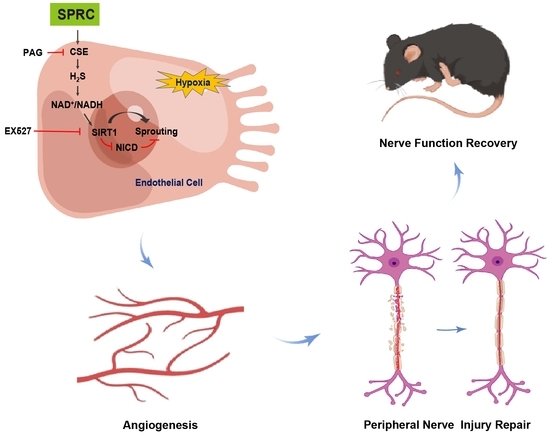
S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Animals
2.3. Sciatic Nerve Crush Injury Procedure and Drug Treatments
2.4. Sciatic Function Index (SFI) Measurement
2.5. Gastrocnemius Muscle Assessment
2.6. Sciatic Nerve Histological Assessment
2.7. Sciatic Nerve Morphological Assessment
2.8. Vascularity of Sciatic Nerve Assessment
2.9. Cell Culture and Treatments
2.10. Cell Viability, Proliferation, Adhesion, Migration and Tube Formation Assay
2.11. H2S Level and NAD+/NADH (the Reduced Form of NAD+) Ratio Measurement
2.12. Immunofluorescence and Co-Immunoprecipitation (Co-IP) of SIRT1 and NICD
2.13. Western Blotting Analysis
2.14. Statistical Analyses
3. Results
3.1. SPRC Accelerated the Motor Function Recovery of Injured Sciatic Nerve and Alleviated the Atrophy Degree of Gastrocnemius Muscle in Mice
3.2. SPRC Facilitated the Viability of SCs, the Outgrowth and Myelination of Regenerated Axons, and Angiogenesis in Rats
3.3. SPRC Facilitated the Viability, Proliferation, Adhesion, Migration and Tube Formation of HUVECs under Hypoxic Condition In Vitro
3.4. SPRC Facilitated Angiogenesis under Hypoxic Condition In Vitro through the H2S/SIRT1/NICD Signaling
3.5. SPRC May Promote Angiogenesis in Injured Sciatic Nerve of Rats In Vivo through the H2S/SIRT1/NICD Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. ALIAmides Update: Palmitoylethanolamide and Its Formulations on Management of Peripheral Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 5330. [Google Scholar] [CrossRef] [PubMed]
- Vaz, K.; Brown, J.; Shah, S. Peripheral nerve lengthening as a regenerative strategy. Neural Regen. Res. 2014, 9, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Noland, S.S.; Bishop, A.T.; Spinner, R.J.; Shin, A.Y. Adult Traumatic Brachial Plexus Injuries. J. Am. Acad. Orthop. Surg. 2019, 27, 705–716. [Google Scholar] [CrossRef]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, P.; Qiao, P.; Zhang, P.; Cai, X.; Tang, L.; Qian, T.; Wang, H. Blood vessel remodeling in late stage of vascular network reconstruction is essential for peripheral nerve regeneration. Bioeng. Transl. Med. 2022, 7, e10361. [Google Scholar] [CrossRef]
- Eren, F.; Öksüz, S.; Küçükodaci, Z.; Kendırlı, M.T.; Cesur, C.; Alarçın, E.; Bektaş, E.; Karagöz, H.; Kerımoğlu, O.; Köse, G.T.; et al. Targeted mesenchymal stem cell and vascular endothelial growth factor strategies for repair of nerve defects with nerve tissue implanted autogenous vein graft conduits. Microsurgery 2015, 36, 578–585. [Google Scholar] [CrossRef]
- Gao, H.; You, Y.; Zhang, G.; Zhao, F.; Sha, Z.; Shen, Y. The Use of Fiber-Reinforced Scaffolds Cocultured with Schwann Cells and Vascular Endothelial Cells to Repair Rabbit Sciatic Nerve Defect with Vascularization. BioMed Res. Int. 2013, 2013, 362918. [Google Scholar] [CrossRef]
- Masgutov, R.; Zeinalova, A.; Bogov, A.; Masgutova, G.; Salafutdinov, I.; Garanina, E.; Syromiatnikova, V.; Idrisova, K.; Mullakhmetova, A.; Andreeva, D.; et al. Angiogenesis and nerve regeneration induced by local administration of plasmid pBud-coVEGF165-coFGF2 into the intact rat sciatic nerve. Neural Regen. Res. 2021, 16, 1882–1889. [Google Scholar] [CrossRef]
- Avolio, E.; Alvino, V.V.; Ghorbel, M.T.; Campagnolo, P. Perivascular cells and tissue engineering: Current applications and untapped potential. Pharmacol. Ther. 2017, 171, 83–92. [Google Scholar] [CrossRef]
- Kan, J.; Guo, W.; Huang, C.; Bao, G.; Zhu, Y.; Zhu, Y.Z. S-Propargyl-Cysteine, a Novel Water-Soluble Modulator of Endogenous Hydrogen Sulfide, Promotes Angiogenesis Through Activation of Signal Transducer and Activator of Transcription 3. Antioxid. Redox Signal. 2014, 20, 2303–2316. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, D.; Krek, W. SIRT1: Linking Adaptive Cellular Responses to Aging-Associated Changes in Organismal Physiology. Physiology 2006, 21, 404–410. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and Sirtuin 3: Physiological Modulators of Metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef]
- Ryu, D.R.; Yu, M.R.; Kong, K.H.; Kim, H.; Kwon, S.H.; Jeon, J.S.; Han, D.C.; Noh, H. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell 2019, 18, e12904. [Google Scholar] [CrossRef]
- Guan, R.; Wang, J.; Cai, Z.; Li, Z.; Wang, L.; Li, Y.; Xu, J.; Li, D.; Yao, H.; Liu, W.; et al. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 2020, 28, 101356. [Google Scholar] [CrossRef]
- Xin, H.; Wang, M.; Tang, W.; Shen, Z.; Miao, L.; Wu, W.; Li, C.; Wang, X.; Xin, X.-M.; Zhu, Y.Z.; et al. Hydrogen Sulfide Attenuates Inflammatory Hepcidin by Reducing IL-6 Secretion and Promoting SIRT1-Mediated STAT3 Deacetylation. Antioxid. Redox Signal. 2016, 24, 70–83. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Wang, C.-Y.; Tang, Y.-Y.; Huang, H.-L.; Kang, X.; Li, X.; Xie, Y.-R.; Tang, X.-Q. Hydrogen Sulfide Inhibits High Glucose-Induced Neuronal Senescence by Improving Autophagic Flux via Up-regulation of SIRT1. Front. Mol. Neurosci. 2019, 12, 194. [Google Scholar] [CrossRef]
- Iso, T.; Hamamori, Y.; Kedes, L. Notch Signaling in Vascular Development. Arter. Thromb. Vasc. Biol. 2003, 23, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Guarani, V.; Deflorian, G.; Franco, C.A.; Krüger, M.; Phng, L.-K.; Bentley, K.; Toussaint, L.; Dequiedt, F.; Mostoslavsky, R.; Schmidt, M.H.H.; et al. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature 2011, 473, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.-R.; Mu, Q.; Rose, P.; Zhu, Y.Z. S-propargyl-cysteine Protects Both Adult Rat Hearts and Neonatal Cardiomyocytes from Ischemia/Hypoxia Injury: The Contribution of the Hydrogen Sulfide-Mediated Pathway. J. Cardiovasc. Pharmacol. 2009, 54, 139–146. [Google Scholar] [CrossRef]
- Inserra, M.M.; Bloch, D.A.; Terris, D.J. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery 1998, 18, 119–124. [Google Scholar] [CrossRef]
- Lawson, S.N.; Waddell, P.J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991, 435, 41–63. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Sainson, R.C.A.; Harris, A.L. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: From basic research to potential therapies. Angiogenesis 2008, 11, 41–51. [Google Scholar] [CrossRef]
- Kangsamaksin, T.; Tattersall, I.; Kitajewski, J. Notch functions in developmental and tumour angiogenesis by diverse mechanisms. Biochem. Soc. Trans. 2014, 42, 1563–1568. [Google Scholar] [CrossRef]
- Benedito, R.; Roca, C.; Sörensen, I.; Adams, S.; Gossler, A.; Fruttiger, M.; Adams, R.H. The Notch Ligands Dll4 and Jagged1 Have Opposing Effects on Angiogenesis. Cell 2009, 137, 1124–1135. [Google Scholar] [CrossRef]
- Yeoh, S.; Warner, W.S.; Merchant, S.S.; Hsu, E.W.; Agoston, D.V.; Mahan, M.A. Incorporating Blood Flow in Nerve Injury and Regeneration Assessment. Front. Surg. 2022, 9, 862478. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Johnson, J.M.; Rone, M.B.; Antel, J.P.; David, S.; Zhang, J. Peripheral Nerve Injury Induces Persistent Vascular Dysfunction and Endoneurial Hypoxia, Contributing to the Genesis of Neuropathic Pain. J. Neurosci. 2015, 35, 3346–3359. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Bibli, S.-I.; Pyriochou, A.; Szabo, C.; Papapetropoulos, A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016, 113, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Li, Y.; Xie, K.-F.; Chang, Y.-H.; Wang, C.; Chen, Y.; Wang, M.-J.; Zhu, Y.-C. S-Propargyl-Cysteine Attenuates Diabetic Cardiomyopathy in db/db Mice Through Activation of Cardiac Insulin Receptor Signaling. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Liao, J.; Hu, W.; Bian, X.; Wu, J.; Zhu, Y.Z. S-Propargyl-Cysteine Remodels the Gut Microbiota to Alleviate Rheumatoid Arthritis by Regulating Bile Acid Metabolism. Front. Cell. Infect. Microbiol. 2021, 11, 670593. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhao, B.; Lin, J.; Liu, H.; Zhou, R.; Lan, D.; Yao, C.; Cong, S.; Tao, S.; Zhu, Y.; et al. SPRC Suppresses Experimental Periodontitis by Modulating Th17/Treg Imbalance. Front. Bioeng. Biotechnol. 2022, 9, 737334. [Google Scholar] [CrossRef]
- Li, Y.-F.; Xiao, C.-S.; Hui, R.-T. Calcium sulfide (CaS), a donor of hydrogen sulfide (H2S): A new antihypertensive drug? Med. Hypotheses 2009, 73, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Zhi, L.; Zhang, H.; Ng, S.-W.; Moore, P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L896–L904. [Google Scholar] [CrossRef]
- Unuma, K.; Aki, T.; Yamashita, A.; Yoshikawa, A.; Uemura, K. Hydrogen sulfide donor NaHS causes bronchitis with enhanced respiratory secretion in rats. J. Toxicol. Sci. 2019, 44, 107–112. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Akakura, S.; Sanokawa-Akakura, R.; Goodwin, S.; Tabibzadeh, S. Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S–Nampt. Exp. Cell Res. 2015, 330, 135–150. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, Z.; Liu, N.; Zhang, L.; Gao, Z.; Sun, X.; Yu, M.; Wu, J.; Yang, F.; Zhao, Y.; et al. Exogenous H2S switches cardiac energy substrate metabolism by regulating SIRT3 expression in db/db mice. J. Mol. Med. 2018, 96, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Navas, L.E.; Carnero, A. NAD+ metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Plowman, G.D. Delta-like 4/Notch Signaling and Its Therapeutic Implications. Clin. Cancer Res. 2007, 13, 7243–7246. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, K.; Satoh, M.; Ii, M.; Silver, M.; Limbourg, F.P.; Mukai, Y.; Rikitake, Y.; Radtke, F.; Gridley, T.; Losordo, D.W.; et al. Critical Role of Endothelial Notch1 Signaling in Postnatal Angiogenesis. Circ. Res. 2007, 100, 70–78. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, J.; Liu, D.; Wan, G.; Gu, X.; Ma, J. Nogo-B promotes angiogenesis and improves cardiac repair after myocardial infarction via activating Notch1 signaling. Cell Death Dis. 2022, 13, 306. [Google Scholar] [CrossRef]
- Park, B.S.; Kim, H.-W.; Rhyu, I.J.; Park, C.; Yeo, S.G.; Huh, Y.; Jeong, N.Y.; Jung, J. Hydrogen sulfide is essential for Schwann cell responses to peripheral nerve injury. J. Neurochem. 2014, 132, 230–242. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- Pan, L.-L.; Liu, X.-H.; Gong, Q.-H.; Zhu, Y.-Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids 2011, 41, 205–215. [Google Scholar] [CrossRef]
- Sidhapuriwala, J.N.; Hegde, A.; Ang, A.D.; Zhu, Y.Z.; Bhatia, M. Effects of S-Propargyl-Cysteine (SPRC) in Caerulein-Induced Acute Pancreatitis in Mice. PLoS ONE 2012, 7, e32574. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-J.; Jia, W.-W.; Liu, X.-H.; Pan, L.-L.; Zhang, Q.-Y.; Yang, D.; Shen, X.-Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]






| Drug | Action |
|---|---|
| S-Propargyl-cysteine (SPRC) | an endogenous hydrogen sulfide (H2S) donor |
| NaHS | an exogenous H2S donor |
| DL-Propargylglycine (PAG) | a specific inhibitor of cystathionine-γ-lyase (CSE) |
| EX527 | a specific inhibitor of sirtuin1 (SIRT1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, H.; Wang, C.; Li, Q.; Ye, Q.; Zhu, Y.; Mao, Y. S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction. Antioxidants 2023, 12, 294. https://doi.org/10.3390/antiox12020294
Xi H, Wang C, Li Q, Ye Q, Zhu Y, Mao Y. S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction. Antioxidants. 2023; 12(2):294. https://doi.org/10.3390/antiox12020294
Chicago/Turabian StyleXi, Haiyan, Chenye Wang, Qixiu Li, Qing Ye, Yizhun Zhu, and Yicheng Mao. 2023. "S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction" Antioxidants 12, no. 2: 294. https://doi.org/10.3390/antiox12020294
APA StyleXi, H., Wang, C., Li, Q., Ye, Q., Zhu, Y., & Mao, Y. (2023). S-Propargyl-Cysteine Ameliorates Peripheral Nerve Injury through Microvascular Reconstruction. Antioxidants, 12(2), 294. https://doi.org/10.3390/antiox12020294