Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of Prx4−/− Mice and Prx4−/−/Srx−/− Mice
2.2. Urethane-Induced Mouse Lung Cancer Protocol and Tissue Processing
2.3. Cell Culture and Establishment of Stable Knockdown Cells
2.4. Intracellular ROS Level Measurement
2.5. Immunoblotting
2.6. Bioinformatics Analysis
2.6.1. Data Source
2.6.2. Expression Analysis of Prx4
2.6.3. Clinical Correlation Analysis
2.6.4. Functional Enrichment Analysis of PRX4
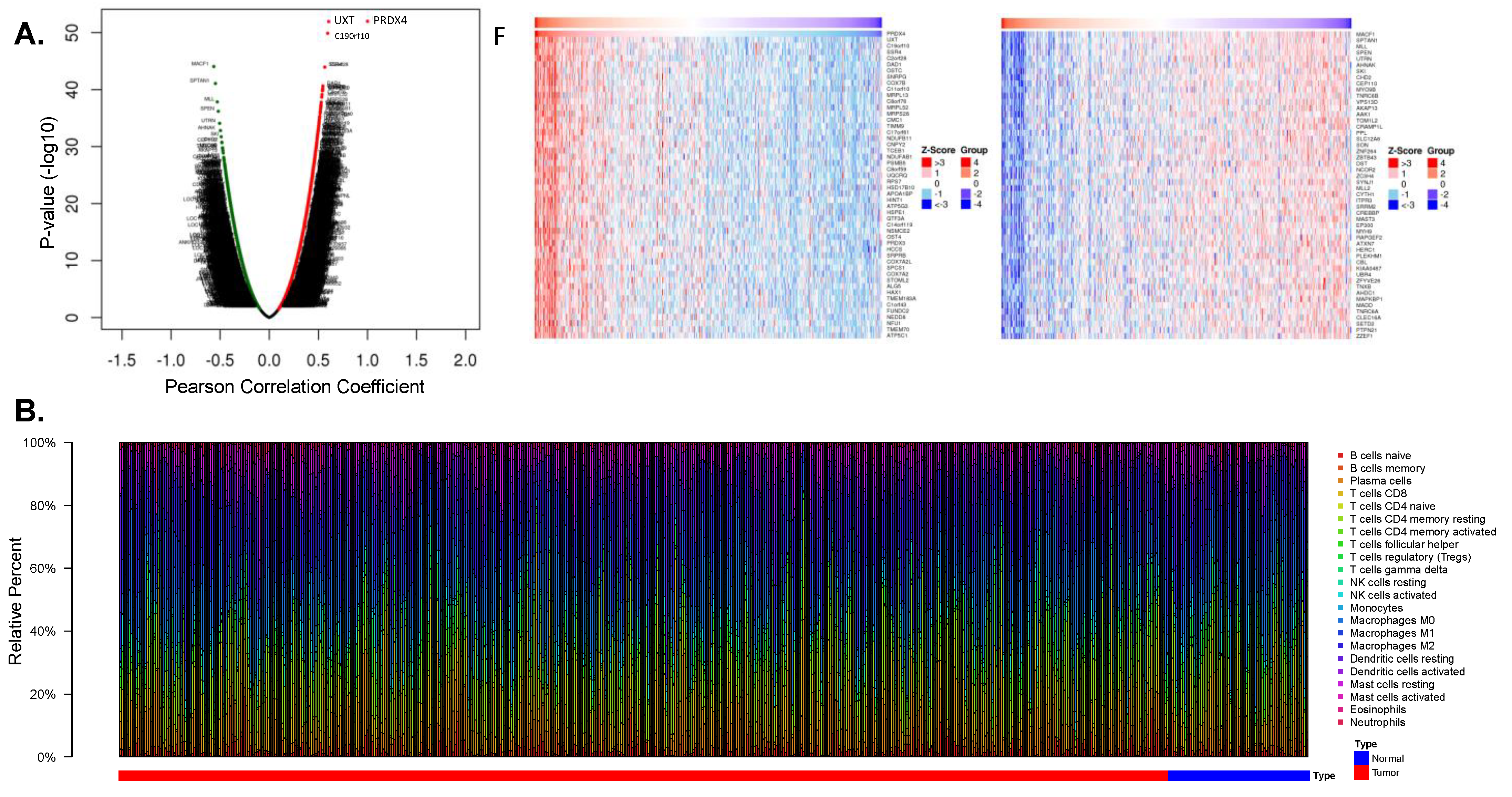
2.6.5. Immune Infiltration Analysis
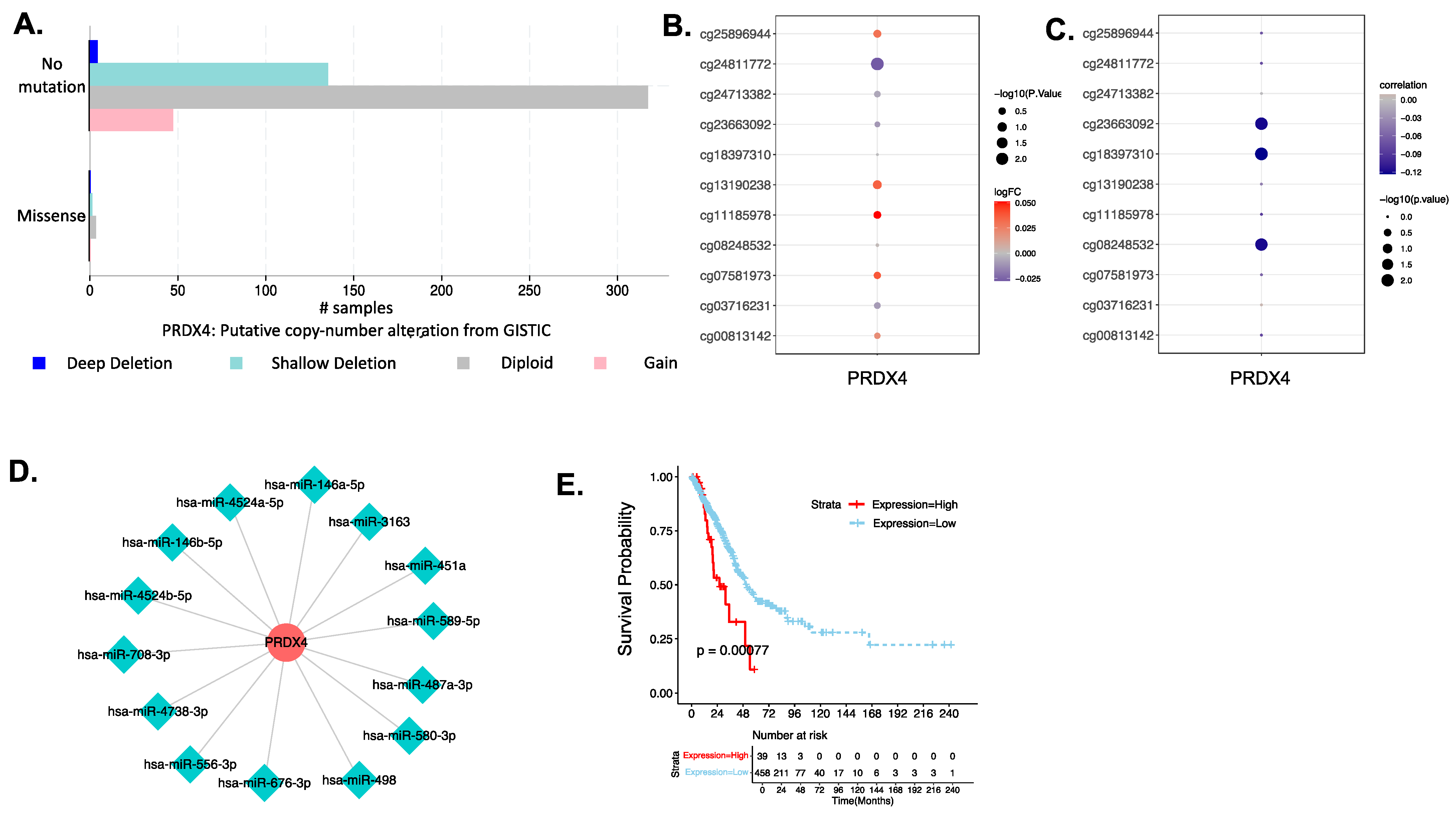
2.6.6. Investigation of the Bio-Mechanisms of Aberrant Prx4 Expression
2.7. Statistical Evaluation
3. Results
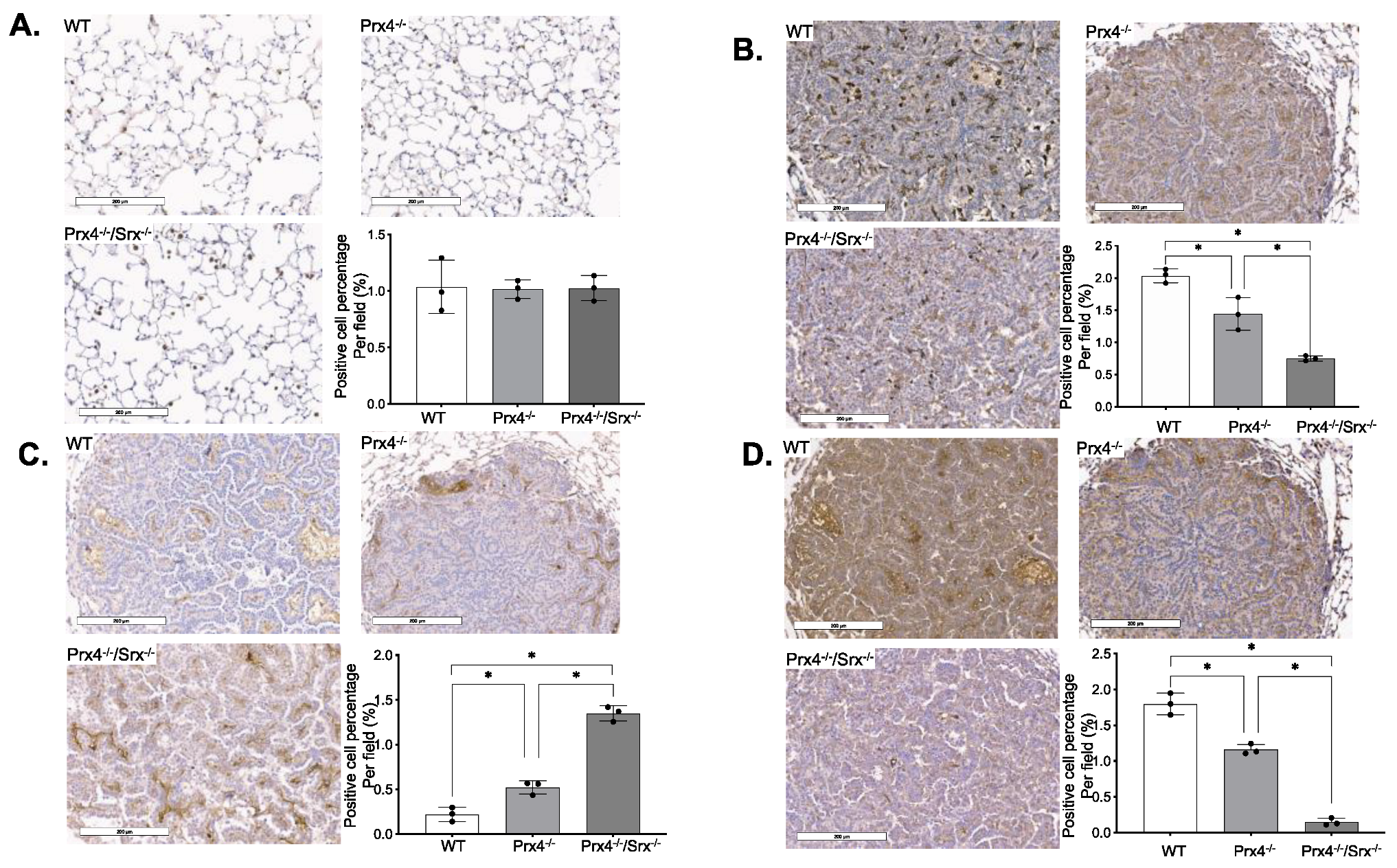
3.1. Loss of Prx4 or Srx/Prx4 Inhibits Tumor Formation and Cell Proliferation
3.2. Depletion of Prx4 or Prx4/Srx Reduces Intratumoral Macrophage Infiltration
3.3. Exposure to Urethane Activates Srx and Prx4 Expression Which Contributes to Cell Transformation
3.4. Bioinformatics Analysis of Prx4
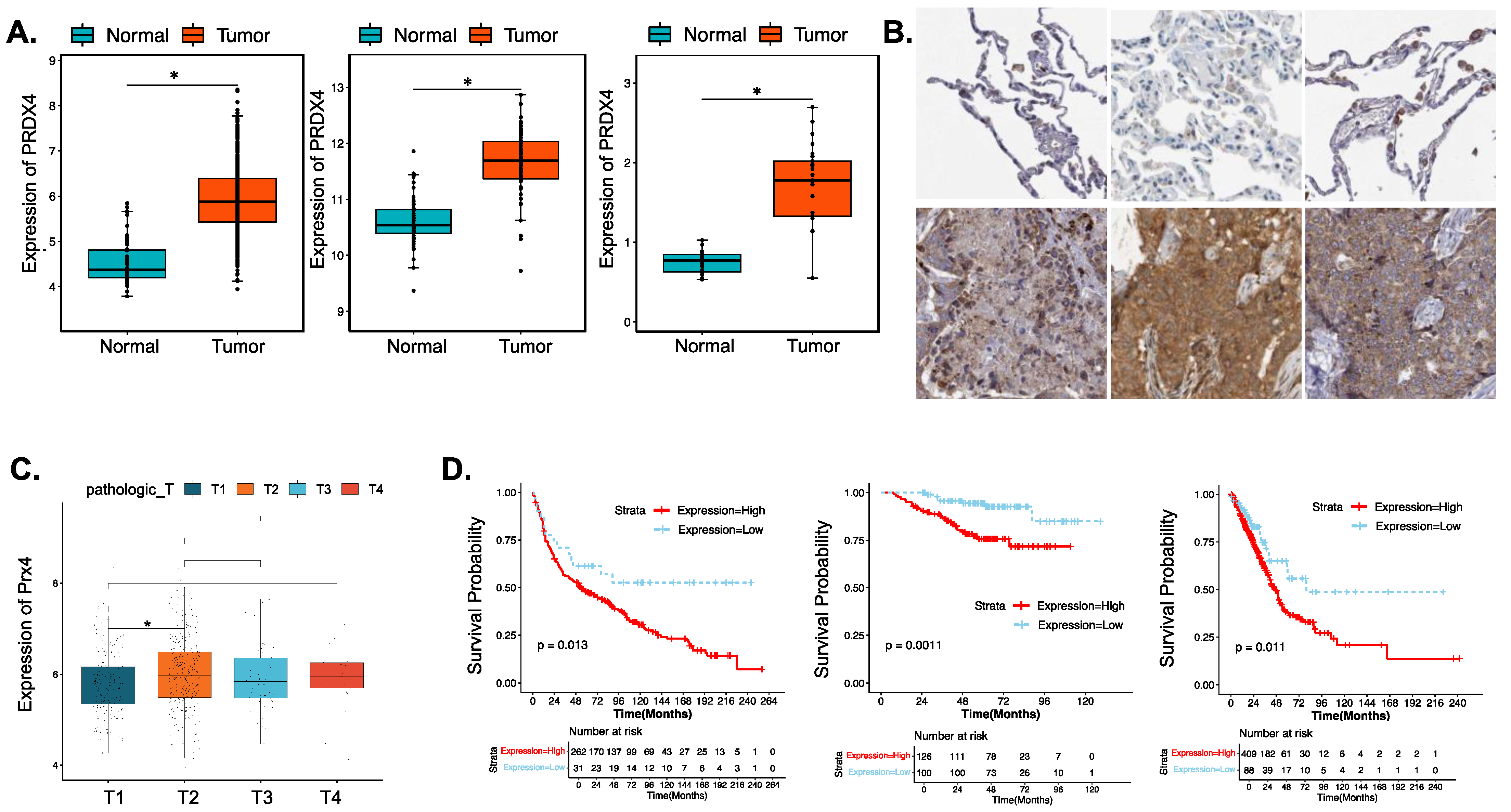
3.4.1. Prx4 Is Significantly Upregulated in LUAD Patients and Is Negatively Correlated with Patient Survival and Prognosis
3.4.2. Prx4 May Affect the Tumor Microenvironment of LUAD
3.4.3. Investigation of the Biological Mechanism of Abnormal Expression of PRX4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Giotopoulou, G.A.; Stathopoulos, G.T. Effects of Inhaled Tobacco Smoke on the Pulmonary Tumor Microenvironment. Tumor Microenviron. 2020, 1225, 53–69. [Google Scholar]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Goldkorn, T.; Filosto, S.; Chung, S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxid. Redox Signal. 2014, 21, 2149–2174. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Chae, H.Z.; Robison, K.; Poole, L.B.; Church, G.; Storz, G.; Rhee, S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: Alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 1994, 91, 7017–7021. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef]
- Chua, P.-J.; Lee, E.-H.; Yu, Y.; Yip, G.W.-C.; Tan, P.-H.; Bay, B.-H. Silencing the Peroxiredoxin III gene inhibits cell proliferation in breast cancer. Int. J. Oncol. 2010, 36, 359–364. [Google Scholar]
- Zhang, Y.; Sun, C.; Xiao, G.; Shan, H.; Tang, L.; Yi, Y.; Yu, W.; Gu, Y. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-Z.; Li, D.-Q.; Hou, Y.-F.; Wu, J.; Lu, J.-S.; Di, G.-H.; Jin, W.; Ou, Z.-L.; Shen, Z.-Z.; Shao, Z.-M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, R76. [Google Scholar] [CrossRef]
- Wang, T.; Tamae, D.; LeBon, T.; Shively, J.E.; Yen, Y.; Li, J.J. The role of peroxiredoxin II in radiation-resistant MCF-7 breast cancer cells. Cancer Res. 2005, 65, 10338–10346. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Matsumoto, A.; Fujii, J.; Taniguchi, N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J. Biochem. 2000, 127, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002, 7, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.; Struck, J.; Köhrle, J.; Müller, B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock 2011, 35, 460–465. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Alkhalaf, A.; Landman, G.W.; van Hateren, K.J.; Groenier, K.H.; Struck, J.; Schulte, J.; Gans, R.O.; Bakker, S.J.; Kleefstra, N. Serum peroxiredoxin 4: A marker of oxidative stress associated with mortality in type 2 diabetes (ZODIAC-28). PLoS ONE 2014, 9, e89719. [Google Scholar] [CrossRef]
- Nawata, A.; Noguchi, H.; Mazaki, Y.; Kurahashi, T.; Izumi, H.; Wang, K.-Y.; Guo, X.; Uramoto, H.; Kohno, K.; Taniguchi, H. Overexpression of peroxiredoxin 4 affects intestinal function in a dietary mouse model of nonalcoholic fatty liver disease. PLoS ONE 2016, 11, e0152549. [Google Scholar] [CrossRef]
- Kam, M.K.; Lee, D.G.; Kim, B.; Lee, H.-S.; Lee, S.-R.; Bae, Y.C.; Lee, D.-S. Peroxiredoxin 4 ameliorates amyloid beta oligomer-mediated apoptosis by inhibiting ER-stress in HT-22 hippocampal neuron cells. Cell Biol. Toxicol. 2019, 35, 573–588. [Google Scholar] [CrossRef]
- Guo, X.; Yamada, S.; Tanimoto, A.; Ding, Y.; Wang, K.-Y.; Shimajiri, S.; Murata, Y.; Kimura, S.; Tasaki, T.; Nabeshima, A. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxid. Redox Signal. 2012, 17, 1362–1375. [Google Scholar] [CrossRef]
- Basu, A.; Banerjee, H.; Rojas, H.; Martinez, S.R.; Roy, S.; Jia, Z.; Lilly, M.B.; De León, M.; Casiano, C.A. Differential expression of peroxiredoxins in prostate cancer: Consistent upregulation of PRDX3 and PRDX4. Prostate 2011, 71, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, X.; Nakamura, Y.; Zhou, X.; Yamaguchi, R.; Zhang, J.; Ishigaki, Y.; Uramoto, H.; Yamada, S. Overexpression of PRDX4 Modulates Tumor Microenvironment and Promotes Urethane-Induced Lung Tumorigenesis. Oxidative Med. Cell. Longev. 2020, 2020, 8262730. [Google Scholar] [CrossRef] [PubMed]
- Gregorieff, A.; Clevers, H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef]
- Wei, Q.; Jiang, H.; Xiao, Z.; Baker, A.; Young, M.R.; Veenstra, T.D.; Colburn, N.H. Sulfiredoxin–peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 7004–7009. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Jiang, H.; Chawsheen, H.A.; Gerard, M.; Toledano, M.B.; Wei, Q. Nrf2-activated expression of sulfiredoxin contributes to urethane-induced lung tumorigenesis. Cancer Lett. 2018, 432, 216–226. [Google Scholar] [CrossRef]
- Kang, S.W.; Baines, I.C.; Rhee, S.G. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem. 1998, 273, 6303–6311. [Google Scholar] [CrossRef]
- Nettleship, A.; Henshaw, P.S.; Meyer, H.L. Induction of pulmonary tumors in mice with ethyl carbamate (urethane). J. Natl. Cancer Inst. 1943, 4, 309–319. [Google Scholar]
- Tuveson, D.A.; Jacks, T. Modeling human lung cancer in mice: Similarities and shortcomings. Oncogene 1999, 18, 5318–5324. [Google Scholar] [CrossRef]
- Hanna, J.M.; Onaitis, M.W. Cell of origin of lung cancer. J. Carcinog. 2013, 12, 6. [Google Scholar]
- Malkinson, A.M. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp. Lung Res. 1998, 24, 541–555. [Google Scholar] [CrossRef]
- Iuchi, Y.; Okada, F.; Tsunoda, S.; Kibe, N.; Shirasawa, N.; Ikawa, M.; Okabe, M.; Ikeda, Y.; Fujii, J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem. J. 2009, 419, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Planson, A.-G.; Palais, G.; Abbas, K.; Gerard, M.; Couvelard, L.; Delaunay, A.; Baulande, S.; Drapier, J.-C.; Toledano, M.B. Sulfiredoxin protects mice from lipopolysaccharide-induced endotoxic shock. Antioxid. Redox Signal. 2011, 14, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wu, L.; Mishra, M.; Chawsheen, H.A.; Wei, Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am. J. Cancer Res. 2014, 4, 445. [Google Scholar] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. In Advanced Protocols in Oxidative Stress II; Springer: New York, NY, USA, 2010; pp. 57–72. [Google Scholar]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Sturm, G.; Finotello, F.; List, M. Immunedeconv: An R package for unified access to computational methods for estimating immune cell fractions from bulk RNA-sequencing data. In Bioinformatics for Cancer Immunotherapy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 223–232. [Google Scholar]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Festing, M.F.; Yang, A.; Malkinson, A. At least four genes and sex are associated with susceptibility to urethane-induced pulmonary adenomas in mice. Genet. Res. 1994, 64, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhou, L.; Lu, L.; Chen, T.; Wei, S.; Lin, X.; Lian, X. Inflammation has a role in urethane-induced lung cancer in C57BL/6J mice. Mol. Med. Rep. 2016, 14, 3323–3328. [Google Scholar] [CrossRef]
- dos Anjos Cassado, A. F4/80 as a major macrophage marker: The case of the peritoneum and spleen. Macrophages 2017, 62, 161–179. [Google Scholar]
- De Larco, J.E.; Todaro, G.J. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA 1978, 75, 4001–4005. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T.; Kang, Z.; Guo, G.; Sun, Y.; Lin, K.; Huang, Q.; Shi, X.; Ni, Z.; Ding, N. Tumor-infiltrating immune cells act as a marker for prognosis in colorectal cancer. Front. Immunol. 2019, 10, 2368. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med. 2003, 167, 1600–1619. [Google Scholar] [CrossRef]
- Burdon, R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef]
- Stakišaitis, D.; Mozūraitė, R.; Kavaliauskaitė, D.; Šlekienė, L.; Balnytė, I.; Juodžiukynienė, N.; Valančiūtė, A. Sex-related differences of urethane and sodium valproate effects on Ki-67 expression in urethane-induced lung tumors of mice. Exp. Ther. Med. 2017, 13, 2741–2750. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.-J.; Chen, H.-Y.; Chen, H.-W.; Ho, C.-C.; Chen, Y.-Y.; Liu, Y.-C.; Hong, T.-H.; Yu, S.-L.; Chen, J.J. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, T.; Kido, T.; Noguchi, S.; Yamada, S.; Noguchi, H.; Guo, X.; Nawata, A.; Wang, K.-Y.; Oda, K.; Takaki, T. The overexpression of peroxiredoxin-4 affects the progression of idiopathic pulmonary fibrosis. BMC Pulm. Med. 2019, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Breton, J.; Planson, A.-G.; Bouton, C.; Bignon, J.; Seguin, C.; Riquier, S.; Toledano, M.B.; Drapier, J.-C. Nitric oxide activates an Nrf2/sulfiredoxin antioxidant pathway in macrophages. Free. Radic. Biol. Med. 2011, 51, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Ley, K. M1 and M2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.-R.; Kim, H.; Jo, Y.-J.; Hong, S.-E.; Lee, J.; Lee, H.I.; Jang, Y.-S.; Oh, S.-H.; Lee, H.J. Effective killing of cancer cells and regression of tumor growth by K27 targeting sulfiredoxin. Free. Radic. Biol. Med. 2016, 101, 384–392. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Cao, Y.; Wang, X.; Guo, Y.; Chen, J.; Horn, J.; Ponomareva, L.V.; Chaiswing, L.; Shaaban, K.A. Frenolicin B targets peroxiredoxin 1 and glutaredoxin 3 to trigger ROS/4E-BP1-mediated antitumor effects. Cell Chem. Biol. 2019, 26, 366–377.e12. [Google Scholar] [CrossRef]
- Lee, T.H.; Jin, J.-O.; Yu, K.J.; Kim, H.S.; Lee, P.C.-W. Inhibition of peroxiredoxin 2 suppresses Wnt/β-catenin signaling in gastric cancer. Biochem. Biophys. Res. Commun. 2019, 512, 250–255. [Google Scholar] [CrossRef]
- Ding, N.; Jiang, H.; Thapa, P.; Hao, Y.; Alshahrani, A.; Allison, D.; Izumi, T.; Rangnekar, V.M.; Liu, X.; Wei, Q. Peroxiredoxin IV plays a critical role in cancer cell growth and radioresistance through the activation of the Akt/GSK3 signaling pathways. J. Biol. Chem. 2022, 298, 102123. [Google Scholar] [CrossRef]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.; Struck, J.; Schulte, J.; Hillege, H.L.; van der Harst, P. Peroxiredoxin 4, A novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J. Am. Heart Assoc. 2012, 1, e002956. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Hussein, O.; Sadvakassova, G.; Guo, Y.; Siegel, P.M.; Komarova, S.V. Breast cancer-derived factors stimulate osteoclastogenesis through the Ca2+/protein kinase C and transforming growth factor-β/MAPK signaling pathways. J. Biol. Chem. 2009, 284, 33662–33670. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, S.; Komarova, S.V. Molecular signaling pathways mediating osteoclastogenesis induced by prostate cancer cells. BMC Cancer 2013, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, S.; Tiedemann, K.; Tabariès, S.; Siegel, P.M.; Komarova, S.V. Peroxiredoxin 4: A novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett. 2015, 361, 262–270. [Google Scholar] [CrossRef] [PubMed]



| Features | H_Prx4 (n = 409) | L_PRDX4 (n = 88) | p Value |
|---|---|---|---|
| age | 0.01047 | ||
| age < 65 | 190 | 28 | |
| age ≥ 65 | 213 | 56 | |
| pathologic_M | 0.3475 | ||
| M0 | 278 | 53 | |
| M1 | 19 | 5 | |
| pathologic_N | 0.473 | ||
| N0 | 259 | 62 | |
| N1 | 82 | 12 | |
| N2 | 58 | 11 | |
| N3 | 2 | 0 | |
| pathologic_T | 0.01243 | ||
| T1 | 131 | 35 | |
| T2 | 227 | 40 | |
| T3 | 36 | 7 | |
| T4 | 15 | 3 | |
| tobacco_smoking_history | 0.7146 | ||
| NO | 56 | 15 | |
| YES | 341 | 71 | |
| gender | 0.7899 | ||
| female | 223 | 46 | |
| male | 186 | 42 | |
| tissue_or_origin | 0.6715 | ||
| Lower lobe | 137 | 31 | |
| Middle lobe | 17 | 4 | |
| Upper lobe | 239 | 52 | |
| tumor_stage | 0.6122 | ||
| stage i | 216 | 51 | |
| stage ii | 102 | 16 | |
| stage iii | 66 | 14 | |
| stage iv | 19 | 6 |
| Cell | Gene | r | p Value | Q Value |
|---|---|---|---|---|
| B cells memory | Prx4 | −0.0357658 | 0.39488518 | 0.39488518 |
| Plasma cells | Prx4 | 0.40876086 | 2.75 × 10−24 | 1.93 × 10−23 |
| T cells CD4 memory activated | Prx4 | 0.18108563 | 1.41 × 10−5 | 1.97 × 10−5 |
| T cells gamma delta | Prx4 | 0.20982468 | 4.51 × 10−7 | 7.90 × 10−7 |
| Monocytes | Prx4 | −0.2933115 | 9.86 × 10−13 | 3.45 × 10−12 |
| Macrophages M2 | Prx4 | −0.1713956 | 4.13 × 10−5 | 4.81 × 10−5 |
| Mast cells resting | Prx4 | −0.2319601 | 2.24 × 10−8 | 5.22 × 10−8 |
| miRNA ID | miRNA Name | geneID | Gene Name | Gene Type | Chromosome | Narrow Start | Narrow End | Broad Start | Broad End | Strand | clipExp Num | DegraExp Num | PITA | RNA22 | miRmap | microT | miRanda | Pancancer Num |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIMAT0000449 | hsa-miR-146a-5p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704483 | 23704489 | 23704469 | 23704490 | + | 5 | 0 | 1 | 0 | 1 | 0 | 1 | 6 |
| MIMAT0001631 | hsa-miR-451a | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704500 | 23704505 | 23704485 | 23704506 | + | 3 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| MIMAT0002178 | hsa-miR-487a-3p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704470 | 23704475 | 23704470 | 23704475 | + | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 6 |
| MIMAT0002809 | hsa-miR-146b-5p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704483 | 23704489 | 23704466 | 23704490 | + | 5 | 0 | 1 | 0 | 1 | 0 | 1 | 3 |
| MIMAT0002824 | hsa-miR-498 | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704476 | 23704482 | 23704476 | 23704482 | + | 4 | 0 | 1 | 0 | 1 | 0 | 0 | 4 |
| MIMAT0003245 | hsa-miR-580-3p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704485 | 23704490 | 23704485 | 23704490 | + | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| MIMAT0004793 | hsa-miR-556-3p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23693402 | 23693407 | 23693402 | 23693407 | + | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| MIMAT0004799 | hsa-miR-589-5p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23704484 | 23704489 | 23704484 | 23704489 | + | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
| MIMAT0015037 | hsa-miR-3163 | ENSG00000123131 | Prx4 | protein_coding | chrX | 23693418 | 23693446 | 23693418 | 23693446 | + | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| MIMAT0018204 | hsa-miR-676-3p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23693424 | 23693452 | 23693424 | 23693452 | + | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| MIMAT0019062 | hsa-miR-4524a-5p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23693414 | 23693420 | 23693393 | 23693421 | + | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| MIMAT0022255 | hsa-miR-4524b-5p | ENSG00000123131 | Prx4 | protein_coding | chrX | 23693414 | 23693420 | 23693393 | 23693421 | + | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| HR | Lower.95 | Upper.95 |
|---|---|---|
| hsa-miR-146a-5p | 0.91033546 | 0.79799012 |
| hsa-miR-146b-5p | 0.94493836 | 0.81559882 |
| hsa-miR-3163 | 12.3304215 | 2.93772613 |
| hsa-miR-451a | 0.98124854 | 0.8963051 |
| hsa-miR-4524a-5p | 0.84779499 | 0.35083517 |
| hsa-miR-4738-3p | 0.85010695 | 0.53104859 |
| hsa-miR-487a-3p | 1.10794343 | 0.95751824 |
| hsa-miR-556-3p | 0.86824518 | 0.68867783 |
| hsa-miR-580-3p | 0.86960466 | 0.65553948 |
| hsa-miR-589-5p | 1.04741951 | 0.86650936 |
| hsa-miR-676-3p | 0.9633758 | 0.78058721 |
| hsa-miR-708-3p | 0.98595142 | 0.86659775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Jiang, H.; Thapa, P.; Ding, N.; Alshahrani, A.; Fujii, J.; Toledano, M.B.; Wei, Q. Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer. Antioxidants 2023, 12, 367. https://doi.org/10.3390/antiox12020367
Hao Y, Jiang H, Thapa P, Ding N, Alshahrani A, Fujii J, Toledano MB, Wei Q. Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer. Antioxidants. 2023; 12(2):367. https://doi.org/10.3390/antiox12020367
Chicago/Turabian StyleHao, Yanning, Hong Jiang, Pratik Thapa, Na Ding, Aziza Alshahrani, Junichi Fujii, Michel B. Toledano, and Qiou Wei. 2023. "Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer" Antioxidants 12, no. 2: 367. https://doi.org/10.3390/antiox12020367
APA StyleHao, Y., Jiang, H., Thapa, P., Ding, N., Alshahrani, A., Fujii, J., Toledano, M. B., & Wei, Q. (2023). Critical Role of the Sulfiredoxin-Peroxiredoxin IV Axis in Urethane-Induced Non-Small Cell Lung Cancer. Antioxidants, 12(2), 367. https://doi.org/10.3390/antiox12020367







