Short-Lived Free Radicals by Low-Frequency Electron Paramagnetic Resonance Spin Trapping in Whole Living Animals. Arch. Biochem. Biophys. 1995, 319, 570–573. [Google Scholar] [CrossRef]
- Arai, H. Oxidative Modification of Lipoproteins. In Lipid Hydroperoxide-Derived Modification of Biomolecules; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; Volume 77, pp. 103–114. [Google Scholar]
- Lee, R.; Margaritis, M.; Channon, K.; Antoniades, C. Evaluating Oxidative Stress in Human Cardiovascular Disease: Methodological Aspects and Considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Libuy, M.; Feliú, F.; Hasson, D. Oxidative Stress-Related Biomarkers in Essential Hypertension and Ischemia-Reperfusion Myocardial Damage. Dis. Markers 2013, 35, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Asselin, C.; Shi, Y.; Clement, R.; Tardif, J.; Rosiers, C.D. Higher circulating 4-hydroxynonenal–protein thioether adducts correlate with more severe diastolic dysfunction in spontaneously hypertensive rats. Redox Rep. 2007, 12, 68–72. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. Protein Glutathionylation in Cardiovascular Diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876. [Google Scholar] [CrossRef] [Green Version]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free. Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Frei, B.; Kim, M.C.; Ames, B.N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. USA 1990, 87, 4879–4883. [Google Scholar] [CrossRef] [Green Version]
- Ulker, S.; McKeown, P.P.; Bayraktutan, U. Vitamins Reverse Endothelial Dysfunction Through Regulation of eNOS and NAD(P)H Oxidase Activities. Hypertension 2003, 41, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Neuzil, J.; Thomas, S.R.; Stocker, R. Requirement for, promotion, or inhibition by alpha-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic. Biol. Med. 1997, 22, 57–71. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [Green Version]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Larson, A.; Witman, M.A.; Guo, Y.; Ives, S.; Richardson, R.S.; Bruno, R.S.; Jalili, T.; Symons, J.D. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr. Res. 2012, 32, 557–564. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Niisato, N.; Marunaka, Y. Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na(+)/K(+)/2Cl(-) cotransporter. Cell. Physiol. Biochem. 2011, 28, 147–156. [Google Scholar] [CrossRef]
- Ye, X.; Tang, X.; Li, F.; Zhu, J.; Wu, M.; Wei, X.; Wang, Y. Green and Oolong Tea Extracts With Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022, 9, 892801. [Google Scholar] [CrossRef]
- Boots, A.W.; Kubben, N.; Haenen, G.; Bast, A. Oxidized quercetin reacts with thiols rather than with ascorbate: Implication for quercetin supplementation. Biochem. Biophys. Res. Commun. 2003, 308, 560–565. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Luo, Z.; Carter, G.; Wellstein, A.; Jose, P.A.; Tomlinson, J.; Leiper, J.; Welch, W.J.; Wilcox, C.S.; Wang, D. NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R399–R406. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibullo, D.; Volti, G.L.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Rose, R.A.; Jordan, S.; Dwyer, T.M.; Hughson, M.D.; Manning, R.D., Jr. N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J. Hypertens. 2006, 24, 2263–2270. [Google Scholar] [CrossRef]
- Song, D.; Hutchings, S.; Pang, C.C. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eur. J. Pharmacol. 2005, 508, 205–210. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal N(G)-Nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am. J. Obstet. Gynecol. 2016, 215, 636.e1–636.e72. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants 2020, 9, 856. [Google Scholar] [CrossRef]
- Girouard, H.; Chulak, C.; LeJossec, M.; Lamontagne, D.; de Champlain, J. Chronic antioxidant treatment improves sympathetic functions and beta-adrenergic pathway in the spontaneously hypertensive rats. J. Hypertens. 2003, 21, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Chulak, C.; Wu, L.; LeJossec, M.; De Champlain, J. N-acetylcysteine improves nitric oxide and α-adrenergic pathways in mesenteric beds of spontaneously hypertensive rats. Am. J. Hypertens. 2003, 16, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural Antioxidants and Hypertension: Promise and Challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Gao, H.-L.; Yu, X.-J.; Qi, J.; Yi, Q.-Y.; Jing, W.-H.; Sun, W.-Y.; Cui, W.; Mu, J.-J.; Yuan, Z.-Y.; Zhao, X.-F.; et al. Oral CoQ10 attenuates high salt-induced hypertension by restoring neurotransmitters and cytokines in the hypothalamic paraventricular nucleus. Sci. Rep. 2016, 6, 30301. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-Targeted Antioxidant MitoQ10 Improves Endothelial Function and Attenuates Cardiac Hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Overvad, K.; Diamant, B.; Holm, L.; Hølmer, G.; Mortensen, S.A.; Stender, S. Coenzyme Q10 in health and disease. Eur. J. Clin. Nutr. 1999, 53, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Langsjoen, P.; Willis, R.; Folkers, K. Treatment of essential hypertension with Coenzyme Q10. Mol. Asp. Med. 1994, 15 (Suppl. 1), s265–s272. [Google Scholar] [CrossRef] [PubMed]
- Digiesi, V.; Cantini, F.; Oradei, A.; Bisi, G.; Guarino, G.; Brocchi, A.; Bellandi, F.; Mancini, M.; Littarru, G. Coenzyme Q10 in essential hypertension. Mol. Asp. Med. 1994, 15, s257–s263. [Google Scholar] [CrossRef]
- Burke, B.E.; Neuenschwander, R.; Olson, R.D. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South. Med. J. 2001, 94, 1112–1117. [Google Scholar] [CrossRef]
- Baker, G.L.M.; Corry, R.J.M.; Autor, A.P. Oxygen Free Radical Induced Damage in Kidneys Subjected to Warm Ischemia and Reperfusion. Ann. Surg. 1985, 202, 628–641. [Google Scholar] [CrossRef]
- Jolly, S.R.; Kane, W.J.; Bailie, M.B.; Abrams, G.D.; Lucchesi, B.R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ. Res. 1984, 54, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Nunes, D.V.; Costa, C.A.; De Bem, G.F.; Cordeiro, V.S.; Santos, I.B.; Carvalho, L.C.; Jordão, A.K.; Cunha, A.C.; Ferreira, V.F.; Moura, R.S.; et al. Tempol, a superoxide dismutase-mimetic drug, prevents chronic ischemic renal injury in two-kidney, one-clip hypertensive rats. Clin. Exp. Hypertens. 2018, 40, 721–729. [Google Scholar] [CrossRef]
- Onuma, S.; Nakanishi, K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism 2004, 53, 1305–1308. [Google Scholar] [CrossRef]
- Park, J.B.; Touyz, R.M.; Chen, X.; Schiffrin, E.L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15 Pt 1, 78–84. [Google Scholar] [CrossRef]
- Savalia, K.; Manickam, D.S.; Rosenbaugh, E.G.; Tian, J.; Ahmad, I.M.; Kabanov, A.V.; Zimmerman, M.C. Neuronal uptake of nanoformulated superoxide dismutase and attenuation of angiotensin II-dependent hypertension after central administration. Free Radic. Biol. Med. 2014, 73, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I.; et al. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Capettini, L.S.; Montecucco, F.; Mach, F.; Stergiopulos, N.; Santos, R.A.; Da Silva, R.F. Role of Renin-Angiotensin System in Inflammation, Immunity and Aging. Curr. Pharm. Des. 2012, 18, 963–970. [Google Scholar] [CrossRef]
- Tao, R.; Vassilopoulos, A.; Parisiadou, L.; Yan, Y.; Gius, D. Regulation of MnSOD Enzymatic Activity by Sirt3 Connects the Mitochondrial Acetylome Signaling Networks to Aging and Carcinogenesis. Antioxid. Redox Signal. 2014, 20, 1646–1654. [Google Scholar] [CrossRef] [Green Version]
- Diaba-Nuhoho, P.; Cour, M.; Hadebe, N.; Marais, D.; Lecour, S.; Blackhurst, D. Chronic and moderate consumption of reduced-alcohol wine confers cardiac benefits in a rat model of pulmonary arterial hypertension. BMC Res. Notes 2021, 14, 324. [Google Scholar] [CrossRef]
- Chin, H.J.; Song, Y.R.; Kim, H.S.; Park, M.; Yoon, H.J.; Na, K.Y.; Kim, Y.; Chae, D.-W.; Kim, S. The Bilirubin Level is Negatively Correlated with the Incidence of Hypertension in Normotensive Korean Population. J. Korean Med. Sci. 2009, 24 (Suppl. 1), S50–S56. [Google Scholar] [CrossRef] [Green Version]
- Joles, J.A.; Wesseling, S.; Braam, B. Renal glutathione S-transferase mu type 1 expression is already reduced in new-born spontaneously hypertensive rats. J. Hypertens. 2010, 28, 633–634. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Zhang, D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J. Hypertens. 2019, 37, 2371–2379. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Antioxidants in Hypertension and Cardiovascular Disease. Mol. Interv. 2010, 10, 354–362. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S. Antioxidants and atherosclerosis: Don’t throw out the baby with the bath water. Circulation 2003, 107, 926–928. [Google Scholar] [CrossRef] [Green Version]
- Czernichow, S.; Bertrais, S.; Blacher, J.; Galan, P.; Briançon, S.; Favier, A.; Safar, M.; Hercberg, S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI.MAX study: Association with plasma antioxidant levels. J. Hypertens. 2005, 23, 2013–2018. [Google Scholar] [CrossRef]
- Kalpdev, A.; Saha, S.C.; Dhawan, V. Vitamin C and E Supplementation Does Not Reduce the Risk of Superimposed PE in Pregnancy. Hypertens. Pregnancy 2011, 30, 447–456. [Google Scholar] [CrossRef]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A nutritive dose of pure (-)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Young, J.M.; Florkowski, C.M.; Molyneux, S.; McEwan, R.G.; Frampton, C.M.; Nicholls, M.G.; Scott, R.S.; George, P.M. A Randomized, Double-Blind, Placebo-Controlled Crossover Study of Coenzyme Q10 Therapy in Hypertensive Patients With the Metabolic Syndrome. Am. J. Hypertens. 2012, 25, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., 2nd; Oates, J.A.; Linton, M.F.; Fazio, S.; Meador, B.P.; Gross, M.D.; Shyr, Y.; Morrow, J.D. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic. Biol. Med. 2007, 43, 1388–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Fortuño, A.; Bidegain, J.; Robador, P.; Hermida, J.; López-Sagaseta, J.; Beloqui, O.; Díez, J.; Zalba, G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: Potential clinical implications in hypertension. Hypertension 2009, 54, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Montezano, A.C.; Touyz, R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012, 44 (Suppl. 1), S2–S16. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. New Engl. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef]
- Yoshioka, M.; Aoyama, K.; Matsushita, T. Effects of ascorbic acid on blood pressure and ascorbic acid metabolism in spontaneously hypertensive rats (SH rats). Int. J. Vitam. Nutr. Res. 1985, 55, 301–307. [Google Scholar]
- Vasdev, S.; Ford, C.; Parai, S.; Longerich, L.; Gadag, V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol. Cell. Biochem. 2001, 218, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ettarh, R.R.; Odigie, I.P.; Adigun, S.A. Vitamin C lowers blood pressure and alters vascular responsiveness in salt-induced hypertension. Can. J. Physiol. Pharmacol. 2002, 80, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Nishi, E.; Campos, R.R.; Bergamaschi, C.; De Almeida, V.R.; Ribeiro, D.A. Vitamin C prevents DNA damage induced by renovascular hypertension in multiple organs of Wistar rats. Hum. Exp. Toxicol. 2010, 29, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant Effects of Vitamins C and E Are Associated With Altered Activation of Vascular NADPH Oxidase and Superoxide Dismutase in Stroke-Prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, S.; Gokce, N.; Holbrook, M.; Huang, A.; Frei, B.; Keaney, J.F., Jr.; Vita, J.A. Treatment of hypertension with ascorbic acid. Lancet 1999, 354, 2048–2049. [Google Scholar] [CrossRef]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation With Vitamins C and E Improves Arterial Stiffness and Endothelial Function in Essential Hypertensive Patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bächler, J.P. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, I.M.; George, V.; Sasse, E.A.; Kochar, M.S. A Randomized, Double-Blind, Controlled Trial of Vitamin C in the Management of Hypertension and Lipids. Am. J. Ther. 2002, 9, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ekpo, E.; Shah, I.; Girling, A.; Jenkins, C.; Sinclair, A. A Double-Blind, Placebo-Controlled Parallel Trial of Vitamin C Treatment in Elderly Patients with Hypertension. Gerontology 1994, 40, 268–272. [Google Scholar] [CrossRef]
- Duffy, S.J.; Gokce, N.; Holbrook, M.; Hunter, L.M.; Biegelsen, E.S.; Huang, A.; Keaney, J.; Vita, J. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H528–H534. [Google Scholar] [CrossRef]
- Solzbach, U.; Hornig, B.; Jeserich, M.; Just, H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 1997, 96, 1513–1519. [Google Scholar] [CrossRef]
- Fotherby, M.D.; Williams, J.C.; Forster, L.A.; Craner, P.; Ferns, G.A. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J. Hypertens. 2000, 18, 411–415. [Google Scholar] [CrossRef]
- Guan, Y.; Dai, P.; Wang, H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef]
- Newaz, M.A.; Nawal, N.; Rohaizan, C.; Muslim, N.; Gapor, A. ?-tocopherol increased nitric oxide synthase activity in blood vessels of spontaneously hypertensive rats. Am. J. Hypertens. 1999, 12 Pt 1, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Pezeshk, A.; Dalhouse, A.D. Vitamin E, membrane fluidity, and blood pressure in hypertensive and normotensive rats. Life Sci. 2000, 67, 1881–1889. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Parai, S.; Longerich, L.; Gadag, V. Dietary vitamin E supplementation lowers blood pressure in spontaneously hypertensive rats. Mol. Cell. Biochem. 2002, 238, 111–117. [Google Scholar] [CrossRef]
- Atarashi, K.; Ishiyama, A.; Takagi, M.; Minami, M.; Kimura, K.; Goto, A.; Omata, M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Am. J. Hypertens. 1997, 10 Pt 2, 116S–119S. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Parai, S.; Gadag, V. Dietary Vitamin E Supplementation Attenuates Hypertension in Dahl Salt-Sensitive Rats. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 103–111. [Google Scholar] [CrossRef]
- Noguchi, T.; Ikeda, K.; Sasaki, Y.; Yamamoto, J.; Yamori, Y. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2004, 31 (Suppl. 2), S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Iino, K.; Abe, K.; Kariya, S.; Kimura, H.; Kusaba, T.; Kusunoki, R.; Saku, J.; Soejima, K.; Nakakura, S.; Nakamura, I.; et al. A Controlled, Double-Blind Study of dl-Alpha-Tocopheryl Nicotinate (Juvela-Nicotinate®) for Treatment of Symptoms in Hypertension and Cerebral Arteriosclerosis. Jpn. Heart J. 1977, 18, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Boshtam, M.; Rafiei, M.; Sadeghi, K.; Sarrafzadegan, N. Vitamin E can Reduce Blood Pressure in Mild Hypertensives. Int. J. Vitam. Nutr. Res. 2002, 72, 309–314. [Google Scholar] [CrossRef]
- Jessup, J.V.; Horne, C.; Yarandi, H.; Quindry, J. The effects of endurance exercise and vitamin E on oxidative stress in the elderly. Biol. Res. Nurs. 2003, 5, 47–55. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Parai, S.; Longerich, L.; Gadag, V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol. Cell. Biochem. 2002, 241, 107–114. [Google Scholar] [CrossRef]
- Seifi, B.; Kadkhodaee, M.; Karimian, S.M.; Zahmatkesh, M.; Shams, S.; Bakhshi, E. Reduction of kidney damage by supplementation of vitamins C and E in rats with deoxycorticosterone-salt-induced hypertension. Iran. J. Kidney Dis. 2009, 3, 197–202. [Google Scholar]
- Xu, J.; Su, L.; Chen, L.; Lin, J. Protection from vascular endothelial dysfunction in acute glycemic load-induced primary hypertension by vitamin C and E. Genet. Mol. Res. 2014, 13, 7246–7255. [Google Scholar] [CrossRef]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Téllez, V.; Villegas-Romero, M.; Rubio-Ruiz, M.E.; Pérez-Torres, I.; Carreón-Torres, E.; Díaz-Díaz, E.; Guarner-Lans, V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2020, 21, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.; Eaton, P.; Burgoyne, J. Blood Pressure-Lowering by the Antioxidant Resveratrol Is Counterintuitively Mediated by Oxidation of cGMP-Dependent Protein Kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef]
- Cheng, P.-W.; Lee, H.-C.; Lu, P.-J.; Chen, H.-H.; Lai, C.-C.; Sun, G.-C.; Yeh, T.-C.; Hsiao, M.; Lin, Y.-T.; Liu, C.-P.; et al. Resveratrol Inhibition of Rac1-Derived Reactive Oxygen Species by AMPK Decreases Blood Pressure in a Fructose-Induced Rat Model of Hypertension. Sci. Rep. 2016, 6, 25342. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, M.; García-Saura, M.F.; Jiménez, R.; Villar, I.C.; Wangensteen, R.; Zarzuelo, A.; Vargas, F.; Duarte, J. Effects of Quercetin Treatment on Vascular Function in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Comparative Study with Verapamil. Planta Med. 2004, 70, 334–341. [Google Scholar] [PubMed]
- Duarte, J.; Jimenez, R.; O’Valle, F.; Galisteo, M.; Pérez-Palencia, R.; Vargas, F.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J. Hypertens. 2002, 20, 1843–1854. [Google Scholar] [CrossRef]
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [Green Version]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [Green Version]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Tain, Y.-L.; Hsu, C.-N.; Huang, L.-T.; Lau, Y.-T. Apocynin attenuates oxidative stress and hypertension in young spontaneously hypertensive rats independent of ADMA/NO pathway. Free Radic. Res. 2012, 46, 68–76. [Google Scholar] [CrossRef]
- Perassa, L.A.; Graton, M.E.; Potje, S.R.; Troiano, J.A.; Lima, M.S.; Vale, G.T.; Pereira, A.A.F.; Nakamune, A.C.M.S.; Sumida, D.H.; Tirapelli, C.; et al. Apocynin reduces blood pressure and restores the proper function of vascular endothelium in SHR. Vasc. Pharmacol. 2016, 87, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Patil, B.M.; Unger, B.S. Apocynin improves endothelial function and prevents the development of hypertension in fructose fed rat. Indian J. Pharmacol. 2009, 41, 208–212. [Google Scholar] [CrossRef]
- Virdis, A.; Neves, M.F.; Amiri, F.; Touyz, R.M.; Schiffrin, E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004, 22, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Beswick, R.A.; Dorrance, A.M.; Leite, R.; Webb, R.C. NADH/NADPH Oxidase and Enhanced Superoxide Production in the Mineralocorticoid Hypertensive Rat. Hypertension 2001, 38, 1107–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Zhang, Y.; Lim, P.S.; Miao, Y.; Tan, C.; McKenzie, K.U.; Schyvens, C.G.; Whitworth, J.A. Apocynin but Not l-Arginine Prevents and Reverses Dexamethasone-Induced Hypertension in the Rat. Am. J. Hypertens. 2006, 19, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.-A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef] [PubMed]
- Negishi, H.; Xu, J.-W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and Green Tea Polyphenols Attenuate Blood Pressure Increases in Stroke-Prone Spontaneously Hypertensive Rats. J. Nutr. 2004, 134, 38–42. [Google Scholar]
- Peng, X.; Zhou, R.; Wang, B.; Yu, X.; Yang, X.; Liu, K.; Mi, M. Effect of green tea consumption on blood pressure: A meta-analysis of 13 randomized controlled trials. Sci. Rep. 2014, 4, 6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Yang, K.; Ding, J.; Chen, G. Effect of green tea supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Medicine 2020, 99, e19047. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Thabane, L.; Mbuagbaw, L.; Liu, A.; Levine, M.A.; Holbrook, A. Effect of green tea supplementation on blood pressure among overweight and obese adults: A systematic review and meta-analysis. J. Hypertens. 2015, 33, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Salari, A.; Ghorbani, Z.; Ashouri, A. The effects of regular consumption of green or black tea beverage on blood pressure in those with elevated blood pressure or hypertension: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 51, 102430. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Connolly, K.; Batacan, R.; Ryan, K.; Vella, R.; Fenning, A. (−)-Epicatechin Reduces Blood Pressure and Improves Left Ventricular Function and Compliance in Deoxycorticosterone Acetate-Salt Hypertensive Rats. Molecules 2018, 23, 1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Zarzuelo, M.J.; Galindo, P.; Quintela, A.M.; López-Sepúlveda, R.; Romero, M.; Tamargo, J.; Vargas, F.; et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free. Radic. Biol. Med. 2012, 52, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kluknavsky, M.; Balis, P.; Skratek, M.; Manka, J.; Bernatova, I. (–)-Epicatechin Reduces the Blood Pressure of Young Borderline Hypertensive Rats During the Post-Treatment Period. Antioxidants 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Litterio, M.C.; Prieto, M.A.V.; Adamo, A.M.; Elesgaray, R.; Oteiza, P.I.; Galleano, M.; Fraga, C.G. (−)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015, 26, 745–751. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Sánchez, M.; Romero, M.; O’Valle, F.; Lopez-Sepulveda, R.; Quintela, A.M.; Galindo, P.; Zarzuelo, M.J.; Bailón, E.; et al. Chronic (−)-epicatechin improves vascular oxidative and inflammatory status but not hypertension in chronic nitric oxide-deficient rats. Br. J. Nutr. 2011, 106, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Galleano, M.; Bernatova, I.; Puzserova, A.; Balis, P.; Sestakova, N.; Pechanova, O.; Fraga, C.G. (-)-Epicatechin reduces blood pressure and improves vasorelaxation in spontaneously hypertensive rats by NO-mediated mechanism. IUBMB Life 2013, 65, 710–715. [Google Scholar] [CrossRef]
- Ellinger, S.; Reusch, A.; Stehle, P.; Helfrich, H.-P. Epicatechin ingested via cocoa products reduces blood pressure in humans: A nonlinear regression model with a Bayesian approach. Am. J. Clin. Nutr. 2012, 95, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; A Kroon, P.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Vasdev, S.; Mian, T.; Ford, C.A.; Longerich, L.; Parai, S. Role of endogenous aldehydes in spontaneously hypertensive and disulfiram-induced hypertensive rats. Nutr. Metab. Cardiovasc. Dis. 1996, 6, 130–140. [Google Scholar]
- Cabassi, A.; Dumont, E.C.; Girouard, H.; Bouchard, J.-F.; Le Jossec, M.; Lamontagne, D.; Besner, J.-G.; de Champlain, J. Effects of chronic N-acetylcysteine treatment on the actions of peroxynitrite on aortic vascular reactivity in hypertensive rats. J. Hypertens. 2001, 19, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Girouard, H.; Denault, C.; Chulak, C.; de Champlain, J. Treatment by N-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am. J. Hypertens. 2004, 17, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Vasdev, S.; Ford, C.A.; Longerich, L.; Gadag, V.; Wadhawan, S. Role of aldehydes in fructose induced hypertension. Mol. Cell. Biochem. 1998, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bourraindeloup, M.; Adamy, C.; Candiani, G.; Cailleret, M.; Bourin, M.-C.; Badoual, T.; Su, J.B.; Adubeiro, S.; Roudot-Thoraval, F.; Dubois-Rande, J.-L.; et al. N -Acetylcysteine Treatment Normalizes Serum Tumor Necrosis Factor-α Level and Hinders the Progression of Cardiac Injury in Hypertensive Rats. Circulation 2004, 110, 2003–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios, V.; Calderón, A.; Navarro-Cid, J.; Lahera, V.; Ruilope, L.M. N -Acetylcysteine Potentiates the Antihypertensive Effect of ACE Inhibitors in Hypertensive Patients. Blood Press. 2002, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Ford, C.A.; Parai, S.; Longerich, L.; Gadag, V. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr. Metab. Cardiovasc. Dis. 2000, 10, 339–346. [Google Scholar] [PubMed]
- Thirunavukkarasu, V.; Nandhini, A.T.A.; Anuradha, C.V. Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose-fed rats. J. Comp. Physiol. B 2004, 174, 587–592. [Google Scholar] [CrossRef]
- Vasdev, S.; Ford, C.A.; Parai, S.; Longerich, L.; Gadag, V. Dietary α-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J. Hypertens. 2000, 18, 567–573. [Google Scholar] [CrossRef]
- Louhelainen, M.; Merasto, S.; Finckenberg, P.; Lapatto, R.; Cheng, Z.J.; Mervaala, E.M. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 947–956. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Longerich, L.; Parai, S.; Gadag, V. Salt-induced hypertension in WKY rats: Prevention by α-lipoic acid supplementation. Mol. Cell. Biochem. 2003, 254, 319–326. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Jin, H.-Y.; Yu, H.-P. Inhibitory effects of alpha-lipoic acid on oxidative stress in the rostral ventrolateral medulla in rats with salt-induced hypertension. Int. J. Mol. Med. 2017, 39, 430–436. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, T.M.; Xia, H.; Filipeanu, C.M.; Braga, V.A.; Lazartigues, E. alpha-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. Am. J. Physiol. Heart Circ. Physiol. 2015, 241, H926–H934. [Google Scholar] [CrossRef]
- Su, Q.; Liu, J.-J.; Cui, W.; Shi, X.-L.; Guo, J.; Li, H.-B.; Huo, C.-J.; Miao, Y.-W.; Zhang, M.; Yang, Q.; et al. Alpha lipoic acid supplementation attenuates reactive oxygen species in hypothalamic paraventricular nucleus and sympathoexcitation in high salt-induced hypertension. Toxicol. Lett. 2015, 241, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Gill, V.; Parai, S.; Gadag, V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol. Cell. Biochem. 2005, 275, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Midaoui, A.E.; Elimadi, A.; Wu, L.; Haddad, P.S.; De Champlain, J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am. J. Hypertens. 2003, 16, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Midaoui, A.; de Champlain, J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension 2002, 39, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.L.H.; Vohra, H.; Zhang, Y.; Sutton, M.; Whitworth, J.A. The Effect of Alpha-Lipoic Acid on Mitochondrial Superoxide and Glucocorticoid-Induced Hypertension. Oxid. Med. Cell. Longev. 2013, 2013, 517045. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, T.; Nakajima, Y.; Tanaka, M.; Otake, S. Effect of coenzyme Q10 on experimental hypertension in rats and dogs. J. Pharmacol. Exp. Ther. 1974, 189, 149–156. [Google Scholar]
- Okamoto, H.; Kawaguchi, H.; Togashi, H.; Minami, M.; Saito, H.; Yasuda, H. Effect of coenzyme Q10 on structural alterations in the renal membrane of stroke-prone spontaneously hypertensive rats. Biochem. Med. Metab. Biol. 1991, 45, 216–226. [Google Scholar] [CrossRef]
- Yamagami, T.; Shibata, N.; Folkers, K. Bioenergetics in clinical medicine. Studies on coenzyme Q10 and essential hypertension. Res. Commun. Chem. Pathol. Pharmacol. 1975, 11, 273–288. [Google Scholar]
- Yamagami, T.; Shibata, N.; Folkers, K. Bioenergetics in clinical medicine. VIII. Adminstration of coenzyme Q10 to patients with essential hypertension. Res. Commun. Chem. Pathol. Pharmacol. 1976, 14, 721–727. [Google Scholar] [PubMed]
- Folkers, K.; Drzewoski, J.; Richardson, P.C.; Ellis, J.; Shizukuishi, S.; Baker, L. Bioenergetics in clinical medicine. XVI. Reduction of hypertension in patients by therapy with coenzyme Q10. Res. Commun. Chem. Pathol. Pharmacol. 1981, 31, 129–140. [Google Scholar] [PubMed]
- Singh, R.; Niaz, M.; Rastogi, S.; Shukla, P.; Thakur, A. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J. Hum. Hypertens. 1999, 13, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dornas, W.C.; Silva, M.; Tavares, R.; de Lima, W.G.; dos Santos, R.C.; Pedrosa, M.L.; Silva, M.E. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: A meta-analysis. J. Hypertens. 2015, 33, 14–23. [Google Scholar] [CrossRef]
- Kamezaki, F.; Tasaki, H.; Yamashita, K.; Tsutsui, M.; Koide, S.; Nakata, S.; Tanimoto, A.; Okazaki, M.; Sasaguri, Y.; Adachi, T.; et al. Gene Transfer of Extracellular Superoxide Dismutase Ameliorates Pulmonary Hypertension in Rats. Am. J. Respir. Crit. Care Med. 2008, 177, 219–226. [Google Scholar] [CrossRef]
- Carillon, J.; Rugale, C.; Rouanet, J.-M.; Cristol, J.-P.; Lacan, D.; Jover, B. Endogenous antioxidant defense induction by melon superoxide dismutase reduces cardiac hypertrophy in spontaneously hypertensive rats. Int. J. Food Sci. Nutr. 2014, 65, 602–609. [Google Scholar] [CrossRef]
- Sugama, I.; Kohagura, K.; Yamazato, M.; Nakamura, T.; Shinzato, T.; Ohya, Y. Superoxide dismutase mimetic, tempol, aggravates renal injury in advanced-stage stroke-prone spontaneously hypertensive rats. J. Hypertens. 2014, 32, 534–541. [Google Scholar] [CrossRef]
- Pires, P.W.; Deutsch, C.; McClain, J.L.; Rogers, C.T.; Dorrance, A.M. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc. Res. 2010, 80, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-K.; Xu, J.-S.; Chen, T.-B.; Xu, M.-M.; Liu, S.-T.; Zhang, C.-X.; Ke, L.-J.; Zhou, J.-W.; Wang, Q.; Rao, P.-F. Effects of TAT-SOD at Acupoints on Essential Hypertension by Monitoring Meridians Electrical Potential. Chin. J. Integr. Med. 2020, 26, 694–700. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [PubMed]


| Antioxidant | Model/Subject/Study Design | Outcome of Study |
|---|---|---|
| Vitamin C | Spontaneously hypertensive rats (SHR) [151,152]. | Blood pressure (BP) ⬇ [151,152]. |
| High salt-treated SHR [153]. | BP ⬇, endothelium-dependent relaxation ⬆ [153]. | |
| Hypertensive Wistar rats [154]. | BP ⬇ [154]. | |
| Stroke-prone SHR [155]. | BP ⬇ [155]. | |
| Humans, essential hypertension [38,156,157,158]. | Systolic blood pressure (SBP) ⬇, endothelial vasodilation ⬆, arterial stiffness ⬇ [38,156,157,158]. | |
| Humans, mild hypertension [159]. | SBP and diastolic BP (DBP) ⬇ [159]. | |
| Humans, elderly patients with hypertension [160]. | Small ⬇ in BP and antioxidant capacity ⬆ [160]. | |
| Humans, systemic review and meta-analysis [136]. | No consistent benefit for the prevention of CVD (hypertension) [136]. | |
| Humans, long-term risk of hypertension [138]. | No clear beneficial effect [138]. | |
| Humans, hypertension [161,162]. | SBP and mean BP ⬇, endothelial vasodilation ⬆ [161,162]. | |
| Humans, elderly patients, ambulatory BP [163]. | Modest effect on BP [163]. | |
| Humans, systemic review and meta-analysis [164]. | SBP ⬇ [164]. | |
| Vitamin E | SHR, Wistar-Kyoto (WKY) rats [165,166,167]. | BP ⬇ [165,166,167]. |
| High salt-treated Dahl salt-sensitive (DSS) rats [168]. | No effect on BP [168]. | |
| DSS rat [169]. | BP ⬇ [169]. | |
| Stroke-prone SHR [170]. | BP ⬇ [170]. | |
| Humans, hypertension and cerebral arteriosclerosis [171]. | SBP ⬇ [171]. | |
| Humans, mild essential hypertension [172]. | SBP, DBP and heart rate ⬇ [172]. | |
| Humans, sedentary elderly patients with mild systolic hypertension [173]. | SBP ⬇ [173]. | |
| Vitamin C and E | Stroke-prone SHR [155]. | BP ⬇ [155]. |
| Fructose-induced hypertensive WKY rats [174]. | BP ⬇ [174]. | |
| DOCA-salt-induced hypertensive rats [175]. | SBP ⬇ [175]. | |
| Humans, essential hypertension [157,158]. | SBP, DBP ⬇, endothelial vasodilation ⬆ and arterial stiffness ⬇ [157,158]. | |
| Humans, essential hypertension [176]. | SBP and DBP ⬇ [176]. | |
| Humans, essential hypertension [139]. | No effect on BP [139]. | |
| Polyphenols | ||
| Resveratrol | SHR, WKY rats [177,178]. | BP ⬇ [177,178]. |
| Rats with sucrose-induced hypertension [179]. | BP ⬇ [179]. | |
| Mice with Ang II-induced hypertension [180]. | BP ⬇ [180]. | |
| Fructose-induced hypertensive rats [181]. | BP ⬇ [181]. | |
| Hypertension induced in Wistar rats [182]. | SBP and DBP ⬇ [182]. | |
| Humans, essential hypertension [140]. | No significant change in BP [140]. | |
| Quercetin | Rats with sucrose-induced hypertension [179]. | BP ⬇ [179]. |
| DOCA-salt hypertensive rats [183]. | BP ⬇ [183]. | |
| L-NAME-induced hypertensive Wistar rats [184]. | BP ⬇ [184]. | |
| SHR [185]. | BP and heart rate ⬇ [185]. | |
| Humans, randomized controlled trial [186]. | DBP and mean arterial pressure ⬇ [186]. | |
| Humans, systemic review and meta-analysis [187]. | SBP and DBP ⬇ [187]. | |
| Humans, randomized controlled trial [188,189]. | SBP ⬇ [188,189]. | |
| Apocynin | SHR, WKY rats [190]. | BP ⬇ [190). |
| SHR [191]. | BP and heart rate ⬇ [191]. | |
| Fructose-treated hypertensive Sprague-Dawley rats [192]. | SBP ⬇ [192]. | |
| ANG II-induced hypertension in mice [193]. | SBP ⬇ [193]. | |
| DOCA-induced hypertensive rats [194]. | SBP ⬇ [194]. | |
| Dexamethasone-induced hypertension in SD rats [195]. | DBP ⬇ [195]. | |
| Green Tea | SHR [196]. | BP and heart rate ⬇ [196]. |
| Stroke-prone SHR [197]. | SBP and DBP ⬇ [197]. | |
| Salt-induced hypertensive Wistar rats [102]. | SBP and DBP ⬇ [102]. | |
| Humans, meta-analysis [198]. | SBP and DBP ⬇ [198]. | |
| Humans, systemic review and meta-analysis [199). | SBP and DBP ⬇ [199]. | |
| Humans, systemic review [200]. | SBP and DBP ⬇ [200]. | |
| Humans, systemic review and meta-analysis [201]. | SBP and DBP ⬇ [201]. | |
| (-)-Epicatechin | DOCA-salt hypertensive rats [202,203]. | SBP ⬇ [202,203]. |
| Borderline hypertensive rats [204]. | SBP ⬇ [204]. | |
| Fructose-induced hypertensive SD rats [205]. | SBP ⬇ [205]. | |
| L-NAME-induced hypertensive Wistar rats [206]. | No significant changes in SBP and heart rate [206]. | |
| SHR, WKY rats [207]. | SBP ⬇ [207]. | |
| Humans, meta-analysis [208,209]. | SBP and DBP ⬇ [208,209]. | |
| Humans, randomized controlled trial [210]. | SBP and DBP ⬇ [210]. | |
| Humans, randomized controlled trial [141]. | No significant changes in SBP [141]. | |
| N-acetyl cysteine | SHR [113,114,211,212,213]. | SBP, mean arterial pressure, heart rate ⬇, but not DBP [113,114,211,212,213]. |
| Fructose-treated hypertensive WKY rats [214]. | Attenuated increase in SBP [214]. | |
| Fructose-treated hypertensive SD rats [110]. | Prevented increases in SBP and DBP [110]. | |
| L-NAME-induced hypertensive SD rats [111]. | BP ⬇ [111]. | |
| Salt-induced hypertensive Wistar rats [215]. | No effect on BP [215]. | |
| Salt-induced hypertensive DSS rats [109]. | BP ⬇ [109]. | |
| Humans, essential hypertension [216]. | 24hr and daytime SBP and DBP ⬇ [216]. | |
| α-lipoic acid | Fructose-treated hypertensive WKY rats [217,218]. | Prevented increase in BP [217,218]. |
| SHR [219,220]. | BP ⬇ [219,220]. | |
| Salt-induced hypertensive WKY rats [221]. | Prevented increase in BP [221]. | |
| Salt-induced hypertensive Wistar rats [222]. | BP ⬇ [222]. | |
| High salt-induced hypertensive mice [223,224]. | BP ⬇ [223,224]. | |
| DSS rats [225]. | BP ⬇ [225]. | |
| Glucose-induced hypertensive SD rats [226,227]. | Prevented increase in BP [226,227]. | |
| Glucocorticoid-induced hypertension in SD rats [228]. | Partially ⬇ SBP [228]. | |
| Coenzyme Q10 | SHR [229]. | BP ⬇ in older animals [229]. |
| Stroke-prone SHR [230]. | SBP ⬇ [230]. | |
| Salt-induced hypertensive SD rats [116]. | BP ⬇ [116]. | |
| Humans, essential hypertension [119,120,231,232,233]. | SBP and DBP ⬇ [119,120,231,232,233]. | |
| Humans, hypertension with coronary artery disease [234]. | SBP and DBP ⬇ [234]. | |
| Humans, isolated systolic hypertension [121]. | SBP ⬇ [121]. | |
| Coenzyme Q10 therapy in humans, hypertensive with metabolic syndrome [142]. | No effect on SBP and DBP [142] | |
| Superoxide dismutases | Meta-analysis using SOD mimetic tempol in hypertensive animal models [235]. | BP ⬇ [235]. |
| EC-SOD in MCT-induced hypertensive rats [236]. | Improved right ventricular SBP [236]. | |
| Poly-l-lysine (PLL50)-polyethylene glycol (PEG) CuZn-SOD nanozyme in mice with Ang II-induced hypertension [127]. | BP ⬇ [127]. | |
| Melon SOD in SHR [237]. | BP ⬇ [237]. | |
| Tempol in hypertension of Wistar rats [124]. | SBP ⬇ [124]. | |
| Tempol in fructose-induced hypertensive SD rats [125]. | BP ⬇ [125]. | |
| Tempol in salt-loaded stroke-prone SHR [126]. | SBP ⬇ [126]. | |
| Tempol in advanced-stage stroke-prone SHR [238,239]. | No effect on SBP [238,239]. | |
| TAT-SOD in humans, essential hypertension [240]. | SBP and DBP ⬇ [240]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. https://doi.org/10.3390/antiox12020281
Amponsah-Offeh M, Diaba-Nuhoho P, Speier S, Morawietz H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants. 2023; 12(2):281. https://doi.org/10.3390/antiox12020281
Chicago/Turabian StyleAmponsah-Offeh, Michael, Patrick Diaba-Nuhoho, Stephan Speier, and Henning Morawietz. 2023. "Oxidative Stress, Antioxidants and Hypertension" Antioxidants 12, no. 2: 281. https://doi.org/10.3390/antiox12020281
APA StyleAmponsah-Offeh, M., Diaba-Nuhoho, P., Speier, S., & Morawietz, H. (2023). Oxidative Stress, Antioxidants and Hypertension. Antioxidants, 12(2), 281. https://doi.org/10.3390/antiox12020281






