Ozone-Induced Biochemical and Molecular Changes in Vitis vinifera Leaves and Responses to Botrytis cinerea Infections
Abstract
:1. Introduction
2. Materials and Methods
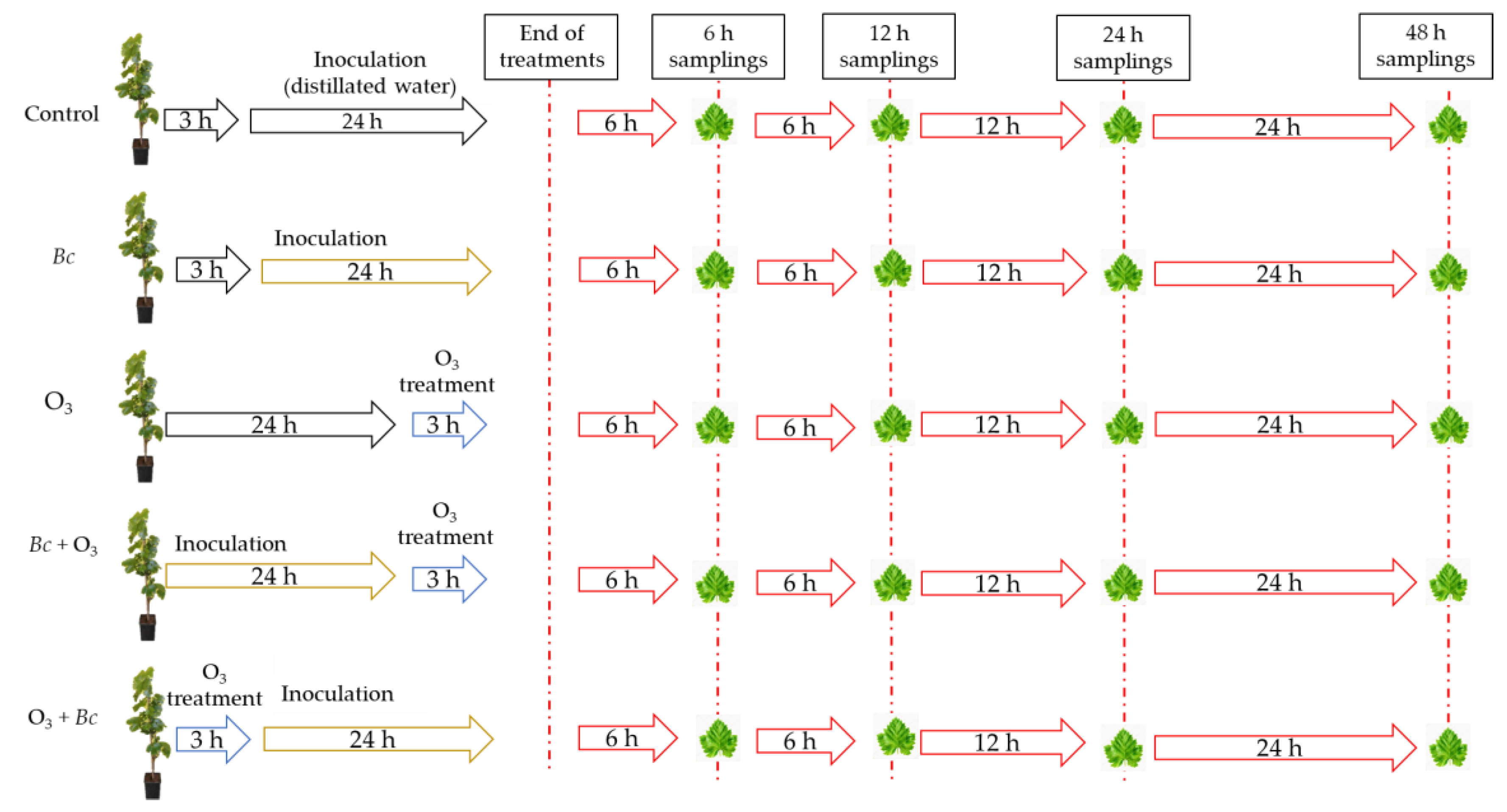
2.1. Biological Material and Experimental Design
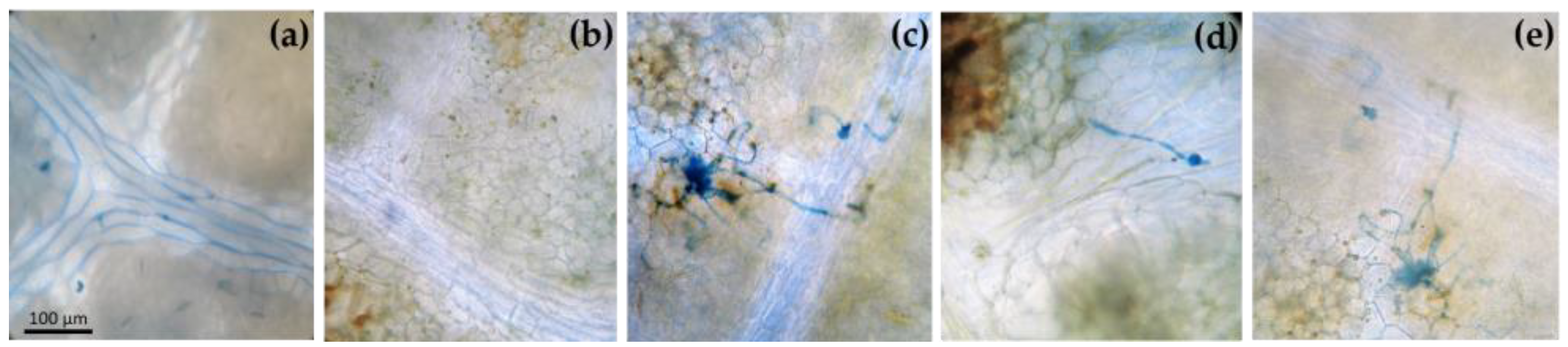
2.2. Microscopic Observations
2.3. Biochemical Analysis
2.4. Molecular Analysis
2.5. Statistical Analysis
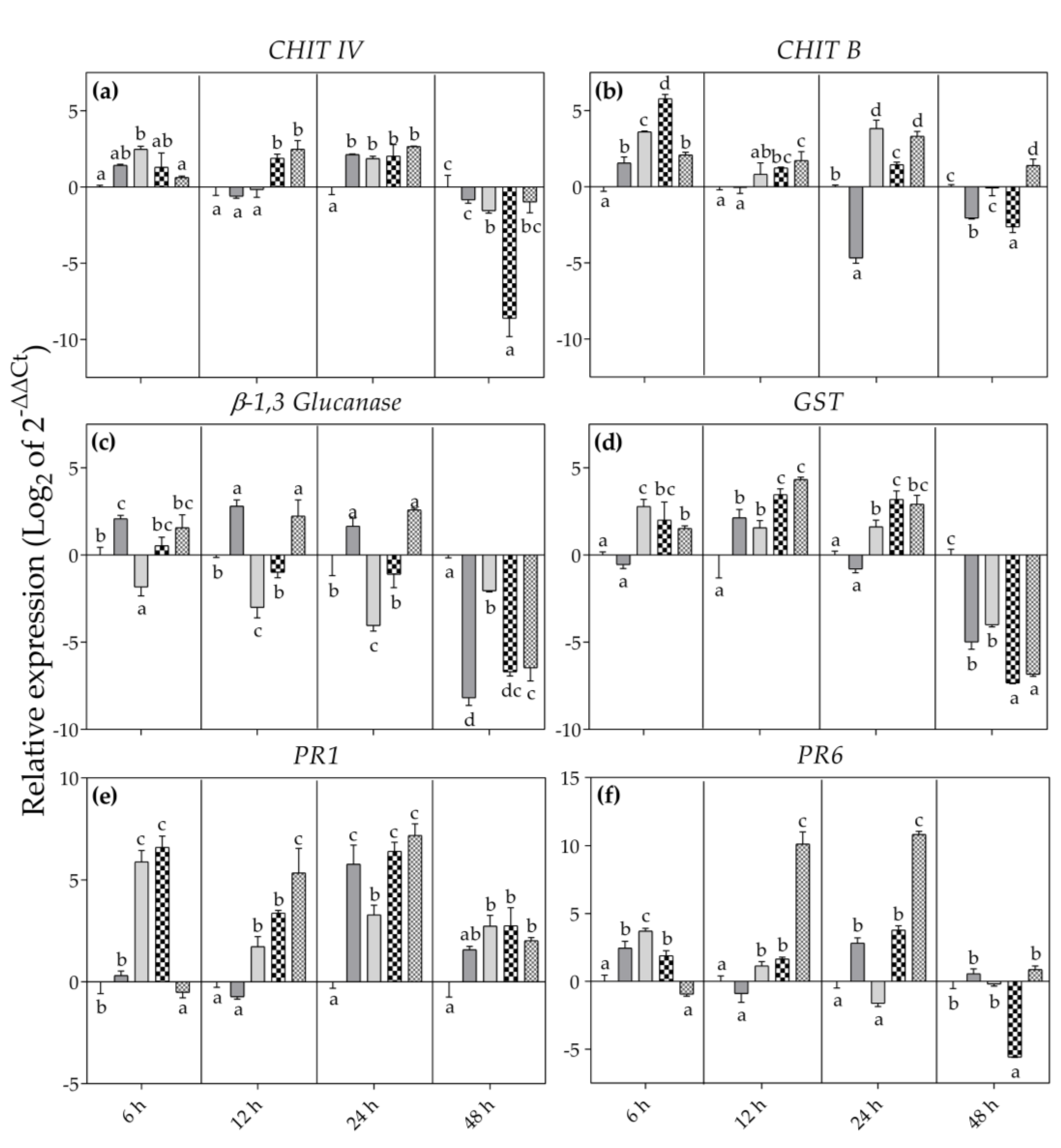
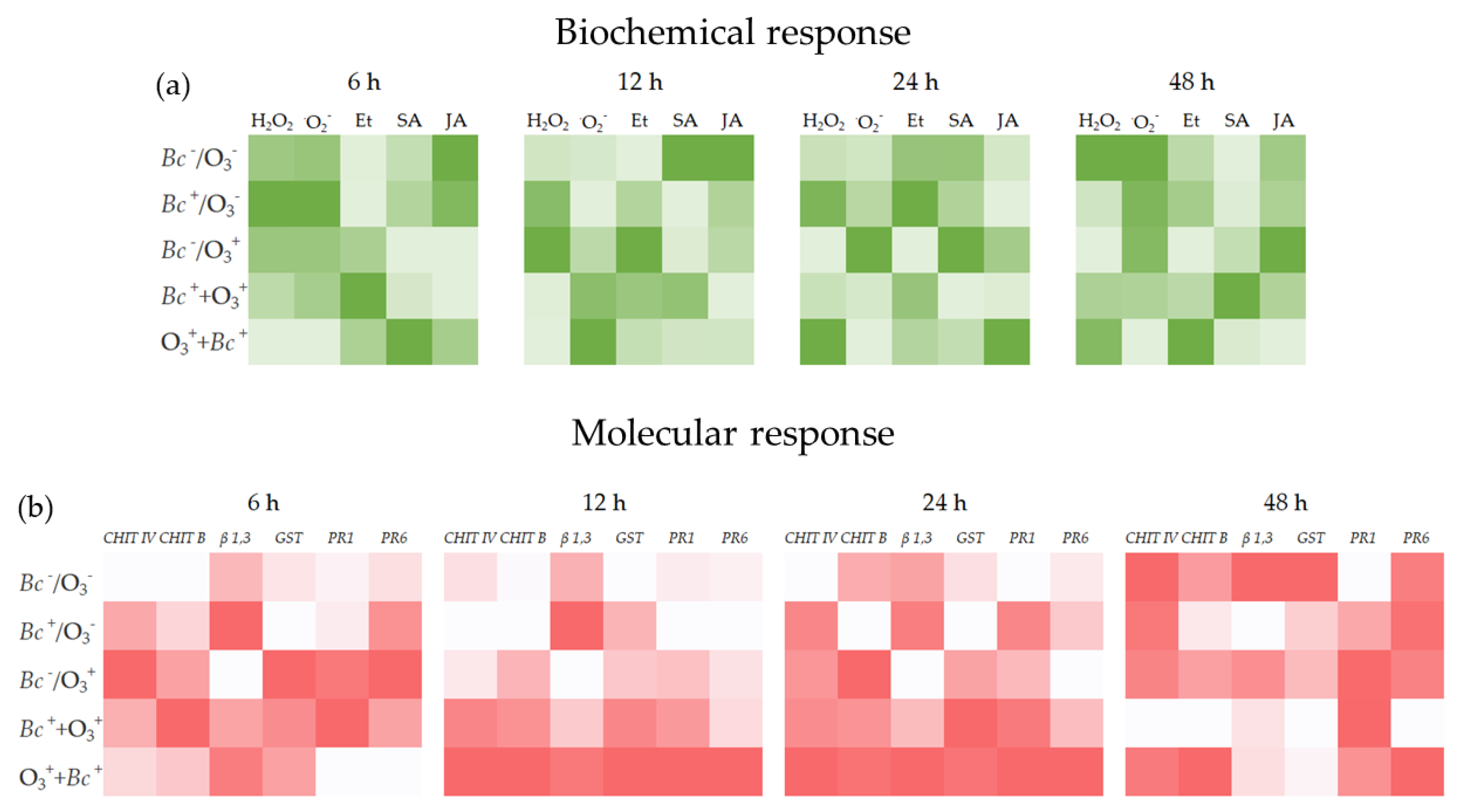
3. Results
3.1. Macroscopic and Microscopic Symptoms
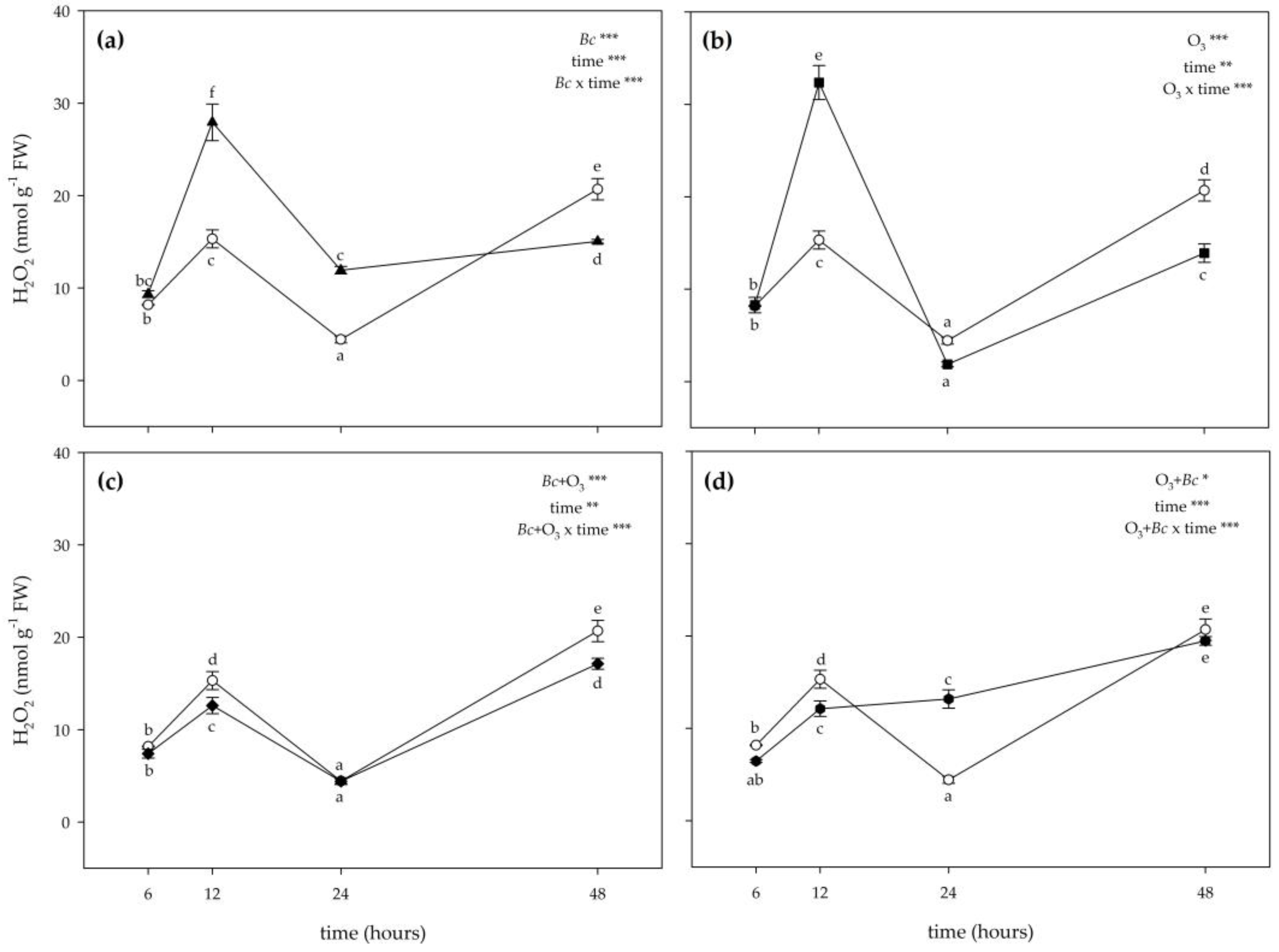
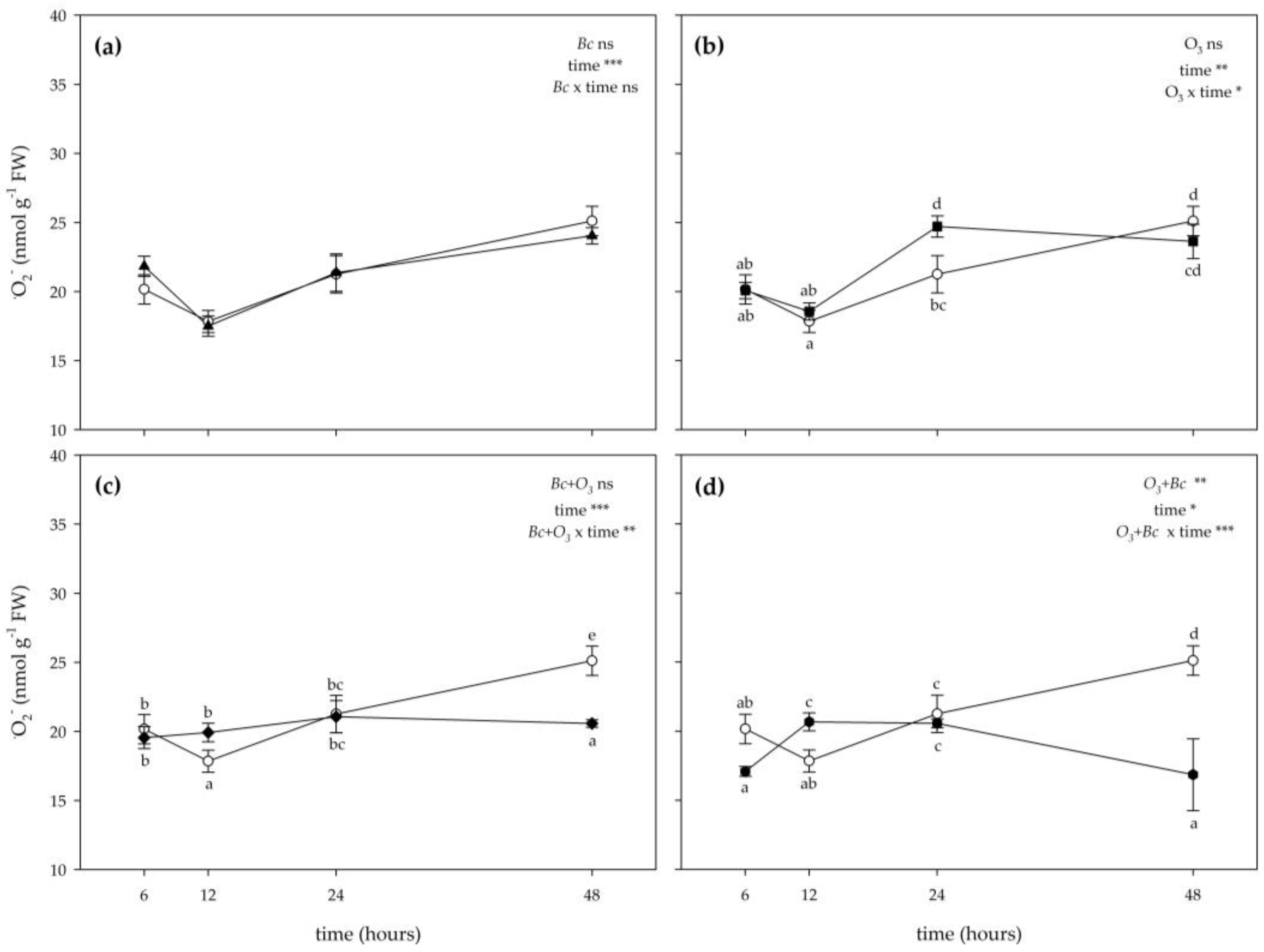
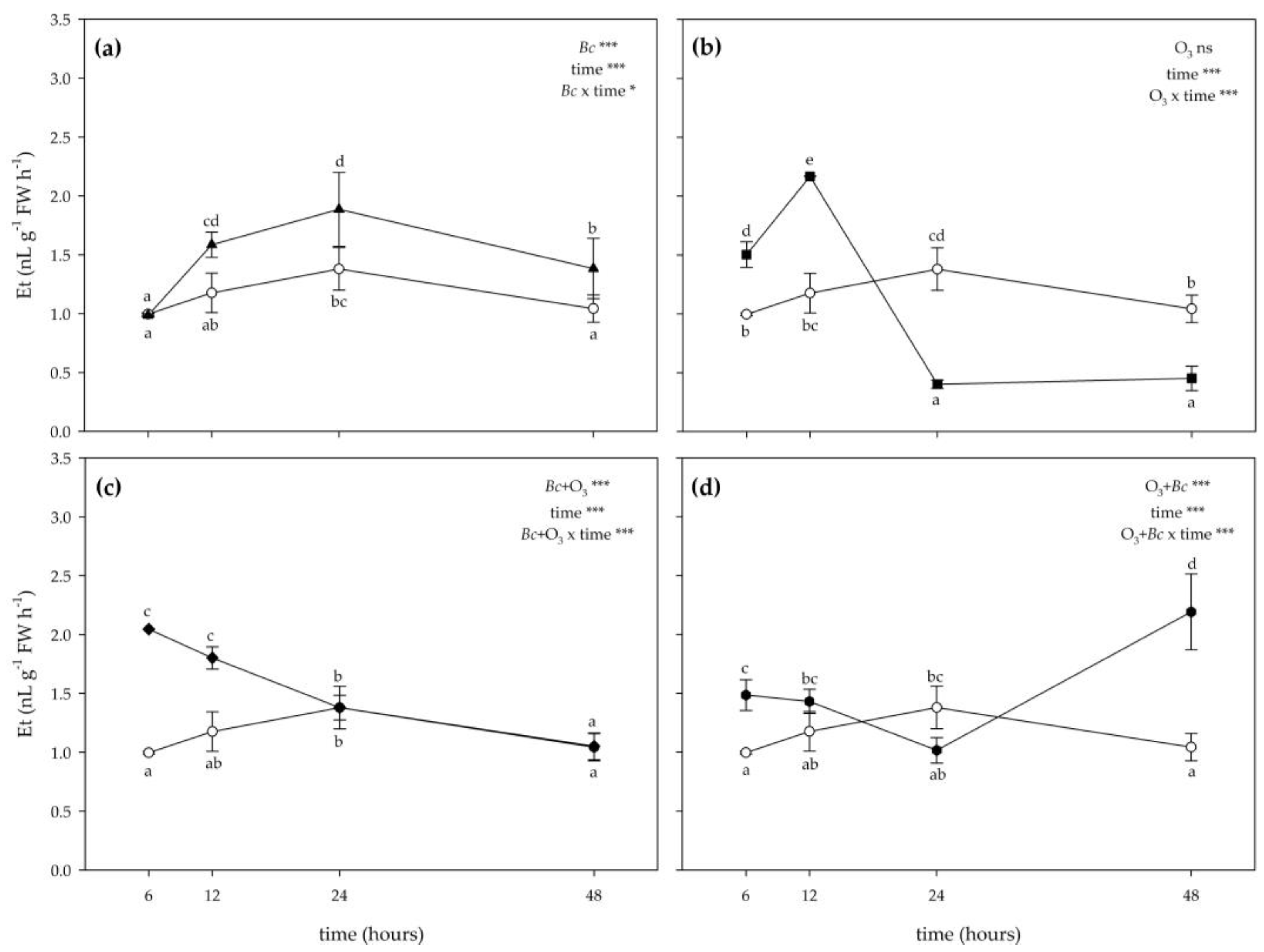
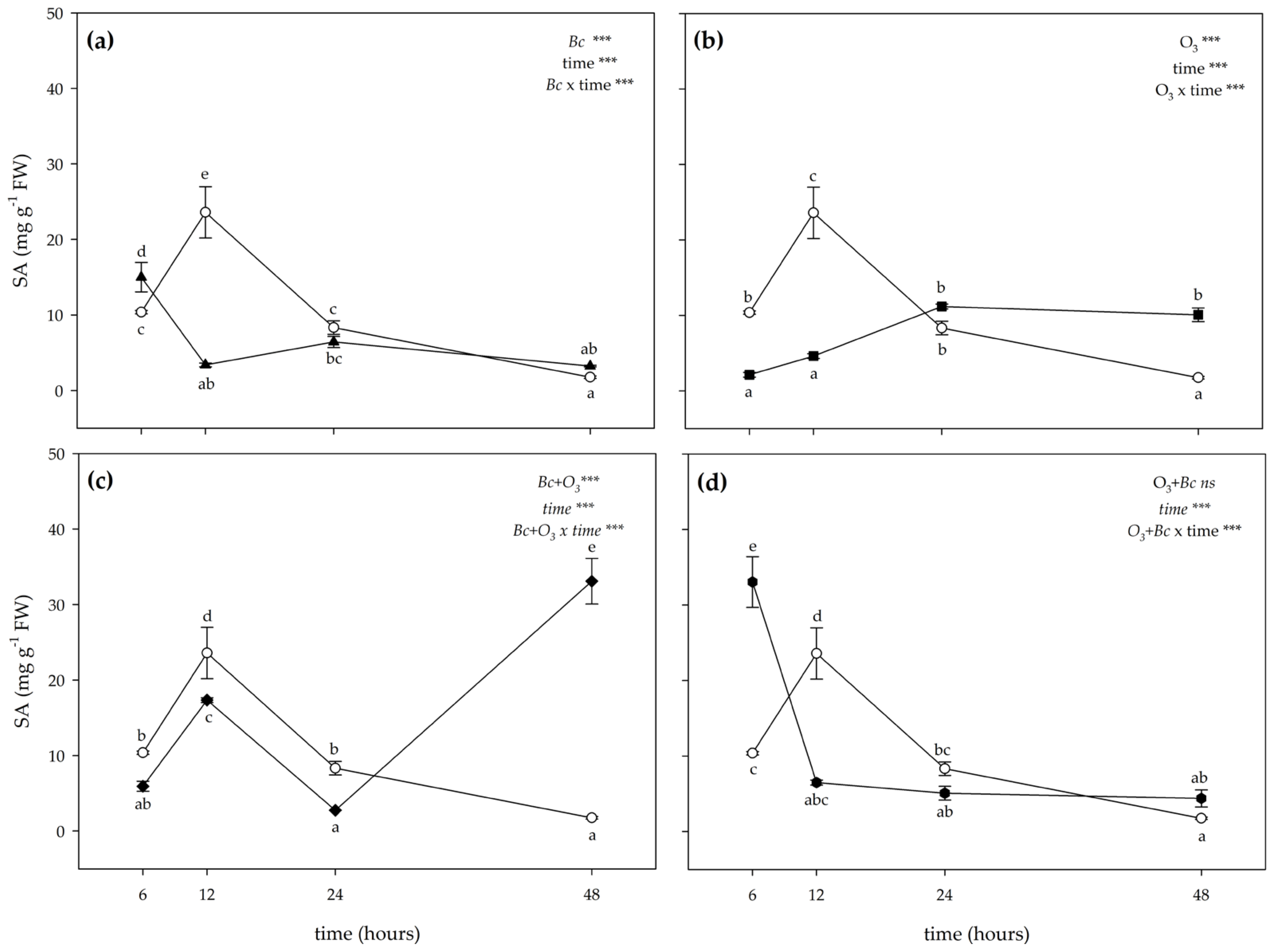
3.2. Biochemical Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Julian, M.; Alston, O.S. Grapes in the World Economy. In The Grape Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24. [Google Scholar]
- Eurostat Vineyards in the EU. Statistics—Statistics Explained. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Vineyards_in_the_EU_-_statistics (accessed on 1 December 2022).
- Román, C.; Peris, M.; Esteve, J.; Tejerina, M.; Cambray, J.; Vilardell, P.; Planas, S. Pesticide Dose Adjustment in Fruit and Grapevine Orchards by DOSA3D: Fundamentals of the System and on-Farm Validation. Sci. Tot. Environ. 2022, 808, 152158. [Google Scholar] [CrossRef] [PubMed]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Barka, E.A. Plasmopara viticola the Causal Agent of Downy Mildew of Grapevine: From Its Taxonomy to Disease Management. Front. Microbiol. 2022, 11, 889472. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop. Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Modesti, M.; Baccelloni, S.; Brizzolara, S.; Aleandri, M.P.; Bellincontro, A.; Mencarelli, F.; Tonutti, P. Effects of Treatments with Ozonated Water in the Vineyard (cv Vermentino) on Microbial Population and Fruit Quality Parameters. BIO Web Conf. 2019, 13, 04011. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in Feed and Food and the Role of Ozone in Their Detoxification and Degradation: An Update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Campayo, A.; Serrano de la Hoz, K.; García-Martínez, M.M.; Sánchez-Martínez, J.F.; Salinas, M.R.; Alonso, G.L. Spraying Ozonated Water on Bobal Grapevines: Effect on Grape Quality. Food Res. Int. 2019, 125, 108540. [Google Scholar] [CrossRef]
- Romeo-Oliván, A.; Pagès, M.; Breton, C.; Lagarde, F.; Cros, H.; Yobrégat, O.; Violleau, F.; Jacques, A. Ozone Dissolved in Water: An Innovative Tool for the Production of Young Plants in Grapevine Nurseries? Ozone Sci. Eng. 2022, 44, 521–535. [Google Scholar] [CrossRef]
- Modesti, M.; Macaluso, M.; Taglieri, I.; Bellincontro, A.; Sanmartin, C. Ozone and Bioactive Compounds in Grapes and Wine. Foods 2021, 10, 2934. [Google Scholar] [CrossRef]
- Risoli, S.; Lauria, G. Ozonated Water Application as an Innovative Tool for Elicitation of Plant Defense Response: A Minireview. Curr. Opin. Environ. Sci. Health 2022, 28, 100375. [Google Scholar] [CrossRef]
- Shezi, S.; Samukelo Magwaza, L.; Mditshwa, A.; Zeray Tesfay, S. Changes in Biochemistry of Fresh Produce in Response to Ozone Postharvest Treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Contribution of Amino Acids to Strawberry Fruit Quality and Their Relevance as Stress Indicators under NaCl Salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- Forney, C.F.; Song, J.; Fan, L.; Hildebrand, P.D.; Jordan, M.A. Ozone and 1-Methylcyclopropene Alter the Postharvest Quality of Broccoli. J. Amer. Soc. Hort. Sci. 2003, 128, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Guzel-Seydim, Z.B.; Greene, A.K.; Seydim, A.C. Use of Ozone in the Food Industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone Effects on Plants in Natural Ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as Eustress for Enhancing Secondary Metabolites and Bioactive Properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Modesti, M.; Forniti, R.; Brunori, E.; Mencarelli, F.; Bellincontro, A.; Tonutti, P. Ozone Treatments to Induce Systemic-Acquired Resistance in Leaves of Potted Vines: Molecular Responses and NIR Evaluation for Identifying Effective Dose and Exposition Duration. Oeno One 2022, 56, 175–187. [Google Scholar] [CrossRef]
- Heath, R.L. Alterations of the Biochemical Pathways of Plants by the Air Pollutant Ozone: Which Are the True Gauges of Injury? Sci. World J. 2007, 7, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L. Modification of the Biochemical Pathways of Plants Induced by Ozone: What Are the Varied Routes to Change? Environ. Pollut. 2008, 155, 453–463. [Google Scholar] [CrossRef]
- Landi, M.; Cotrozzi, L.; Pellegrini, E.; Remorini, D.; Tonelli, M.; Trivellini, A.; Nali, C.; Guidi, L.; Massai, R.; Vernieri, P.; et al. When “Thirsty” Means “Less Able to Activate the Signalling Wave Trigged by a Pulse of Ozone”: A Case of Study in Two Mediterranean Deciduous Oak Species with Different Drought Sensitivity. Sci. Tot. Environ. 2019, 657, 379–390. [Google Scholar] [CrossRef]
- Blanco-Ward, D.; Ribeiro, A.; Paoletti, E.; Miranda, A.I. Assessment of Tropospheric Ozone Phytotoxic Effects on the Grapevine (Vitis vinifera L.): A Review. Atmos. Environ. 2021, 244, 117924. [Google Scholar] [CrossRef]
- Soja, G.; Reichenauer, T.G.; Eid, M.; Soja, A.M.; Schaber, R.; Gangl, H. Long-Term Ozone Exposure and Ozone Uptake of Grapevines in Open-Top Chambers. Atmos. Environ. 2004, 38, 2313–2321. [Google Scholar] [CrossRef]
- Valletta, A.; Salvatori, E.; Rita Santamaria, A.; Nicoletti, M.; Toniolo, C.; Caboni, E.; Bernardini, A.; Pasqua, G.; Manes, F. Ecophysiological and Phytochemical Response to Ozone of Wine Grape Cultivars of Vitis vinifera L. Nat. Prod. Res. 2016, 30, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, E.; Campanella, A.; Paolocci, M.; Trivellini, A.; Gennai, C.; Muganu, M.; Nali, C.; Lorenzini, G. Functional Leaf Traits and Diurnal Dynamics of Photosynthetic Parameters Predict the Behavior of Grapevine Varieties towards Ozone. PLoS ONE 2015, 10, e0135056. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.V.; Davis, K.R. Ozone-Induced Cell Death Occurs via Two Distinct Mechanisms in Arabidopsis: The Role of Salicylic Acid. Plant. J. 1999, 17, 603–614. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Pellegrini, E.; D’Angiolillo, F.; Petersen, M.; Nali, C.; Pistelli, L.; Lorenzini, G. Ozone-Elicited Secondary Metabolites in Shoot Cultures of Melissa officinalis L. Plant Cell Tissue Organ Cult. 2015, 120, 617–629. [Google Scholar] [CrossRef]
- Huang, Z.H.; Wang, Z.L.; Shi, B.L.; Wei, D.; Chen, J.X.; Wang, S.L.; Gao, B.J. Simultaneous Determination of Salicylic Acid, Jasmonic Acid, Methyl Salicylate, and Methyl Jasmonate from Ulmus pumila Leaves by GC-MS. Int. J. Anal. Chem. 2015, 2015, 698630. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, E.; Trivellini, A.; Campanella, A.; Francini, A.; Lorenzini, G.; Nali, C.; Vernieri, P. Signaling Molecules and Cell Death in Melissa officinalis Plants Exposed to Ozone. Plant Cell Rep. 2013, 32, 1965–1980. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Coolen, S.; Van Pelt, J.A.; Van Wees, S.C.M.; Pieterse, C.M.J. Mining the Natural Genetic Variation in Arabidopsis thaliana for Adaptation to Sequential Abiotic and Biotic Stresses. Planta 2019, 249, 1087–1105. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temme, N.; Tudzynski, P. Does Botrytis cinerea Ignore H2O2-Induced Oxidative Stress during Infection? Characterization of Botrytis Activator Protein 1. MPMI 2009, 22, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.A. ROS in Biotic Interactions. Physiol. Plant 2010, 138, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, L.; Pellegrini, E.; Guidi, L.; Landi, M.; Lorenzini, G.; Massai, R.; Remorini, D.; Tonelli, M.; Trivellini, A.; Vernieri, P.; et al. Losing the Warning Signal: Drought Compromises the Cross-Talk of Signaling Molecules in Quercus ilex Exposed to Ozone. Front. Plant Sci. 2017, 8, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berrios, L.; Rentsch, J.D. Linking Reactive Oxygen Species (ROS) to Abiotic and Biotic Feedbacks in Plant Microbiomes: The Dose Makes the Poison. Int. J. Mol. Sci. 2022, 23, 4402. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing Softly: A Roadmap of Botrytis cinerea Pathogenicity. Trends Plant Sci. 2022, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, K.; Zamora, O.; Vaahtera, L.; Overmyer, K.; Brosche, M. Dissecting Contrasts in Cell Death, Hormone, and Defense Signaling in Response to Botrytis cinerea and Reactive Oxygen Species. Mol. Plant Microbe Interact. 2021, 34, 75–87. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-Related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Shigenaga, A.M.; Argueso, C.T. No Hormone to Rule Them All: Interactions of Plant Hormones during the Responses of Plants to Pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Balint-Kurti, P. The Plant Hypersensitive Response: Concepts, Control and Consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.E.-L., Upadhyay, R.S., Sharma, G.D., Manoharachary, C., Gupta, V.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 457–470. ISBN 978-3-030-41870-0. [Google Scholar]
- Datta, S.K.; Muthukrishnan, S. Pathogenesis-Related Proteins in Plants; CRC Press: Boca Raton, FL, USA, 1999; 304p. [Google Scholar]
- Sharma, Y.K.; Leont, J.; Raskint, I.; Davis, K.R. Ozone-Induced Responses in Arabidopsis thaliana: The Role of Salicylic Acid in the Accumulation of Defense-Related Transcripts and Induced Resistance. Proc. Natl. Acad. Sci. USA 1996, 93, 5099–5104. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modesti, M.; Marchica, A.; Pisuttu, C.; Risoli, S.; Pellegrini, E.; Bellincontro, A.; Mencarelli, F.; Tonutti, P.; Nali, C. Ozone-Induced Biochemical and Molecular Changes in Vitis vinifera Leaves and Responses to Botrytis cinerea Infections. Antioxidants 2023, 12, 343. https://doi.org/10.3390/antiox12020343
Modesti M, Marchica A, Pisuttu C, Risoli S, Pellegrini E, Bellincontro A, Mencarelli F, Tonutti P, Nali C. Ozone-Induced Biochemical and Molecular Changes in Vitis vinifera Leaves and Responses to Botrytis cinerea Infections. Antioxidants. 2023; 12(2):343. https://doi.org/10.3390/antiox12020343
Chicago/Turabian StyleModesti, Margherita, Alessandra Marchica, Claudia Pisuttu, Samuele Risoli, Elisa Pellegrini, Andrea Bellincontro, Fabio Mencarelli, Pietro Tonutti, and Cristina Nali. 2023. "Ozone-Induced Biochemical and Molecular Changes in Vitis vinifera Leaves and Responses to Botrytis cinerea Infections" Antioxidants 12, no. 2: 343. https://doi.org/10.3390/antiox12020343
APA StyleModesti, M., Marchica, A., Pisuttu, C., Risoli, S., Pellegrini, E., Bellincontro, A., Mencarelli, F., Tonutti, P., & Nali, C. (2023). Ozone-Induced Biochemical and Molecular Changes in Vitis vinifera Leaves and Responses to Botrytis cinerea Infections. Antioxidants, 12(2), 343. https://doi.org/10.3390/antiox12020343







