Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies
Abstract
:1. NOX2 Isoform of NADPH Oxidase: Activation and Regulation
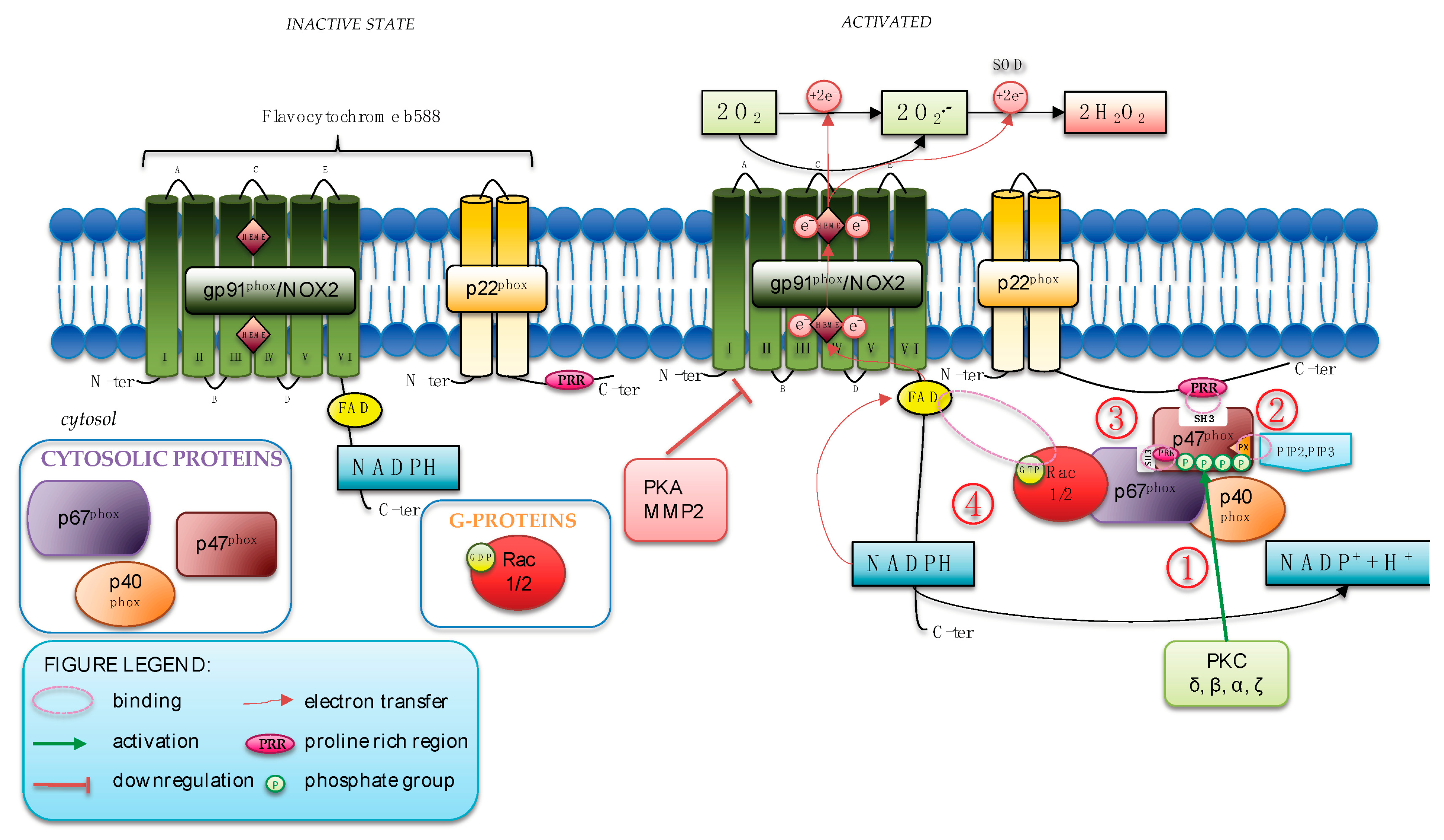
1.1. NOX2 Structure
1.2. NOX2 Activation and Regulation
2. The Immune Function of NOX2: Reactive Oxygen Species and Antimicrobial Activity
3. Deficit of NOX2: Human, Murine, and Cellular Models
3.1. NOX2 Deficiency: Human Model
3.2. NOX2 Deficiency: Mouse Models
3.3. NOX2 Deficiency: Cellular Models
4. NOX2-Derived Reactive Oxygen Species-Mediated Diseases
4.1. NOX2 and Carcinogenesis
4.2. NOX2 and Neurodegenerative Diseases
4.3. NOX2 and Cardiovascular Diseases
5. NOX2 as a Therapeutic Target: Pharmacological Approaches from Natural to Synthetic Small Molecules
5.1. Peptide-Based Inhibitors
5.1.1. Direct Inhibitor
5.1.2. Indirect Inhibitor
5.2. Drug-Like Small Molecules and Drugs
5.2.1. Direct Inhibitor
5.2.2. Indirect Inhibitors
5.3. Small Molecules of Natural Origin
5.3.1. Direct Inhibitors
5.3.2. Indirect Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. Nadph oxidases (Nox): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, C.W.; Gerard, R.W. The extra respiration of phagocytosis. Am. J. Physiol. Content 1932, 103, 235–236. [Google Scholar] [CrossRef]
- Sbarra, A.J.; Karnovsky, M.L. The Biochemical Basis of Phagocytosis. J. Biol. Chem. 1959, 234, 1355–1362. [Google Scholar] [CrossRef]
- Bridges, R.A.; Berendes, H.; Good, R.A. A fatal granulomatous disease of childhood: The clinical, pathological, and laboratory features of a new syndrome. AMA J. Dis. Child. 1959, 97, 387–408. Available online: https://pubmed.ncbi.nlm.nih.gov/13636694 (accessed on 8 December 2022). [CrossRef] [PubMed]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002, 59, 1428–1459. [Google Scholar] [CrossRef]
- Breitenbach, M.; Rinnerthaler, M.; Weber, M.; Breitenbach-Koller, H.; Karl, T.; Cullen, P.; Basu, S.; Haskova, D.; Hasek, J. The defense and signaling role of NADPH oxidases in eukaryotic cells: Review. Wien. Med. Wochenschr. 2018, 168, 286–299. [Google Scholar] [CrossRef]
- Noreng, S.; Ota, N.; Sun, Y.; Ho, H.; Johnson, M.; Arthur, C.P.; Schneider, K.; Lehoux, I.; Davies, C.W.; Mortara, K.; et al. Structure of the core human NADPH oxidase NOX2. Nat. Commun. 2022, 13, 6079. [Google Scholar] [CrossRef]
- Liu, R.; Song, K.; Wu, J.X.; Geng, X.P.; Zheng, L.; Gao, X.; Peng, H.; Chen, L. Structure of human phagocyte NADPH oxidase in the resting state. Elife 2022, 11, e83743. [Google Scholar] [CrossRef]
- Segal, A.W.; Heyworth, P.G.; Cockcroft, S.; Barrowman, M.M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature 1985, 316, 547–549. [Google Scholar] [CrossRef]
- Belambri, S.A.; Marzaioli, V.; Hurtado-Nedelec, M.; Pintard, C.; Liang, S.; Liu, Y.; Boussetta, T.; Gougerot-Pocidalo, M.A.; Ye, R.D.; Dang, P.M.C.; et al. Impaired p47phox phosphorylation in neutrophils from patients with p67phox-deficient chronic granulomatous disease. Blood 2022, 139, 2512–2522. [Google Scholar] [CrossRef]
- Paclet, M.H.; Laurans, S.; Dupré-Crochet, S. Regulation of Neutrophil NADPH Oxidase, NOX2: A Crucial Effector in Neutrophil Phenotype and Function. Front. Cell Dev. Biol. 2022, 10, 945749. [Google Scholar] [CrossRef]
- Noubade, R.; Wong, K.; Ota, N.; Rutz, S.; Eidenschenk, C.; Valdez, P.A.; Ding, J.; Peng, I.; Sebrell, A.; Caplazi, P.; et al. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 2014, 509, 235–239. [Google Scholar] [CrossRef]
- Graham, D.B.; Becker, C.E.; Doan, A.; Goel, G.; Villablanca, E.J.; Knights, D.; Mok, A.; Ng, A.C.Y.; Doench, J.G.; Root, D.E.; et al. Functional genomics identifies negative regulatory nodes controlling phagocyte oxidative burst. Nat. Commun. 2015, 6, 7838. [Google Scholar] [CrossRef]
- Raad, H.; Mouawia, H.; Hassan, H.; El-Seblani, M.; Arabi-Derkawi, R.; Boussetta, T.; Gougerot-Pocidalo, M.A.; Dang, P.M.C.; El-Benna, J. The protein kinase A negatively regulates reactive oxygen species production by phosphorylating gp91phox/NOX2 in human neutrophils. Free Radic. Biol. Med. 2020, 160, 19–27. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Sanguigni, V.; Violi, F.; Carnevale, R. A novel role of MMP2 in regulating platelet NOX2 activation. Free Radic. Biol. Med. 2020, 152, 355–362. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Cangemi, R.; Loffredo, L.; Sanguigni, V.; Stefanutti, C.; Basili, S.; Violi, F. Atorvastatin inhibits gp91phox circulating levels in patients with hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 360–367. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 2789. [Google Scholar] [CrossRef]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Direct evidence for catalase as the predominant H2O2-removing enzyme in human erythrocytes. Blood 1997, 90, 4973–4978. [Google Scholar] [CrossRef]
- Mitozo, P.A.; De Souza, L.F.; Loch-Neckel, G.; Flesch, S.; Maris, A.F.; Figueiredo, C.P.; Dos Santos, A.R.S.; Farina, M.; Dafre, A.L. A study of the relative importance of the peroxiredoxin-, catalase-, and glutathione-dependent systems in neural peroxide metabolism. Free Radic. Biol. Med. 2011, 51, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Nauseef, W.M. Myeloperoxidase in human neutrophil host defence. Cell. Microbiol. 2014, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.L.; Summers, C.; Chilvers, E.R.; Condliffe, A.M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Investig. 2018, 48, e12967. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Chiriaco, M.; Salfa, I.; Di Matteo, G.; Rossi, P.; Finocchi, A. Chronic granulomatous disease: Clinical, molecular, and therapeutic aspects. Pediatr. Allergy Immunol. 2016, 27, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Rae, J.; Newburger, P.E.; Dinauer, M.C.; Noack, D.; Hopkins, P.J.; Kuruto, R.; Curnutte, J.T. X-linked chronic granulomatous disease: Mutations in the CYBB gene encoding the gp91-phox component of respiratory-burst oxidase. Am. J. Hum. Genet. 1998, 62, 1320–1331. [Google Scholar] [CrossRef]
- Mollin, M.; Beaumel, S.; Vigne, B.; Brault, J.; Roux-Buisson, N.; Rendu, J.; Barlogis, V.; Catho, G.; Dumeril, C.; Fouyssac, F.; et al. Clinical, functional and genetic characterization of 16 patients suffering from chronic granulomatous disease variants—Identification of 11 novel mutations in CYBB. Clin. Exp. Immunol. 2020, 203, 247. [Google Scholar] [CrossRef]
- Arnadottir, G.A.; Norddahl, G.L.; Gudmundsdottir, S.; Agustsdottir, A.B.; Sigurdsson, S.; Jensson, B.O.; Bjarnadottir, K.; Theodors, F.; Benonisdottir, S.; Ivarsdottir, E.V.; et al. A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease. Nat. Commun. 2018, 9, 4447. [Google Scholar] [CrossRef]
- Thomas, D.C.; Charbonnier, L.M.; Schejtman, A.; Aldhekri, H.; Coomber, E.L.; Dufficy, E.R.; Beenken, A.E.; Lee, J.C.; Clare, S.; Speak, A.O.; et al. EROS/CYBC1 mutations: Decreased NADPH oxidase function and chronic granulomatous disease. J. Allergy Clin. Immunol. 2019, 143, 782–785.e1. [Google Scholar] [CrossRef]
- Thomas, D.C.; Clare, S.; Sowerby, J.M.; Pardo, M.; Juss, J.K.; Goulding, D.A.; van der Weyden, L.; Storisteanu, D.; Prakash, A.; Espéli, M.; et al. Eros is a novel transmembrane protein that controls the phagocyte respiratory burst and is essential for innate immunity. J. Exp. Med. 2017, 214, 1111–1128. [Google Scholar] [CrossRef]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Leiding, J.W.; Holland, S.M. Chronic Granulomatous Disease; University of Washington: Seattle, DC, USA, 1993. [Google Scholar]
- Deffert, C.; Cachat, J.; Krause, K.H. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell. Microbiol. 2014, 16, 1168–1178. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef]
- Hartl, D.; Rieber, N.; Hector, A.; Kuijpers, T.; Roos, D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin. Dev. Immunol. 2012, 2012, 252460. [Google Scholar] [CrossRef]
- Violi, F.; Carnevale, R.; Loffredo, L.; Pignatelli, P.; Gallin, J.I. NADPH Oxidase-2 and Atherothrombosis: Insight From Chronic Granulomatous Disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 218–225. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Sanguigni, V.; Plebani, A.; Rossi, P.; Pignata, C.; Martire, B.; Finocchi, A.; Pietrogrande, M.C.; Azzari, C.; et al. Different degrees of NADPH oxidase 2 regulation and in vivo platelet Activation: Lesson from chronic granulomatous disease. J. Am. Heart Assoc. 2014, 3, e000920. [Google Scholar] [CrossRef]
- Loffredo, L.; Carnevale, R.; Sanguigni, V.; Plebani, A.; Rossi, P.; Pignata, C.; De Mattia, D.; Finocchi, A.; Martire, B.; Pietrogrande, M.C.; et al. Does NADPH Oxidase Deficiency Cause Artery Dilatation in Humans? Antioxid. Redox Signal. 2012, 18, 1491–1496. [Google Scholar] [CrossRef]
- Pollock, J.D.; Williams, D.A.; Gifford, M.A.C.; Li, L.L.; Du, X.; Fisherman, J.; Orkin, S.H.; Doerschuk, C.M.; Dinauer, M.C. Mouse model of X–linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995, 9, 202–209. [Google Scholar] [CrossRef]
- Tosetti, B.; Ward, B.; Grumme, D.; Herb, M.; Schramm, M.; Utermöhlen, O.; Heukamp, L.C.; Krönke, M.; Krut, O. NOX2 Deficiency Permits Sustained Survival of S. aureus in Macrophages and Contributes to Severity of Infection. Front. Immunol. 2021, 12, 633629. [Google Scholar] [CrossRef]
- Whitmore, L.C.; Hilkin, B.M.; Goss, K.L.; Wahle, E.M.; Colaizy, T.T.; Boggiatto, P.M.; Varga, S.M.; Miller, F.J.; Moreland, J.G. NOX2 protects against prolonged inflammation, lung injury, and mortality following systemic insults. J. Innate Immun. 2013, 5, 565–580. [Google Scholar] [CrossRef]
- Olofsson, P.; Holmberg, J.; Tordsson, J.; Lu, S.; Åkerström, B.; Holmdahl, R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat. Genet. 2003, 33, 25–32. [Google Scholar] [CrossRef]
- Gelderman, K.A.; Hultqvist, M.; Olsson, L.M.; Bauer, K.; Pizzolla, A.; Olofsson, P.; Holmdahl, R. Rheumatoid arthritis: The role of reactive oxygen species in disease development and therapeutic strategies. Antioxid. Redox Signal. 2007, 9, 1541–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Olsson, L.M.; Urbonaviciute, V.; Yang, M.; Bäckdahl, L.; Holmdahl, R. Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic. Biol. Med. 2018, 125, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Idol, R.A.; Bhattacharya, S.; Huang, G.; Song, Z.; Huttenlocher, A.; Keller, N.P.; Dinauer, M.C. Neutrophil and Macrophage NADPH Oxidase 2 Differentially Control Responses to Inflammation and to Aspergillus fumigatus in Mice. J. Immunol. 2022, 209, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Huang, G.; Paracatu, L.C.; Grimes, D.; Gu, J.; Luke, C.J.; Clemens, R.A.; Dinauer, M.C. NADPH oxidase controls pulmonary neutrophil infiltration in the response to fungal cell walls by limiting LTB4. Blood 2020, 135, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Bhattacharya, S.; Huang, G.; Greenberg, Z.J.; Yang, W.; Bagaitkar, J.; Schuettpelz, L.; Dinauer, M.C. NADPH oxidase 2 limits amplification of IL-1β-G-CSF axis and an immature neutrophil subset in murine lung inflammation. Blood Adv. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Lee, K.; Won, H.Y.; Bae, M.A.; Hong, J.H.; Hwang, E.S. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc. Natl. Acad. Sci. USA 2011, 108, 9548–9553. [Google Scholar] [CrossRef]
- Kienhöfer, D.; Hahn, J.; Stoof, J.; Csepregi, J.Z.; Reinwald, C.; Urbonaviciute, V.; Johnsson, C.; Maueröder, C.; Podolska, M.J.; Biermann, M.H.; et al. Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight 2017, 2, e92920. [Google Scholar] [CrossRef]
- Khmaladze, I.; Kelkka, T.; Guerard, S.; Wing, K.; Pizzolla, A.; Saxena, A.; Lundqvist, K.; Holmdahl, M.; Nandakumar, K.S.; Holmdahl, R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3669–E3678. [Google Scholar] [CrossRef]
- Zhen, L.; King, A.A.J.; Xiao, Y.; Chanock, S.J.; Orkin, S.H.; Dinauer, M.C. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91(phox). Proc. Natl. Acad. Sci. USA 1993, 90, 9832–9836. [Google Scholar] [CrossRef]
- Beaumel, S.; Stasia, M.J. The X-CGD PLB-985 cell model for NOX2 structure-function analysis. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1982, pp. 153–171. [Google Scholar]
- Benyoucef, A.; Marchitto, L.; Touzot, F. CRISPR gene-engineered CYBBko THP-1 cell lines highlight the crucial role of NADPH-induced reactive oxygen species for regulating inflammasome activation. J. Allergy Clin. Immunol. 2020, 145, 1690–1693.e5. [Google Scholar] [CrossRef] [Green Version]
- Brault, J.; Vigne, B.; Stasia, M.J. Ex vivo models of chronic granulomatous disease. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1982, pp. 587–622. [Google Scholar]
- Brault, J.; Vaganay, G.; Le Roy, A.; Lenormand, J.L.; Cortes, S.; Stasia, M.J. Therapeutic effects of proteoliposomes on X-linked chronic granulomatous disease: Proof of concept using macrophages differentiated from patient-specific induced pluripotent stem cells. Int. J. Nanomed. 2017, 12, 2161–2177. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; Huang, L.; Su, Y.; Kong, L.; Ji, P.; Sun, R.; Wang, C.; Li, W.; Li, W. Stress level of glucocorticoid exacerbates neuronal damage and Aβ production through activating NLRP1 inflammasome in primary cultured hippocampal neurons of APP-PS1 mice. Int. Immunopharmacol. 2022, 110, 108972. [Google Scholar] [CrossRef]
- Yang, H.; Bai, J.; zhan, C.; Liu, S.; Gao, Y.; Zhong, L.; Lv, Y.; Chi, J.; Liu, J.; Yang, X.; et al. Hydrogen Attenuates Thyroid Hormone-Induced Cardiac Hypertrophy in Rats by regulating angiotensin II type 1 receptor and NADPH oxidase 2 mediated oxidative stress. Eur. J. Pharmacol. 2022, 922, 174917. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Geraily, G.; Amini, P.; Tooli, L.F.; Shabeeb, D. Melatonin Modulates Regulation of NOX2 and NOX4 Following Irradiation in the Lung. Curr. Clin. Pharmacol. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Nilsen, J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008, 29, 463–475. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 8127362. [Google Scholar] [CrossRef]
- Shimizu, H.; Katsurahara, K.; Inoue, H.; Shiozaki, A.; Kosuga, T.; Kudou, M.; Arita, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; et al. NADPH Oxidase 2 Has a Crucial Role in Cell Cycle Progression of Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2022, 29, 8677–8687. [Google Scholar] [CrossRef]
- Kitamoto, K.; Miura, Y.; Karnan, S.; Ota, A.; Konishi, H.; Hosokawa, Y.; Sato, K. Inhibition of NADPH oxidase 2 induces apoptosis in osteosarcoma: The role of reactive oxygen species in cell proliferation. Oncol. Lett. 2018, 15, 7955–7962. [Google Scholar] [CrossRef]
- Wiktorin, H.G.; Aydin, E.; Hellstrand, K.; Martner, A. Nox2-derived reactive oxygen species in cancer. Oxid. Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.P.; Vinh, A.; Johnson, I.R.D.; Luong, R.; Drummond, G.R.; Sobey, C.G.; Tiganis, T.; Williams, E.D.; O’Leary, J.J.; Brooks, D.A.; et al. NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development. Oncotarget 2018, 9, 35378–35393. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-derived reactive oxygen species in NK cell–mediated control of murine melanoma metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Van der Weyden, L.; Speak, A.O.; Swiatkowska, A.; Clare, S.; Schejtman, A.; Santilli, G.; Arends, M.J.; Adams, D.J. Pulmonary metastatic colonisation and granulomas in NOX2-deficient mice. J. Pathol. 2018, 246, 300–310. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Cooney, S.J.; Bermudez-Sabogal, S.L.; Byrnes, K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation 2013, 10, 155. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Kleikers, P.W.M.; Radermacher, K.A.; Scheurer, P.; Hermans, J.J.R.; Schiffers, P.; Ho, H.; Wingler, K.; Schmidt, H.H.H.W. The NOX toolbox: Validating the role of NADPH oxidases in physiology and disease. Cell. Mol. Life Sci. 2012, 69, 2327–2343. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Liu, Y.; Wang, T.; Wei, S.J.; Block, M.L.; Wilson, B.; Liu, B.; Hong, J.S. NADPH Oxidase Mediates Lipopolysaccharide-induced Neurotoxicity and Proinflammatory Gene Expression in Activated Microglia. J. Biol. Chem. 2004, 279, 1415–1421. [Google Scholar] [CrossRef]
- Wilkinson, B.L.; Landreth, G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflammation 2006, 3, 30. [Google Scholar] [CrossRef]
- Fragoso-Morales, L.G.; Correa-Basurto, J.; Rosales-Hernández, M.C. Implication of nicotinamide adenine dinucleotide phosphate (Nadph) oxidase and its inhibitors in alzheimer’s disease murine models. Antioxidants 2021, 10, 218. [Google Scholar] [CrossRef]
- Moreira, A.P.; Vizuete, A.F.K.; Zin, L.E.F.; de Marques, C.O.; Pacheco, R.F.; Leal, M.B.; Gonçalves, C.A. The Methylglyoxal/RAGE/NOX-2 Pathway is Persistently Activated in the Hippocampus of Rats with STZ-Induced Sporadic Alzheimer’s Disease. Neurotox. Res. 2022, 40, 395–409. [Google Scholar] [CrossRef]
- Malkov, A.; Popova, I.; Ivanov, A.; Jang, S.S.; Yoon, S.Y.; Osypov, A.; Huang, Y.; Zilberter, Y.; Zilberter, M. Aβ initiates brain hypometabolism, network dysfunction and behavioral abnormalities via NOX2-induced oxidative stress in mice. Commun. Biol. 2021, 4, 1504. [Google Scholar] [CrossRef]
- Keeney, M.T.; Hoffman, E.K.; Farmer, K.; Bodle, C.R.; Fazzari, M.; Zharikov, A.; Castro, S.L.; Hu, X.; Mortimer, A.; Kofler, J.K.; et al. NADPH oxidase 2 activity in Parkinson’s disease. Neurobiol. Dis. 2022, 170, 105754. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Yang, L.; Ji, T.; Zhang, N.; Dong, X.; Chen, X.; Ma, J.; Gao, W.; Huang, S.; et al. NOX2-derived hydrogen peroxide impedes the AMPK/Akt-mTOR signaling pathway contributing to cell death in neuronal cells. Cell. Signal. 2022, 94, 110330. [Google Scholar] [CrossRef]
- Yan, J.; Huang, J.; Liu, A.; Wu, J.; Fan, H.; Shen, M.; Lai, X.; Ma, H.; Sun, W.; Yang, J.; et al. Atorvastatin improves motor function, anxiety and depression by NOX2-mediated autophagy and oxidative stress in MPTP-lesioned mice. Aging 2021, 13, 831–845. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Xie, L.; Qian, C.; Ye, Y.; Mao, H.; Wang, B.; Zhang, H.; Zhang, Y.; He, X.; et al. Tristetraprolin destabilizes NOX2 mRNA and protects dopaminergic neurons from oxidative damage in Parkinson’s disease. FASEB J. 2020, 34, 15047–15061. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, L.; Wei, X.; Wang, Y.; Ren, Z.; Zeng, S.; Zhang, X.; Wen, H.; Gao, C.; Liu, H. NOD2 promotes dopaminergic degeneration regulated by NADPH oxidase 2 in 6-hydroxydopamine model of Parkinson’s disease. J. Neuroinflammation 2018, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marrali, G.; Casale, F.; Salamone, P.; Fuda, G.; Ilardi, A.; Manera, U.; Calvo, A.; Zibetti, M.; Lopiano, L.; Chiò, A. NADPH oxidases 2 activation in patients with Parkinson’s disease. Park. Relat. Disord. 2018, 49, 110–111. [Google Scholar] [CrossRef]
- Belarbi, K.; Cuvelier, E.; Destée, A.; Gressier, B.; Chartier-Harlin, M.C. NADPH oxidases in Parkinson’s disease: A systematic review. Mol. Neurodegener. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Baratta, F.; Castellani, V.; Bartimoccia, S.; Nocella, C.; D’erasmo, L.; Cocomello, N.; Barale, C.; Scicali, R.; Di Pino, A.; et al. Proprotein convertase subtilisin kexin type 9 inhibitors reduce platelet activation modulating ox-LDL pathways. Int. J. Mol. Sci. 2021, 22, 7193. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef]
- Loffredo, L.; Martino, F.; Carnevale, R.; Pignatelli, P.; Catasca, E.; Perri, L.; Calabrese, C.M.; Palumbo, M.M.; Baratta, F.; Del Ben, M.; et al. Obesity and hypercholesterolemia are associated with NOX2 generated oxidative stress and arterial dysfunction. J. Pediatr. 2012, 161, 1004–1009. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef]
- Haddad, P.; Dussault, S.; Groleau, J.; Turgeon, J.; Maingrette, F.; Rivard, A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: Effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis 2011, 217, 340–349. [Google Scholar] [CrossRef]
- Miller, A.A.; De Silva, T.M.; Judkins, C.P.; Diep, H.; Drummond, G.R.; Sobey, C.G. Augmented superoxide production by nox2-containing nadph oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke 2010, 41, 784–789. [Google Scholar] [CrossRef]
- Judkins, C.P.; Diep, H.; Broughton, B.R.S.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE -/- mice. Am. J. Physiol. Hear. Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef] [Green Version]
- Loffredo, L.; Martino, F.; Zicari, A.M.; Carnevale, R.; Battaglia, S.; Martino, E.; Cammisotto, V.; Peruzzi, M.; De Castro, G.; Duse, M.; et al. Enhanced NOX-2 derived oxidative stress in offspring of patients with early myocardial infarction. Int. J. Cardiol. 2019, 293, 56–59. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, F.; Peng, J.; Fang, Z.; Liu, Q.; Zhou, S. KMT2B-dependent RFK transcription activates the TNF-α/NOX2 pathway and enhances ferroptosis caused by myocardial ischemia-reperfusion. J. Mol. Cell. Cardiol. 2022, 173, 75–91. [Google Scholar] [CrossRef]
- Korn, A.; Baylan, U.; Simsek, S.; Schalkwijk, C.G.; Niessen, H.W.M.; Krijnen, P.A.J. Myocardial infarction coincides with increased NOX2 and Nε-(carboxymethyl) lysine expression in the cerebral microvasculature. Open Hear. 2021, 8, e001842. [Google Scholar] [CrossRef]
- Tanzilli, G.; Arrivi, A.; Placanica, A.; Viceconte, N.; Cammisotto, V.; Nocella, C.; Barillà, F.; Torromeo, C.; Pucci, G.; Acconcia, M.C.; et al. Glutathione infusion before and 3 days after primary angioplasty blunts ongoing NOX2-mediated inflammatory response. J. Am. Heart Assoc. 2021, 10, e020560. [Google Scholar] [CrossRef]
- Jin, Q.; Jiang, Y.; Fu, L.; Zheng, Y.; Ding, Y.; Liu, Q. Wenxin Granule Ameliorates Hypoxia/Reoxygenation-Induced Oxidative Stress in Mitochondria via the PKC- δ/NOX2/ROS Pathway in H9c2 Cells. Oxid. Med. Cell. Longev. 2020, 2020, 3245483. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, L.; Ding, F.; Yang, F.; Ma, W.; Han, Z.; Hua, B.; Wang, X.; Yu, Y.; Huang, Q.; et al. Blocking Nox2 improves mesenchymal stem cells therapy in myocardial infarction via antagonizing oxidant and promoting survival. J. Cell. Physiol. 2018, 233, 7004–7015. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Platelet oxidative stress and thrombosis. Thromb. Res. 2012, 129, 378–381. [Google Scholar] [CrossRef]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef]
- Versaci, F.; Biondi-Zoccai, G.; Giudici, A.D.; Mariano, E.; Trivisonno, A.; Sciarretta, S.; Valenti, V.; Peruzzi, M.; Cavarretta, E.; Frati, G.; et al. Climate changes and ST-elevation myocardial infarction treated with primary percutaneous coronary angioplasty. Int. J. Cardiol. 2019, 294, 1–5. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.M.; Wingler, K.; Schmidt, H.H.H.W. Evolution of NADPH oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Cheng, G.; Zielonka, M.; Ganesh, T.; Sun, A.; Joseph, J.; Michalski, R.; O’Brien, W.J.; Lambeth, J.D.; Kalyanaraman, B. High-throughput assays for superoxide and hydrogen peroxide design of a screening workflow to identify inhibitors of NADPH oxidases. J. Biol. Chem. 2014, 289, 16176–16189. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Dürr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Massari, M.; Marchese, S.; Ceccon, M.; Aalbers, F.S.; Corana, F.; Valente, S.; Mai, A.; Magnani, F.; Mattevi, A. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biol. 2020, 32, 101466. [Google Scholar] [CrossRef]
- Bechor, E.; Zahavi, A.; Berdichevsky, Y.; Pick, E. p67phox-derived self-assembled peptides prevent Nox2 NADPH oxidase activation by an auto-inhibitory mechanism. J. Leukoc. Biol. 2021, 109, 657–673. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Chatterjee, S.; Feinstein, S.I. A peptide inhibitor of nadph oxidase (Nox2) activation markedly decreases mouse lung injury and mortality following administration of lipopolysaccharide (lps). Int. J. Mol. Sci. 2019, 20, 2395. [Google Scholar] [CrossRef]
- Hirano, K.; Chen, W.S.; Chueng, A.L.W.; Dunne, A.A.; Seredenina, T.; Filippova, A.; Ramachandran, S.; Bridges, A.; Chaudry, L.; Pettman, G.; et al. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid. Redox Signal. 2015, 23, 358–374. [Google Scholar] [CrossRef]
- Xue, N.; Wang, L.; Wang, B.; Hu, J.; Zhang, S. NOX2 oxidase inhibitor GSK2795039 possess antiviral activity against H1N1 influenza A virus in vitro and vivo. Microb. Pathog. 2023, 174, 105942. [Google Scholar] [CrossRef]
- Fan, L.M.; Liu, F.; Du, J.; Geng, L.; Li, J.M. Inhibition of endothelial Nox2 activation by LMH001 protects mice from angiotensin II-induced vascular oxidative stress, hypertension and aortic aneurysm. Redox Biol. 2022, 51, 102269. [Google Scholar] [CrossRef]
- Li, D.; Cong, Z.; Yang, C.; Zhu, X. Inhibition of LPS-induced Nox2 activation by VAS2870 protects alveolar epithelial cells through eliminating ROS and restoring tight junctions. Biochem. Biophys. Res. Commun. 2020, 524, 575–581. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ali, M.M.; Miranda, E.R.; Mey, J.T.; Blackburn, B.K.; Haus, J.M.; Phillips, S.A. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol. 2017, 13, 288–300. [Google Scholar] [CrossRef]
- Bosco, E.E.; Kumar, S.; Marchioni, F.; Biesiada, J.; Kordos, M.; Szczur, K.; Meller, J.; Seibel, W.; Mizrahi, A.; Pick, E.; et al. Rational design of small molecule inhibitors targeting the Rac GTPase-p67 phox signaling axis in inflammation. Chem. Biol. 2012, 19, 228–242. [Google Scholar] [CrossRef]
- Akbar, H.; Duan, X.; Piatt, R.; Saleem, S.; Davis, A.K.; Tandon, N.N.; Bergmeier, W.; Zheng, Y. Small molecule targeting the Rac1-NOX2 interaction prevents collagen-related peptide and thrombin-induced reactive oxygen species generation and platelet activation. J. Thromb. Haemost. 2018, 16, 2083–2096. [Google Scholar] [CrossRef]
- Smith, S.M.E.; Min, J.; Ganesh, T.; Diebold, B.; Kawahara, T.; Zhu, Y.; McCoy, J.; Sun, A.; Snyder, J.P.; Fu, H.; et al. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem. Biol. 2012, 19, 752–763. [Google Scholar] [CrossRef]
- Chew, P.; Yuen, D.Y.C.; Stefanovic, N.; Pete, J.; Coughlan, M.T.; Jandeleit-Dahm, K.A.; Thomas, M.C.; Rosenfeldt, F.; Cooper, M.E.; De Haan, J.B. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes 2010, 59, 3198–3207. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, E.; Saha, J.; Csányi, G.; Al Ghouleh, I.; Sahoo, S.; Rodríguez, A.; Wipf, P.; Pagano, P.J.; Skoda, E.M. Bridged tetrahydroisoquinolines as selective NADPH oxidase 2 (Nox 2) inhibitors. Medchemcomm 2013, 4, 1085–1092. [Google Scholar] [CrossRef]
- Gatto, G.J.; Ao, Z.; Kearse, M.G.; Zhou, M.; Morales, C.R.; Daniels, E.; Bradley, B.T.; Goserud, M.T.; Goodman, K.B.; Douglas, S.A.; et al. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013, 28, 95–104. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Nocella, C.; Vicario, T.; Loffredo, L.; Angelico, F.; Violi, F. Rosuvastatin reduces platelet recruitment by inhibiting NADPH oxidase activation. Biochem. Pharmacol. 2012, 84, 1635–1642. [Google Scholar] [CrossRef]
- Liu, F.C.; Yu, H.P.; Chen, P.J.; Yang, H.W.; Chang, S.H.; Tzeng, C.C.; Cheng, W.J.; Chen, Y.R.; Chen, Y.L.; Hwang, T.L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef]
- Gautam, J.; Ku, J.M.; Regmi, S.C.; Jeong, H.; Wang, Y.; Banskota, S.; Park, M.H.; Nam, T.-G.; Jeong, B.S.; Kim, J.A. Dual inhibition of NOX2 and receptor tyrosine kinase by BJ-1301 enhances anticancer therapy efficacy via suppression of autocrine-stimulatory factors in lung cancer. Mol. Cancer Ther. 2017, 16, 2144–2156. [Google Scholar] [CrossRef]
- Cha, D.R.; Cha, J.J.; Min, H.S.; Kim, K.T.; Kim, J.E.; Ghee, J.Y.; Kim, H.W.; Lee, J.E.; Han, J.Y.; Lee, G.; et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Investig. 2017, 97, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, F.L.M.; Walum, E.; Wikström, P.; Arner, A. A small molecule inhibitor of Nox2 and Nox4 improves contractile function after ischemia–reperfusion in the mouse heart. Sci. Rep. 2021, 11, 11970. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Li, Q.; Wu, S.; Pan, J.; Liao, Y.; Zheng, X.; Zeng, W. Dexmedetomidine Alleviates Hypoxia-Induced Synaptic Loss and Cognitive Impairment via Inhibition of Microglial NOX2 Activation in the Hippocampus of Neonatal Rats. Oxid. Med. Cell. Longev. 2021, 2021, 6643171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Chen, P.; Chen, A.; Deng, J.; Wei, J.; Zeng, W.; Zheng, X. Dexmedetomidine Attenuates Apoptosis and Neurological Deficits by Modulating Neuronal NADPH Oxidase 2-Derived Oxidative Stress in Neonates Following Hypoxic Brain Injury. Antioxidants 2022, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, Z.; Jia, J.; Chen, A.; Gao, Y.; Qian, J.; Ge, J. Rosuvastatin Alleviates Coronary Microembolization-Induced Cardiac Injury by Suppressing Nox2-Induced ROS Overproduction and Myocardial Apoptosis. Cardiovasc. Toxicol. 2022, 22, 341–351. [Google Scholar] [CrossRef]
- Ding, M.; Fang, Q.H.; Cui, Y.T.; Shen, Q.L.; Liu, Q.; Wang, P.H.; Yu, D.M.; Li, C.J. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J. Diabetes Complicat. 2019, 33, 267–277. [Google Scholar] [CrossRef]
- Pignatelli, P.; Baratta, F.; Buzzetti, R.; D’Amico, A.; Castellani, V.; Bartimoccia, S.; Siena, A.; D’Onofrio, L.; Maddaloni, E.; Pingitore, A.; et al. The Sodium–Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study. Antioxidants 2022, 11, 1878. [Google Scholar] [CrossRef]
- Parente, J.E.; Wong, K.; Davis, P. Effect of gold compounds on nadph oxidase system of human neutrophils. Inflammation 1986, 10, 303–310. [Google Scholar] [CrossRef]
- Seredenina, T.; Chiriano, G.; Filippova, A.; Nayernia, Z.; Mahiout, Z.; Fioraso-Cartier, L.; Plastre, O.; Scapozza, L.; Krause, K.H.; Jaquet, V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic. Biol. Med. 2015, 86, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Jaquet, V.; Marcoux, J.; Forest, E.; Leidal, K.G.; McCormick, S.; Westermaier, Y.; Perozzo, R.; Plastre, O.; Fioraso-Cartier, L.; Diebold, B.; et al. NADPH oxidase (NOX) isoforms are inhibited by celastrol with a dual mode of action. Br. J. Pharmacol. 2011, 164, 507–520. [Google Scholar] [CrossRef]
- Qi, S.; Feng, Z.; Li, Q.; Qi, Z.; Zhang, Y. Myricitrin Modulates NADPH Oxidase-Dependent ROS Production to Inhibit Endotoxin-Mediated Inflammation by Blocking the JAK/STAT1 and NOX2/p47phox Pathways. Oxid. Med. Cell. Longev. 2017, 2017, 9738745. [Google Scholar] [CrossRef]
- Wang, Z.C.; Niu, K.M.; Wu, Y.J.; Du, K.R.; Qi, L.W.; Zhou, Y.B.; Sun, H.J. A dual Keap1 and p47phox inhibitor Ginsenoside Rb1 ameliorates high glucose/ox-LDL-induced endothelial cell injury and atherosclerosis. Cell Death Dis. 2022, 13, 824. [Google Scholar] [CrossRef]
- Gustafson, S.J.; Dunlap, K.L.; McGill, C.M.; Kuhn, T.B. A nonpolar blueberry fraction blunts NADPH oxidase activation in neuronal cells exposed to tumor necrosis factor-α. Oxid. Med. Cell. Longev. 2012, 2012, 768101. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1410–1416. [Google Scholar] [CrossRef]
- Hsu, H.T.; Tseng, Y.T.; Wong, W.J.; Liu, C.M.; Lo, Y.C. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial A549 cells. BMC Complement. Altern. Med. 2018, 18, 211. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, L.; Deng, X.; Liang, Q.; Xu, Y.; Deng, R.; Lv, L.; Ji, M.; Hao, Z.; He, J. The Antioxidant Rosmarinic Acid Ameliorates Oxidative Lung Damage in Experimental Allergic Asthma via Modulation of NADPH Oxidases and Antioxidant Enzymes. Inflammation 2020, 43, 1902–1912. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Yang, L.; Zhang, D.; Li, L.; Dong, X.; Li, Y.; Qun, S.; Li, W. Ginsenoside Rg1 attenuates cerebral ischemia-reperfusion injury due to inhibition of NOX2-mediated calcium homeostasis dysregulation in mice. J. Ginseng Res. 2022, 46, 515–525. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Yang, B.; Ma, S.; Zhang, C.; Sun, J.; Zhang, D.; Chang, S.; Lin, Y.; Zhao, G. Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-ĸB Signaling Pathway. Front. Pharmacol. 2021, 12, 2518. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, E.; Park, B.C.; Kim, S.J. Novel potential NOX2 inhibitors, Dudleya brittonii water extract and polygalatenoside A inhibit intracellular ROS generation and growth of melanoma. Biomed. Pharmacother. 2022, 150, 112967. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; He, Y.; Zheng, Q.; Wang, M.; Wu, Y.; Zhang, Y.; Wang, C. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur. J. Pharmacol. 2017, 797, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Pan, Y.; Wang, X.; Ding, Y.; Zhou, C.; Shah, A.M.; Zhao, G.; Zhang, M. Celastrol Alleviates Aortic Valve Calcification Via Inhibition of NADPH Oxidase 2 in Valvular Interstitial Cells. JACC Basic Transl. Sci. 2020, 5, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases PMA-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, W.; Huang, C.; Jian, J.; Zhang, Y.; Liu, Q.; Chen, P.; Zhu, X. Ursolic acid alleviates Kupffer cells pyroptosis in liver fibrosis by the NOX2/NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 2022, 113, 109321. [Google Scholar] [CrossRef]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 9268083. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Rao, K.; Liu, K.; Zhang, Y.; Liu, X.; Wang, T.; Wang, S.; Liu, Z.; Liu, J. Curcumin ameliorates atrophy of seminal vesicle via reduction of oxidative stress in castrated mice. PeerJ 2019, 7, e7192. [Google Scholar] [CrossRef]
- Huang, S.L.; Chen, P.Y.; Wu, M.J.; Tai, M.H.; Ho, C.T.; Yen, J.H. Curcuminoids Modulate the PKCδ/NADPH Oxidase/Reactive Oxygen Species Signaling Pathway and Suppress Matrix Invasion during Monocyte-Macrophage Differentiation. J. Agric. Food Chem. 2015, 63, 8838–8848. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef]
- Wongeakin, N.; Bhattarakosol, P.; Patumraj, S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 expressions. Biomed Res. Int. 2014, 2014, 161346. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ni, D.; Liu, Y.; Lu, S. Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, E.; Csanyi, G.; Pagano, P.J. NADPH oxidase inhibitors: A decade of discovery from Nox2ds to HTS. Cell. Mol. Life Sci. 2012, 69, 2315–2325. [Google Scholar] [CrossRef]
- Rey, F.E.; Cifuentes, M.E.; Kiarash, A.; Quinn, M.T.; Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2- and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef]
- Csányi, G.; Cifuentes-Pagano, E.; Al Ghouleh, I.; Ranayhossaini, D.J.; Egaña, L.; Lopes, L.R.; Jackson, H.M.; Kelley, E.E.; Pagano, P.J. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic. Biol. Med. 2011, 51, 1116–1125. [Google Scholar] [CrossRef]
- Fisher, A.B.; Dodia, C.; Feinstein, S.I. Identification of small peptides that inhibit NADPH oxidase (Nox2) activation. Antioxidants 2018, 7, 181. [Google Scholar] [CrossRef]
- Vázquez-Medina, J.P.; Dodia, C.; Weng, L.; Mesaros, C.; Blair, I.A.; Feinstein, S.I.; Chatterjee, S.; Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6 modulates NADPH oxidase 2 activation via lysophosphatidic acid receptor signaling in the pulmonary endothelium and alveolar macrophages. FASEB J. 2016, 30, 2885–2898. [Google Scholar] [CrossRef]
- Chatterjee, S.; Feinstein, S.I.; Dodia, C.; Sorokina, E.; Lien, Y.C.; Nguyen, S.; Debolt, K.; Speicher, D.; Fisher, A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011, 286, 11696–11706. [Google Scholar] [CrossRef]
- Megino-Luque, C.; Moiola, C.P.; Molins-Escuder, C.; López-Gil, C.; Gil-Moreno, A.; Matias-Guiu, X.; Colas, E.; Eritja, N. Small-molecule inhibitors (Smis) as an effective therapeutic strategy for endometrial cancer. Cancers 2020, 12, 2751. [Google Scholar] [CrossRef]
- Weiss, W.A.; Taylor, S.S.; Shokat, K.M. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat. Chem. Biol. 2007, 3, 739–744. [Google Scholar] [CrossRef]
- Zang, J.; Cambet, Y.; Jaquet, V.; Bach, A. Chemical synthesis of a reported p47phox/p22phox inhibitor and characterization of its instability and irreproducible activity. Front. Pharmacol. 2023, 13, 5476. [Google Scholar] [CrossRef]
- Ten Freyhaus, H.; Huntgeburth, M.; Wingler, K.; Schnitker, J.; Bäumer, A.T.; Vantler, M.; Bekhite, M.M.; Wartenberg, M.; Sauer, H.; Rosenkranz, S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006, 71, 331–341. [Google Scholar] [CrossRef]
- Bedard, K.; Jaquet, V. Cell-free screening for Nox inhibitors. Chem. Biol. 2012, 19, 664–665. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.G.; Arnold, M.J.; Kelley, C.; Spacek, L.; Buelt, A.; Natarajan, S.; Donahue, M.P.; Vagichev, E.; Ballard-Hernandez, J.; Logan, A.; et al. Management of dyslipidemia for cardiovascular disease risk reduction: Synopsis of the 2020 updated U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med. 2020, 173, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; Ku, J.M.; Park, H.G.; Porter, N.A.; Jeong, B.S. New synthetic route to N-tocopherol derivatives: Synthesis of pyrrolopyridinol analogue of α-tocopherol from pyridoxine. Org. Biomol. Chem. 2011, 9, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.M.R.F.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef]
- Barzegar, A. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol. Biol. Res. Commun. 2016, 5, 87–95. [Google Scholar]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef]
- Song, C.Y.; Xu, Y.G.; Lu, Y. qiang Use of Tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ. Sci. B 2020, 21, 280–290. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tang, G.Y. Why polyphenols have promiscuous actions? An investigation by chemical bioinformatics. Nat. Prod. Commun. 2016, 11, 655–656. [Google Scholar] [CrossRef]
- Hu, Y.; Bajorath, J. What is the likelihood of an active compound to be promiscuous? Systematic assessment of compound promiscuity on the basis of pubchem confirmatory bioassay data. AAPS J. 2013, 15, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Roche, O.; Schneider, P.; Zuegge, J.; Guba, W.; Kansy, M.; Alanine, A.; Bleicher, K.; Danel, F.; Gutknecht, E.M.; Rogers-Evans, M.; et al. Development of a virtual screening method for identification of “frequent hitters” in compound libraries. J. Med. Chem. 2002, 45, 137–142. [Google Scholar] [CrossRef]

| Compound Name | Pathology | Main Effects | Suggested Mechanism of Inhibition | Type of Study: Range of Concentrations Tested | Ref. |
|---|---|---|---|---|---|
| PEPTIDE-BASED INHIBITORS | |||||
| DIRECT INHIBITION | |||||
| p67phox-derived self-assembled peptides | Not applicable | ↓ NOX2 activation | p67phox inhibitory peptides | In vitro study: 0.19–50 μM in cell free assay | [109] |
| INDIRECT INHIBITION | |||||
| Peroxiredoxin 6 (Prdx6)-inhibitory peptides | Acute lung injury | ↓ ROS production ↓ Phospholipase A2 ↓ LPS-mediated lung injury | Inhibition of Prdx6-PLA2 activity by the SP-A peptide | Animal study: 2 µg/g | [110] |
| DRUG-LIKE SMALL MOLECULES AND DRUGS | |||||
| DIRECT INHIBITION | |||||
| GSK2795039 | Inflammation/acute pancreatitis | ↓ ROS formation ↓ NOX2 activity ↓ Amylase levels | Competition for the NADPH binding site of NOX2 | Animal study: 100 mg/kg | [111] |
| GSK2795039 | Influenza A viruses infection | ↓ ROS formation ↓ NOX2 activity ↓ Viral burden | Competition for the NADPH binding site of NOX2 | Animal study: 100 mg/kg In vitro study: 0–80 μM in A549 cells | [112] |
| LMH001 | Vascular oxidative stress, hypertension, and aortic aneurysm | ↓ AngII-induced ROS production ↓ NOX2 activity ↓ Hypertension ↓ Aortic walls inflammation ↓ Incidences of aortic aneurysm | Blocking p47/p22 binding | Animal study: 2.5 mg/kg In vitro study: 0–100 μM in PBMC | [113] |
| VAS2870 | ARDS | ↓ NOX2 expression ↓ ROS production ↑ ZO-1 | Covalent ligands of the dehydrogenase domain | In vitro study: 0–20 μM in A549 cells | [114] |
| VAS2870 | Hyperinsulinemia-induced microvascular endothelial cell dysfunction | ↓ ROS production ↓ NOX2 expression ↓ p47phox phosphorylation ↑ NO ↑ FID | Covalent ligands of the dehydrogenase domain | In vitro study: 2 μM in arterioles from human skeletal muscle tissue and HAMECs | [115] |
| Phox-I1 | Not applicable | ↓ RAC1 binding ↓ NOX2-mediated superoxide production | Binding to p67phox | In vitro study: 10 μM in neutrophils | [116] |
| Phox-I1 | Thrombosis | ↓ ROS production ↓ P-selectin release ↓ Platelet aggregation ↓ Akt phosphorylation | Binding to p67phox | In vitro study: 3–10 μM in platelets | [117] |
| Ebselen | Not applicable | ↓ NOX2 activity | Inhibition of p47phox and p67phox translocation to membranes | In vitro study: 10 µM in human neuthrophils | [118] |
| Ebselen | Diabetes-associated atherosclerosis/renal injury | ↓ NOX2 expression ↓ Oxidative stress ↓ Fibrosis ↓ Inflammation | Inhibition of p47phox translocation to membranes | Animal study: 10 mg/kg | [119] |
| Tetrahydroisoquinoline analogs (compounds 11 g and 11 h) | Not applicable | ↓ NOX2 activity | Disruption of p22phox binding to p47phox | In vitro study: 3–300 μM in COS-NOX2 cells | [120] |
| Perhexiline | Not applicable | ↓ NOX2 activity | Unknown | In vitro study: 1 nM–100 μM in human neutrophils | [121] |
| Rosuvastatin | Hypercholesterolemia | ↓ NOX2 activity ↓ Platelet isoprostanes ↓ Platelet recruitment ↓ Platelet PLA2 | Inhibition of p47phox translocation to membranes | Human study: 20 mg In vitro study: 0.1–10 µM in human platelets | [122] |
| Atorvastatin | Hypercholesterolemia | ↓ NOX2 activity ↓ Platelet isoprostanes ↓ Platelet recruitment ↓ Platelet PLA2 | Inhibition of p47phox translocation to membranes and Rac1 | Human study: 40 mg In vitro study: 1–10 µM in human platelets | [90] |
| INDIRECT INHIBITION | |||||
| CYR5099 | Inflammatory arthritis | ↓ ROS production ↓ Neutrophil infiltration ↓ Edema | Inhibition of NOX2 upstream pathways. | Animal study: 10–25 mg/kg In vitro study: 1–15 μM in human neutrophils | [123] |
| BJ-1301 | Lung cancer | ↓ ROS ↓ NOX2 activity ↓ Cell proliferation Regression of tumor growth | Inhibition of NOX2 upstream pathways. | Animal study: 1–5 mg/kg In vitro study: 0.1–1 μM in endothelial and lung cancer cells | [124] |
| APX-115 | Diabetic nephropathy | ↓ NOX2 expression ↓ 8-isoprostane level ↑ Insulin resistance ↓ Mesangial expansion | Attenuation of NOX2 protein expression | Animal study: 60 mg/kg | [125] |
| GLX481304 | Ischemia–reperfusion | ↓ ROS production ↑ Contractile function in cells and whole heart | Unknown | In vitro study: 1.57 μM in cardiomyocytes | [126] |
| Dexmedetomidine | Perinatal Hypoxia | ↓ ROS production ↓ NOX2 activity ↓ 4-hydroxynonenal ↓ Proinflammatory cytokines | Reduction in NOX2 expression | Animal study: 25 mg/kg In vitro study: 1 μM in BV2 microglial cells | [127] |
| Dexmedetomidine | Hypoxic-ischemic brain injury | ↓ ROS production ↓ NOX2 activity ↓ MDA ↓ 8-OHdG ↑ Antioxidant activity | Reduction in NOX2 expression | Animal study: 25 mg/kg In vitro study: 1 μM in primary hippocampal neurons | [128] |
| Rosuvastatin | Coronary Microembolism-induced cardiac injury | ↓ ROS production ↓ NOX2 activity ↑ pro-apoptotic proteins ↓ anti-apoptotic Bcl-2 | Reduction in NOX2 expression | Animal study: 10 or 20 mg/kg In vitro study: 10 or 20 μM in cardiomyocyte | [129] |
| Atorvastatin | Parkinson’s disease | ↓ NOX2 activity ↓ α-synuclein Ser129 expression ↓ LC3II/I expression ↑ Muscle capacity ↓ Anxiety ↓ Depression | Reduction in NOX2 expression | Animal study: 10 mg/kg | [81] |
| GLP-1Ra (Liraglutide) | Diabetes mellitus | ↓ NOX2 activity ↓ JNK1/2 phosphorylation ↓ AMPKα phosphorylation ↓ β-cell apoptosis | Reduction in NOX2 expression | Animal study: 0.2 mg/kg | [130] |
| Gliflozins (dapagliflozin) | Type 2 diabetes mellitus | ↓ NOX2 activity ↓ ROS production ↓ Platelet activation ↓ Thrombus formation ↑ Antioxidant power | Inhibition of NOX2 upstream pathways | Human study: 10 mg In vitro study: 10 or 20 μM in platelets | [131] |
| Auranofin | Not applicable | ↓ Superoxide anion generation | Inhibition of NOX2 upstream pathways | Human study In vitro study: 0.5–4 μg AU/mL in PBMC | [132] |
| N-substituted Phenothiazine | Not applicable | ↓ NOX2 activity | Unknown | In vitro study: 0.35–50 µM in PLB-985 | [133] |
| COMPOUNDS OF NATURAL ORIGIN | |||||
| DIRECT INHIBITION | |||||
| Celastrol | Not applicable | ↓ H2O2 production ↓ NOX2 activity | Disruption of the binding of the PRR of p22phox to the tandem SH3 domain of p47phox | In vitro study: 0.10–100 μM in human neuthophils | [134] |
| Myricitrin | Acute lung injury | ↓ NO production ↓ TNF-α, IL-6 ↓ Intracellular ROS production | Inhibition of NOX2/p47phox assembly | In vitro: 0–500 μg/mL in RAW264.7 macrophage cells | [135] |
| Ginsenoside Rb1 | Atherosclerosis | ↓ p47phox phosphorylation ↓ ROS production | Repression of p47phox activity | Animal study: 50 mg/kg In vitro study: 0–30 μM in endothelial cells | [136] |
| INDIRECT INHIBITION | |||||
| Blueberry-derived polyphenols | Central nervous system | ↓ ROS production | Modulation of lipid raft formation and p67phox colocalization | In vitro study: 5 μg/mL in human neuroblastoma cells | [137] |
| Resveratrol | Senescence of aorta cells induced by HFS | ↓ Senescence of aorta cells ↓ ROS production ↓ Expression of p47phox subunit | Downregulation of p47phox protein expression | Animal study: 50 or 100 mg/kg In vitro study: 0.1 or 1 μM in cultured BAECs | [138] |
| Resveratrol | Inflammation | ↓ Expression of NOX2 ↓ ROS production | Downregulation of PKC-α protein expression | In vitro study: 1, 5, and 10 μM in lung epithelial A549 cells | [139] |
| Rosmarinic acid | OVA-induced lung diseases | ↓ IL-4, IL-5, and IL-13 ↓ ROS production ↓ NOX2 expression | Downregulation of mRNA NOX2 expression | Animal study: 10, 20, or 40 mg/kg | [140] |
| Ginsenoside Rg1 | Cerebral ischemia-reperfusion injury | ↓ Oxidative stress ↓ NOX2 expression ↓ Calcium overload | Downregulation of NOX2 and NOX2-related protein expression | Animal study: 5, 10 mg/kg In vitro study: 5, 10 μM in HT22 cells | [141] |
| Ginsenoside Rg1 | Alzheimer’s disease | ↓ ROS production ↓ NOX2 expression ↓ p-Tau level ↓ APP expression, ↓ Aβ generation | Downregulation of NOX2, p22phox, and p47phox mRNA and protein | Animal study: 5, 10 mg/kg | [142] |
| Higenamine | Neuropathic pain | ↓ ROS production ↓ MDA levels ↓ TNF-α, and IL-6 levels ↑ SOD and GSH | Downregulation of NOX2 protein expression | Animal study: 25/50/100 mg/Kg In vitro study: 100/200/400 µM in RSC96 | [143] |
| Dudleya brittonii water extract (DBWE) Polygalatenoside A | Growth of melanoma | ↓ Intracellular ROS generation ↓ Mitochondrial activity ↓ ROS generation | Antioxidant effect | In vitro study: 0–90 ng/mL in B16–F10 melanoma cells and NIH/3T3 fibroblasts In vitro study: 0–10 µM in B16–F10 melanoma cells and NIH/3T3 fibroblasts | [144] |
| Celastrol | Ang II-mediated endothelial dysfunction | ↓ ROS generation ↓ NOX2/AT1 pathway ↑ endothelial cell activity | Inhibition of NOX2 upstream pathways (ERK1/2/Nrf2) | In vitro study: 50 nM in HUVEC | [145] |
| Celastrol | Calcific aortic valve disease | ↓ ROS generation ↓ Glycogen synthase kinase 3 beta/β-catenin pathway | Downregulation of NOX2 protein expression | Animal study: 1 mg/kg In vitro study: 10 nM in AVICs | [146] |
| Carnosine | Inflammation | ↓ ROS generation ↓ Akt phosphorylation ↓ TNF-α and IL-6 mRNAs ↑ IL-4, IL-10, TGF-β1 | Downregulation of NOX2 gene expression | In vitro study: 5–20 mM in RAW 264.7 macrophages | [147] |
| Ursolic Acid | Liver inflammation and fibrosis | ↓ NOX2/NLRP3 signalling pathway ↓ Liver fibrosis | Downregulation of NOX2 gene expression | Animal study: 50 mg/kg | [148] |
| Curcumin | Depression | ↓ NOX2 expression ↓ 4-HNE ↓ MDA | Downregulation of NOX2 protein expression | Animal study: 100 mg/kg | [149] |
| Curcumin | Seminal vesicle atrophy | ↓ NOX2 expression ↓ MDA | Downregulation of NOX2 protein expression | Animal study: 100 mg/kg | [150] |
| Curcumin | Atherosclerosis | ↓ NOX2 expression ↓ p47phox membrane translocation ↓ PKCδ activation | Downregulation of PKC-δ protein expression | In vitro study: 20 µM in monocytes-macrophages | [151] |
| Curcumin | Lung cancer cells invasiveness | ↓ NOX2 expression ↓ p47phox membrane translocation ↓ PKCα activation ↓ MMP9 expression ↓ cell invasiveness | Downregulation of PKC-α protein expression | In vitro study: 0–60 µM in lung cancer A549 cells | [152] |
| Curcumin | Diabetes-induced vascular inflammation | ↓ NOX2 expression ↓ ROS formation ↓ ICAM-1 ↓leukocyte-endothelium interaction | Downregulation of p47phox protein expression | Animal study: 300 mg/Kg | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocella, C.; D’Amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G.; et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. https://doi.org/10.3390/antiox12020429
Nocella C, D’Amico A, Cammisotto V, Bartimoccia S, Castellani V, Loffredo L, Marini L, Ferrara G, Testa M, Motta G, et al. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants. 2023; 12(2):429. https://doi.org/10.3390/antiox12020429
Chicago/Turabian StyleNocella, Cristina, Alessandra D’Amico, Vittoria Cammisotto, Simona Bartimoccia, Valentina Castellani, Lorenzo Loffredo, Leonardo Marini, Giulia Ferrara, Matteo Testa, Giulio Motta, and et al. 2023. "Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies" Antioxidants 12, no. 2: 429. https://doi.org/10.3390/antiox12020429
APA StyleNocella, C., D’Amico, A., Cammisotto, V., Bartimoccia, S., Castellani, V., Loffredo, L., Marini, L., Ferrara, G., Testa, M., Motta, G., Benazzi, B., Zara, F., Frati, G., Sciarretta, S., Pignatelli, P., Violi, F., Carnevale, R., & Group, S. (2023). Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants, 12(2), 429. https://doi.org/10.3390/antiox12020429











