β Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Docking Analysis
2.2. Cell Culture
2.3. Cell Treatment
2.4. Metabolic and Biochemical Assays
2.5. Transcriptomic Silencing
2.6. Transcriptomic Profiling
2.7. Proteome Profiling
2.8. Statistical Analysis and Sample Size
3. Results
3.1. Determining the Proteome Profile of TLR4/IL1R-Stimulated Primary Human OA Chondrocytes
3.2. Predicting the Binding Affinity of β Boswellic Acid to Potential Proteome Profile Targets
3.3. BBA Effects on Cell Viability and ROS Secretion in Chondrocytes
3.4. BBA Effects on TLR4/IL1R-Mediated IIRs in Primary Human OA Chondrocytes
3.5. BBA Effects on TLR4/IL1R-Mediated IIRs in Primary Human OA Osteoblasts
3.6. BBA Effects on TLR4/IL1R-Mediated IIRs in Human OA Synoviocytes
3.7. BBA Effects in Joint Cells on the NLRP3 Inflammasome Pathway
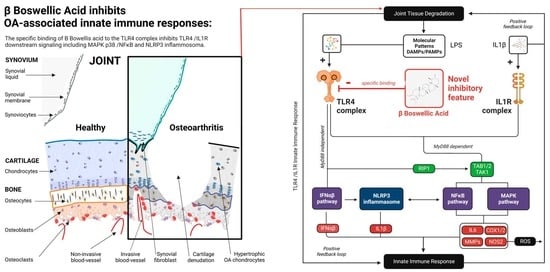
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Disease Incidence, Prevalence and Disability. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2004; Volume 3, pp. 28–37. [Google Scholar]
- United Nations. World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/ (accessed on 2 February 2023).
- Thomas, E.; Peat, G.; Croft, P. Defining and Mapping the Person with Osteoarthritis for Population Studies and Public Health. Rheumatology 2014, 53, 338–345. [Google Scholar] [CrossRef]
- Oo, W.M.; Yu, S.P.-C.; Daniel, M.S.; Hunter, D.J. Disease-Modifying Drugs in Osteoarthritis: Current Understanding and Future Therapeutics. Expert Opin. Emerg. Drugs 2018, 23, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Saarakkala, S.; Finnilä, M.; Karsdal, M.A.; Bay-Jensen, A.-C.; van Spil, W.E. Recent Advances in Understanding the Phenotypes of Osteoarthritis. F1000Research 2019, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-Modifying Treatments for Osteoarthritis (DMOADs) of the Knee and Hip: Lessons Learned from Failures and Opportunities for the Future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis: Literature Update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Schaible, H.-G. Osteoarthritis Pain. Recent Advances and Controversies. Curr. Opin. Support. Palliat. Care 2018, 12, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.; Zhang, Y.; Jiang, W.; Shen, J.; Xu, S.; Cai, D.; Shen, J.; Huang, B.; Li, M.; et al. Tyrosine Kinase Fyn Promotes Osteoarthritis by Activating the β-Catenin Pathway. Ann. Rheum. Dis. 2018, 77, 935–943. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/β-Catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Saito, T.; Tanaka, S. Molecular Mechanisms Underlying Osteoarthritis Development: Notch and NF-ΚB. Arthritis. Res. Ther. 2017, 19, 94. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis. Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Kania, K.; Rafipay, A.J.; Sambale, M.; Kuwahara, S.T.; Collins, F.L.; Smeeton, J.; Serowoky, M.A.; Rowley, L.; Wang, H.; et al. Identification of the Skeletal Progenitor Cells Forming Osteophytes in Osteoarthritis. Ann. Rheum. Dis. 2020, 79, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.; Vajda, E.; Nagy, E.E. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 Signalling in Osteoarthritis-Finding Targets for Candidate DMOADs. Nat. Rev. Rheumatol. 2014, 11, 159–170. [Google Scholar] [CrossRef]
- Sohn, D.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma Proteins Present in Osteoarthritic Synovial Fluid Can Stimulate Cytokine Production via Toll-like Receptor 4. Arthritis. Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef]
- Rousseau, J.C.; Garnero, P. Biological Markers in Osteoarthritis. Bone 2012, 51, 265–277. [Google Scholar] [CrossRef]
- Litwic, A.; Registrar, S.; Edwards, M.; Clinical, M. Europe PMC Funders Group Epidemiology and Burden of Osteoarthritis. Epidemiology 2013, 44, 185–199. [Google Scholar] [CrossRef]
- Sinyeue, C.; Matsui, M.; Oelgemöller, M.; Bregier, F.; Chaleix, V.; Sol, V.; Lebouvier, N. Synthesis and Investigation of Flavanone Derivatives as Potential New Anti-Inflammatory Agents. Molecules 2022, 27, 1781. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Alonso-Pérez, A.; Guillán-Fresco, M.; Jorge-Mora, A.; Crespo-Golmar, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Gualillo, O.; Belén Bravo, S.; Gómez Bahamonde, R. Amitriptyline Blocks Innate Immune Responses Mediated by Toll-like Receptor 4 and IL-1 Receptor: Preclinical and Clinical Evidence in Osteoarthritis and Gout. Br. J. Pharmacol. 2022, 179, 270–286. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Crespo-Golmar, A.; Gualillo, O.; Jorge-Mora, A.; Bravo, S.B.; Gómez, R. Repurposing Drugs to Inhibit Innate Immune Responses Associated with TLR4, IL1, and NLRP3 Signaling in Joint Cells. Biomed. Pharm. 2022, 155, 113671. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, L.; Wang, Q.; Chen, H.; Xu, G. Traditional Chinese Medicine for Knee Osteoarthritis: An Overview of Systematic Review. PLoS ONE 2017, 12, e0189884. [Google Scholar] [CrossRef]
- Kessler, C.S.; Pinders, L.; Michalsen, A.; Cramer, H. Ayurvedic Interventions for Osteoarthritis: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2015, 35, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Pathania, M.; Bhardwaj, P.; Pathania, N.; Rathaur, V. A Review on Exploring Evidence-Based Approach to Harnessing the Immune System in Times of Corona Virus Pandemic: Best of Modern and Traditional Indian System of Medicine. J. Fam. Med. Prim. Care 2020, 9, 3826. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Wong, H.H.; Wagner, C.A.; Lahey, L.J.; Robinson, W.H.; Sokolove, J. Oral and Topical Boswellic Acid Attenuates Mouse Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 128–132. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Anti-Inflammatory and Anti-Cancer Activities of Frankincense: Targets, Treatments and Toxicities. Semin. Cancer Biol. 2022, 80, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Rathod, I.S.; Suhagia, B.N.; Patel, D.A.; Parmar, V.K.; Shah, B.K.; Vaishnavi, V.M. Estimation of Boswellic Acids from Market Formulations of Boswellia Serrata Extract and 11-Keto Beta-Boswellic Acid in Human Plasma by High-Performance Thin-Layer Chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 848, 232–238. [Google Scholar] [CrossRef]
- Gerbeth, K.; Meins, J.; Kirste, S.; Momm, F.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Determination of Major Boswellic Acids in Plasma by High-Pressure Liquid Chromatography/Mass Spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 998–1005. [Google Scholar] [CrossRef]
- Riva, A.; Allegrini, P.; Franceschi, F.; Togni, S.; Giacomelli, L.; Eggenhoffner, R. A Novel Boswellic Acids Delivery Form (Casperome®) in the Management of Musculoskeletal Disorders: A Review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5258–5263. [Google Scholar] [CrossRef]
- Gupta, S.; Ahsan, A.U.; Wani, A.; Khajuria, V.; Nazir, L.A.; Sharma, S.; Bhagat, A.; Raj Sharma, P.; Bhardwaj, S.; Peerzada, K.J.; et al. The Amino Analogue of β-Boswellic Acid Efficiently Attenuates the Release of pro-Inflammatory Mediators than Its Parent Compound through the Suppression of NF-ΚB/IκBα Signalling Axis. Cytokine 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia Serrata: An Overall Assessment of in Vitro, Preclinical, Pharmacokinetic and Clinical Data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef]
- Thummuri, D.; Jeengar, M.K.; Shrivastava, S.; Areti, A.; Yerra, V.G.; Yamjala, S.; Komirishetty, P.; Naidu, V.G.M.; Kumar, A.; Sistla, R. Boswellia Ovalifoliolata Abrogates ROS Mediated NF-ΚB Activation, Causes Apoptosis and Chemosensitization in Triple Negative Breast Cancer Cells. Environ. Toxicol. Pharmacol. 2014, 38, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Umar, K.; Sarwar, A.H.M.G.; Khan, A.; Ahmad, N.; Ahmad, S.; Katiyar, C.K.; Husain, S.A.; Khan, H.A. Boswellia Serrata Extract Attenuates Inflammatory Mediators and Oxidative Stress in Collagen Induced Arthritis. Phytomedicine 2014, 21, 847–856. [Google Scholar] [CrossRef]
- Kimmatkar, N.; Thawani, V.; Hingorani, L.; Khiyani, R. Efficacy and Tolerability of Boswellia Serrata Extract in Treatment of Osteoarthritis of Knee--a Randomized Double Blind Placebo Controlled Trial. Phytomedicine 2003, 10, 3–7. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K.; Narayanan, |.; Narayanan, K.; Nagabhushanam, K. A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial to Assess the Safety and Efficacy of a Novel Boswellia Serrata Extract in the Management of Osteoarthritis of the Knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of Toll-like Receptor (TLR) 4 Inhibitor TAK-242 as a New Potential Anti-Rheumatoid Arthritis Drug. Thromb. Haemost. 2020, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Loiarro, M.; Ruggiero, V.; Sette, C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediat. Inflamm. 2010, 2010, 12. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB System. WIREs Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Frazão, J.B.; Errante, P.R.; Condino-Neto, A. Toll-Like Receptors’ Pathway Disturbances Are Associated with Increased Susceptibility to Infections in Humans. Arch. Immunol. Ther. Exp. 2013, 61, 427–443. [Google Scholar] [CrossRef]
- Abbaszade, I.; Liu, R.Q.; Yang, F.; Rosenfeld, S.A.; Ross, O.H.; Link, J.R.; Ellis, D.M.; Tortorella, M.D.; Pratta, M.A.; Hollist, J.M.; et al. Cloning and Characterization of ADAMTS11, an Aggrecanase from the ADAMTS Family. J. Biol. Chem. 1999, 274, 23443–23450. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, W.B.; Van De Loo, F.A.J.; Zwarts, W.A.; Otterness, I.G. Effects of Murine Recombinant Interleukin 1 on Intact Homologous Articular Cartilage: A Quantitative and Autoradiographic Study. Ann. Rheum. Dis. 1988, 47, 855–863. [Google Scholar] [CrossRef]
- Van de Loo, A.A.J.; Van den Berg, W.B. Effects of Murine Recombinant Interleukin 1 on Synovial Joints in Mice: Measurement of Patellar Cartilage Metabolism and Joint Inflammation. Ann. Rheum. Dis. 1990, 49, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Modulation by Interleukin 1 and Tumor Necrosis Factor α of Production of Collagenase, Tissue Inhibitor of Metalloproteinases and Collagen Types in Differentiated and Dedifferentiated Articular Chondrocytes. BBA Mol. Cell Res. 1990, 1052, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Balavoine, J.F.; de Rochemonteix, B.; Williamson, K. Prostaglandin E2 and Collagenase Production by Fibroblasts and Synovial Cells Is Regulated by Urine-Derived Human Interleukin 1 and Inhibitor(s). J. Clin. Investig. 1986, 78, 1120–1124. [Google Scholar] [CrossRef]
- Gadher, S.J.; Eyre, D.R.; Duance, V.C.; Wotton, S.F.; Heck, L.W.; Schmid, T.M.; Woolley, D.E. Susceptibility of Cartilage Collagens Type II, IX, X, and XI to Human Synovial Collagenase and Neutrophil Elastase. Eur. J. Biochem. 1988, 175, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.A. Articular Cartilage Cultured with Catabolin (Pig Interleukin 1) Synthesizes a Decreased Number of Normal Proteoglycan Molecules. Biochem. J. 1985, 227, 869–878. [Google Scholar] [CrossRef]
- Tortorella, M.D.; Burn, T.C.; Pratta, M.A.; Abbaszade, I.; Hollis, J.M.; Liu, R.; Rosenfeld, S.A.; Copeland, R.A.; Decicco, C.P.; Wynn, R.; et al. Purification and Cloning of Aggrecanase-1: A Member of the ADAMTS Family of Proteins. Science 1999, 284, 1664–1666. [Google Scholar] [CrossRef]
- Mertens, M.; Singh, J.A. Anakinra for Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2009, 1, CD005121. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in Osteoarthritis: Time for a Critical Review of the Literature. F1000Research 2019, 8, 934. [Google Scholar] [CrossRef]
- Alonso-Pérez, A.; Franco-Trepat, E.; Guillán-Fresco, M.; Jorge-Mora, A.; López, V.; Pino, J.; Gualillo, O.; Gómez, R. Role of Toll-Like Receptor 4 on Osteoblast Metabolism and Function. Front. Physiol. 2018, 9, 504. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-ΚB Signalling Pathway in Osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Gomez, R.; Bianco, G.; Scotece, M.; Lear, P.; Dieguez, C.; Gomez-Reino, J.; Lago, F.; Gualillo, O. Expanding the Adipokine Network in Cartilage: Identification and Regulation of Novel Factors in Human and Murine Chondrocytes. Ann. Rheum. Dis. 2011, 70, 551–559. [Google Scholar] [CrossRef]
- Gómez, R.; Conde, J.; Scotece, M.; Gómez-Reino, J.J.; Lago, F.; Gualillo, O. What’s New in Our Understanding of the Role of Adipokines in Rheumatic Diseases? Nat. Rev. Rheumatol. 2011, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-B Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Bai, F.; Chen, X.; Yang, H.; Xu, H.G. Acetyl-11-Keto-β-Boswellic Acid Promotes Osteoblast Differentiation by Inhibiting Tumor Necrosis Factor-α and Nuclear Factor-ΚB Activity. J. Craniofacial Surg. 2018, 29, 1996–2002. [Google Scholar] [CrossRef]
- Al-Bahlani, S.; Burney, I.A.; Al-Dhahli, B.; Al-Kharusi, S.; Al-Kharousi, F.; Al-Kalbani, A.; Ahmed, I. Boswellic Acid Sensitizes Gastric Cancer Cells to Cisplatin-Induced Apoptosis via P53-Mediated Pathway. BMC Pharmacol. Toxicol. 2020, 21, 64. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Volante, A.; Tava, A.; Masini, M.A.; Burlando, B. The Major Boswellia Serrata Active 3-Acetyl-11-Keto-β-Boswellic Acid Strengthens Interleukin-1α Upregulation of Matrix Metalloproteinase-9 via JNK MAP Kinase Activation. Phytomedicine 2017, 36, 176–182. [Google Scholar] [CrossRef]
- Goswami, D.; Das Mahapatra, A.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia Serrata Oleo-Gum-Resin and β-Boswellic Acid Inhibits HSV-1 Infection in Vitro through Modulation of NF-KB and P38 MAP Kinase Signaling. Phytomedicine 2018, 51, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yu, X.; Zhang, C.; Cai, Y.; Cao, Y.; Wang, S.; Shen, J. Drug Repurposing Screen Identifies Novel Classes of Drugs with Anticancer Activity in Mantle Cell Lymphoma. Comb. Chem. High Throughput Screen. 2019, 22, 483–495. [Google Scholar] [CrossRef]
- Park, K. A Review of Computational Drug Repurposing. Transl. Clin. Pharmacol. 2019, 27, 59. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Lee, H.S.E.; Moon, S.; Ko, K.M.; Koh, J.H.; Seok, J.K.; Min, J.; Heo, T.; Kang, H.C.; Cho, Y.; et al. Direct Binding to NLRP3 Pyrin Domain as a Novel Strategy to Prevent NLRP3-Driven Inflammation and Gouty Arthritis. Arthritis Rheumatol. 2020, 72, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 23 May 2022).
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological Macromolecular Structures Enabling Research and Education in Fundamental Biology, Biomedicine, Biotechnology and Energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef]
- Xu, G.; Lo, Y.C.; Li, Q.; Napolitano, G.; Wu, X.; Jiang, X.; Dreano, M.; Karin, M.; Wu, H. Crystal Structure of Inhibitor of Κb Kinase β. Nature 2011, 472, 325–330. [Google Scholar] [CrossRef]
- Sharif, H.; Wang, L.; Wang, W.L.; Magupalli, V.G.; Andreeva, L.; Qiao, Q.; Hauenstein, A.V.; Wu, Z.; Núñez, G.; Mao, Y.; et al. Structural Mechanism for NEK7-Licensed Activation of NLRP3 Inflammasome. Nature 2019, 570, 338–343. [Google Scholar] [CrossRef]
- Sandall, C.F.; Ziehr, B.K.; MacDonald, J.A. ATP-Binding and Hydrolysis in Inflammasome Activation. Molecules 2020, 25, 4572. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, L.Y.; Brough, D.; Freeman, S. Inhibiting the Nlrp3 Inflammasome. Molecules 2020, 25, 5533. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-Chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A Hierarchical Approach to All-Atom Protein Loop Prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Carrasco, J.P. When Virtual Screening Yields Inactive Drugs: Dealing with False Theoretical Friends. Chem. Med. Chem. 2022, 17, e202200278. [Google Scholar] [CrossRef]
- Cerón-Carrasco, J.P.; Jacquemin, D. Using Theory to Extend the Scope of Azobenzene Drugs in Chemotherapy: Novel Combinations for a Specific Delivery. ChemMedChem 2021, 16, 1764–1774. [Google Scholar] [CrossRef]
- Villalvilla, A.; Silva, A.; Largo, R.; Gualillo, O. 6-Shogaol Inhibits Chondrocytes ’ Innate Immune Responses and Cathepsin-K Activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef]
- Alonso-Pérez, A.; Guillán-Fresco, M.; Franco-Trepat, E.; Jorge-Mora, A.; López-Fagúndez, M.; Pazos-Pérez, A.; Crespo-Golmar, A.; Caeiro-Rey, J.R.; Gómez, R. Improved Protocol to Study Osteoblast and Adipocyte Differentiation Balance. Biomedicines. 2022, 11, 31. [Google Scholar] [CrossRef]
- Hussey, S.E.; Liang, H.; Costford, S.R.; Klip, A.; DeFronzo, R.A.; Sanchez-Avila, A.; Ely, B.; Musi, N. TAK-242, a Small-Molecule Inhibitor of Toll-like Receptor 4 Signalling, Unveils Similarities and Differences in Lipopolysaccharide- and Lipid-Induced Inflammation and Insulin Resistance in Muscle Cells. Biosci. Rep. 2012, 33, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hannon, G.J.; Rossi, J.J. Unlocking the Potential of the Human Genome with RNA Interference. Nature 2004, 431, 371–378. [Google Scholar] [CrossRef]
- Omega Biotek, E.Z.N. A Total RNA Kit I Product Manual. 2012. Available online: https://www.omegabiotek.com/wp-content/uploads/2017/08/R6834-Quick-Guide.pdf (accessed on 2 February 2023).
- AppliedBiosystems High-Capacity RNA-to-CDNA Kit Product Information Sheet. 2018. Available online: https://tools.thermofisher.com/content/sfs/manuals/4387949_RNAtocDNA_PI.pdf (accessed on 2 February 2023).
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- QuantStudio 3 and 5 Real-Time PCR System Software|Thermo Fisher Scientific—ES. Available online: https://www.thermofisher.com/es/es/home/global/forms/life-science/quantstudio-3-5-software.html (accessed on 2 February 2023).
- QuantStudio Design & Analysis. Available online: https://apps.thermofisher.com/apps/da2/#/home/welcome (accessed on 5 May 2021).
- Couselo-Seijas, M.; López-Canoa, J.N.; Agra-Bermejo, R.M.; Díaz-Rodriguez, E.; Fernandez, A.L.; Martinez-Cereijo, J.M.; Durán-Muñoz, D.; Bravo, S.B.; Velo, A.; González-Melchor, L.; et al. Cholinergic Activity Regulates the Secretome of Epicardial Adipose Tissue: Association with Atrial Fibrillation. J. Cell. Physiol. 2019, 234, 10512–10522. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Franco, D.; Serrano, M.P.; Maggiolino, A.; Landete-Castillejos, T.; De Palo, P.; Lorenzo, J.M. A Proteomic-Based Approach for the Search of Biomarkers in Iberian Wild Deer (Cervus Elaphus) as Indicators of Meat Quality. J. Proteom. 2019, 205, 103422. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a Next Generation Search Engine That Uses Sequence Temperature Values and Feature Probabilities to Identify Peptides from Tandem Mass Spectra. Mol. Cell. Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Chisanga, D.; Alessandro, R.; Ang, C.S.; Askenase, P.; Batagov, A.O.; Benito-Martin, A.; Camussi, G.; Clayton, A.; et al. A Novel Community Driven Software for Functional Enrichment Analysis of Extracellular Vesicles Data. J. Extracell. Vesicles 2017, 6, 1321455. [Google Scholar] [CrossRef]
- Komander, D. The Emerging Complexity of Protein Ubiquitination. Biochem. Soc. Trans. 2009, 37, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.W.; Harrison, S.C. The Structure of the NF-Kappa B P50:DNA-Complex: A Starting Point for Analyzing the Rel Family. FEBS Lett. 1995, 369, 113–117. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Ohto, U.; Fukase, K.; Miyake, K.; Shimizu, T. Structural Basis of Species-Specific Endotoxin Sensing by Innate Immune Receptor TLR4/MD-2. Proc. Natl. Acad. Sci. USA 2012, 109, 7421–7426. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Martínez-Banaclocha, H.; de Torre-Minguela, C.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 Closes the Active Conformation of NLRP3 to an Inactive. Nat. Chem. Biol. 2019, 15, 560. [Google Scholar] [CrossRef]
- Chi, C.T.; Lee, M.H.; Weng, C.F.; Leong, M.K. In Silico Prediction of PAMPA Effective Permeability Using a Two-QSAR Approach. Int. J. Mol. Sci. 2019, 20, 3170. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.L.; Duan, J.; Smith, E.; von Bargen, C.D.; Sherman, W.; Repasky, M.P. AutoQSAR: An Automated Machine Learning Tool for Best-Practice Quantitative Structure-Activity Relationship Modeling. Futur. Med. Chem. 2016, 8, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Cernanec, J.M.; Weinberg, J.B.; Batinic-Haberle, I.; Guilak, F.; Fermor, B. Influence of Oxygen Tension on Interleukin 1-Induced Peroxynitrite Formation and Matrix Turnover in Articular Cartilage. J. Rheumatol. 2007, 34, 401–407. [Google Scholar] [PubMed]
- Davies, C.M.; Guilak, F.; Weinberg, J.B.; Fermor, B. Reactive Nitrogen and Oxygen Species in Interleukin-1-Mediated DNA Damage Associated with Osteoarthritis. Osteoarthr. Cartil. 2008, 16, 624. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Spencer, J.P.E.; Zhu, Y.Z.; Armstrong, J.S.; Schantz, J.T. Peroxynitrite-Modified Collagen-II Induces P38/ERK and NF-ΚB-Dependent Synthesis of Prostaglandin E2 and Nitric Oxide in Chondrogenically Differentiated Mesenchymal Progenitor Cells. Osteoarthr. Cartil. 2006, 14, 460–470. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, G.; Wu, Y.; Xiong, Y. Inflammasome Complexes: Crucial Mediators in Osteoimmunology and Bone Diseases. Int. Immunopharmacol. 2022, 110, 109072. [Google Scholar] [CrossRef]
- Li, X.; Qin, J. Modulation of Toll?Interleukin 1 Receptor Mediated Signaling. J. Mol. Med. 2005, 83, 258–266. [Google Scholar] [CrossRef]
- Martin, M.U.; Wesche, H. Summary and Comparison of the Signaling Mechanisms of the Toll/Interleukin-1 Receptor Family. Biochim. Biophys. Acta BBA Mol. Cell Res. 2002, 1592, 265–280. [Google Scholar] [CrossRef]
- Pascart, T.; Richette, P. Current and Future Therapies for Gout. Expert Opin. Pharmacother. 2017, 18, 1201–1211. [Google Scholar] [CrossRef]
- Park, H.; Hong, J.; Yin, Y.; Joo, Y.; Kim, Y.; Shin, J.; Kwon, H.H.; Shin, N.; Shin, H.J.; Beom, J.; et al. TAP2, a Peptide Antagonist of Toll-like Receptor 4, Attenuates Pain and Cartilage Degradation in a Monoiodoacetate-Induced Arthritis Rat Model. Sci. Rep. 2020, 10, 17451. [Google Scholar] [CrossRef]
- Ha, S.H.; Kim, H.K.; Anh, N.T.T.; Kim, N.; Ko, K.S.; Rhee, B.D.; Han, J. Time-Dependent Proteomic and Genomic Alterations in Toll-like Receptor-4-Activated Human Chondrocytes: Increased Expression of Lamin A/C and Annexins. Korean J. Physiol. Pharmacol. 2017, 21, 531–546. [Google Scholar] [CrossRef] [Green Version]
- Haglund, L.; Bernier, S.M.; Önnerfjord, P.; Recklies, A.D. Proteomic Analysis of the LPS-Induced Stress Response in Rat Chondrocytes Reveals Induction of Innate Immune Response Components in Articular Cartilage. Matrix Biol. 2008, 27, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tardif, G.; Paré, F.; Gotti, C.; Roux-Dalvai, F.; Droit, A.; Zhai, G.; Sun, G.; Fahmi, H.; Pelletier, J.-P.; Martel-Pelletier, J. Mass Spectrometry-Based Proteomics Identify Novel Serum Osteoarthritis Biomarkers. Arthritis. Res. Ther. 2022, 24, 120. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/Oxidative Stress Signaling in Osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and Therapeutic Implications of Cellular Senescence in Osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-KappaB: Players, Pathways, Perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C. Celecoxib for the Treatment of Musculoskeletal Arthritis. Expert Opin. Pharmacother. 2019, 20, 1689–1702. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.B.; Roelofs, M.F.; Radstake, T.R.D.J.; Matera, G.; Popa, C.; van der Meer, J.W.M.; Netea, M.G.; van den Berg, W.B. Inhibition of Toll-like Receptor 4 Breaks the Inflammatory Loop in Autoimmune Destructive Arthritis. Arthritis Rheum. 2007, 56, 2957–2967. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 Inflammasome in Inflammatory Diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Szamosi, S.; Kovács, G.E.; Kocsis, E.; Benkő, S. The NLRP3 Inflammasome—Interleukin 1 Pathway as a Therapeutic Target in Gout. Arch. Biochem. Biophys. 2019, 670, 82–93. [Google Scholar] [CrossRef]
- Qing, Y.-F.; Zhang, Q.-B.; Zhou, J.-G. Innate Immunity Functional Gene Polymorphisms and Gout Susceptibility. Gene 2013, 524, 412–414. [Google Scholar] [CrossRef]
- Demidowich, A.P.; Davis, A.I.; Dedhia, N.; Yanovski, J.A. Colchicine to Decrease NLRP3-Activated Inflammation and Improve Obesity-Related Metabolic Dysregulation. Med. Hypotheses 2016, 92, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Trepat, E.; Alonso-Pérez, A.; Guillán-Fresco, M.; López-Fagúndez, M.; Pazos-Pérez, A.; Crespo-Golmar, A.; Belén Bravo, S.; López-López, V.; Jorge-Mora, A.; Cerón-Carrasco, J.P.; et al. β Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine. Antioxidants 2023, 12, 371. https://doi.org/10.3390/antiox12020371
Franco-Trepat E, Alonso-Pérez A, Guillán-Fresco M, López-Fagúndez M, Pazos-Pérez A, Crespo-Golmar A, Belén Bravo S, López-López V, Jorge-Mora A, Cerón-Carrasco JP, et al. β Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine. Antioxidants. 2023; 12(2):371. https://doi.org/10.3390/antiox12020371
Chicago/Turabian StyleFranco-Trepat, Eloi, Ana Alonso-Pérez, María Guillán-Fresco, Miriam López-Fagúndez, Andrés Pazos-Pérez, Antía Crespo-Golmar, Susana Belén Bravo, Verónica López-López, Alberto Jorge-Mora, José P. Cerón-Carrasco, and et al. 2023. "β Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine" Antioxidants 12, no. 2: 371. https://doi.org/10.3390/antiox12020371
APA StyleFranco-Trepat, E., Alonso-Pérez, A., Guillán-Fresco, M., López-Fagúndez, M., Pazos-Pérez, A., Crespo-Golmar, A., Belén Bravo, S., López-López, V., Jorge-Mora, A., Cerón-Carrasco, J. P., Lois Iglesias, A., & Gómez, R. (2023). β Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine. Antioxidants, 12(2), 371. https://doi.org/10.3390/antiox12020371