Structural Characterization of Polysaccharides from Coriandrum sativum Seeds: Hepatoprotective Effect against Cadmium Toxicity In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Raw Material
2.2. Chemicals
2.3. Polysaccharides Extraction and Purification Procedure
2.4. Polysaccharides Characterization
2.4.1. Colorimetric Assays
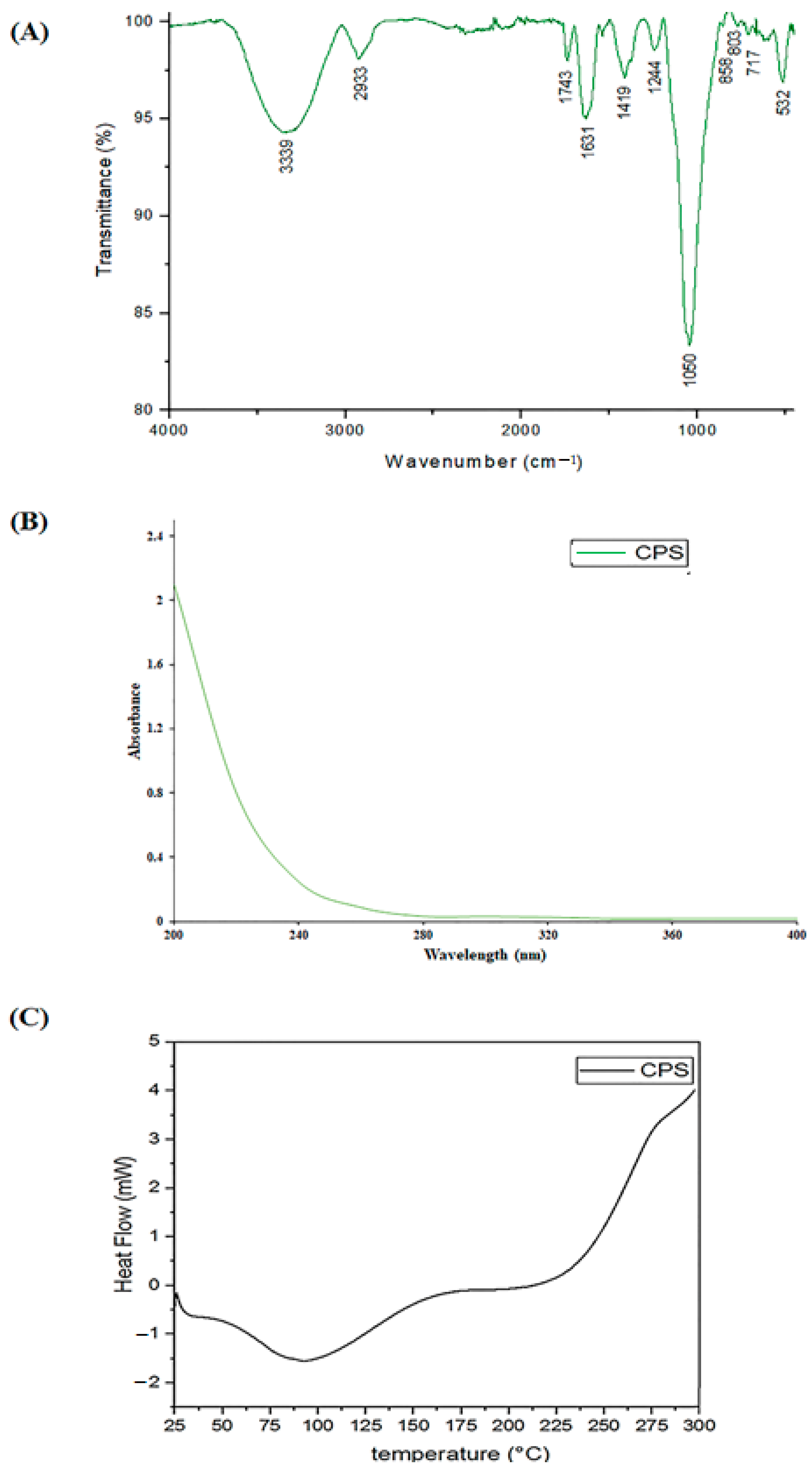
2.4.2. DSC, UV–vis and FT-IR Experiments
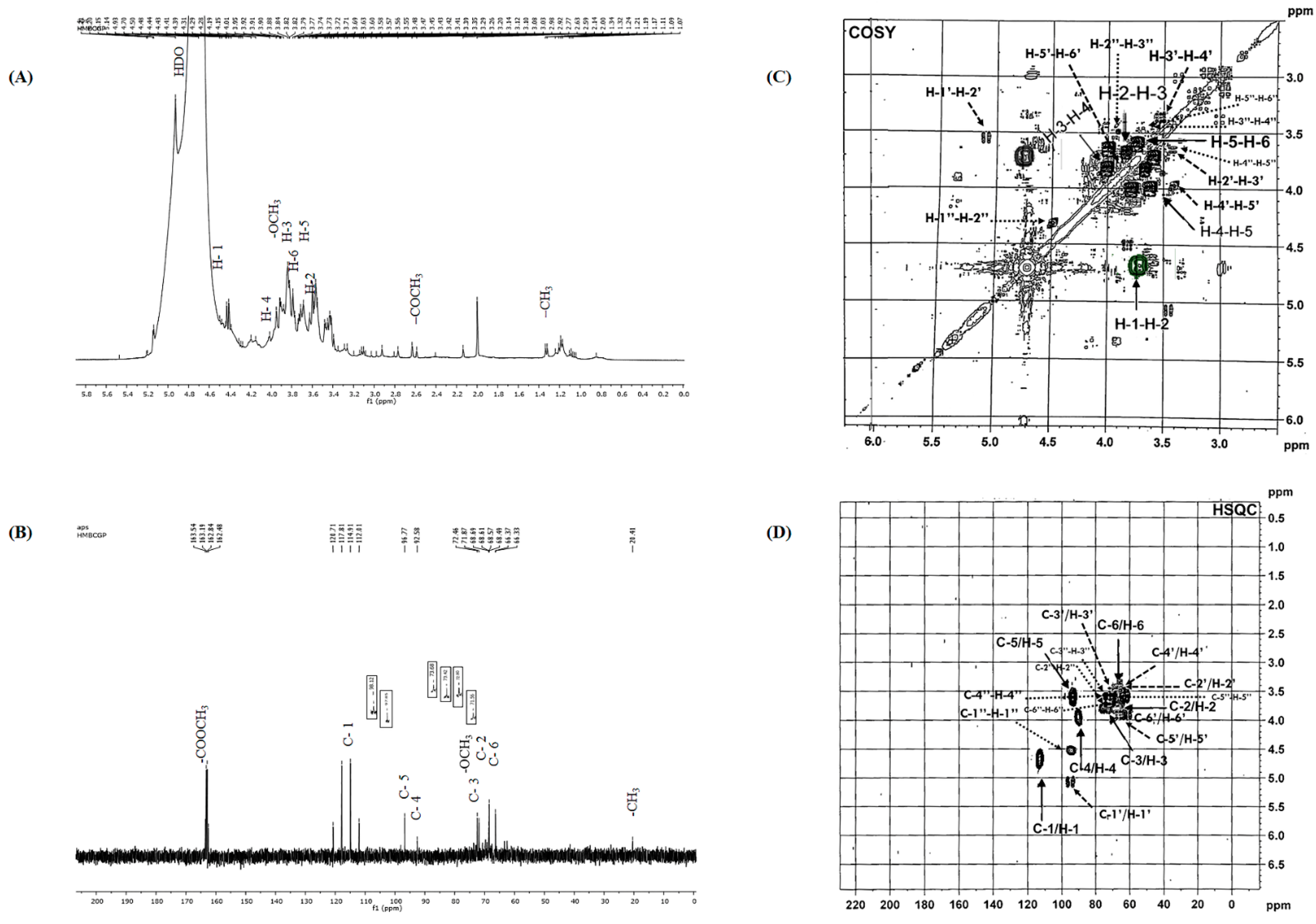
2.4.3. NMR Analysis
2.4.4. Monosaccharide Composition
2.4.5. Size Exclusion Chromatography
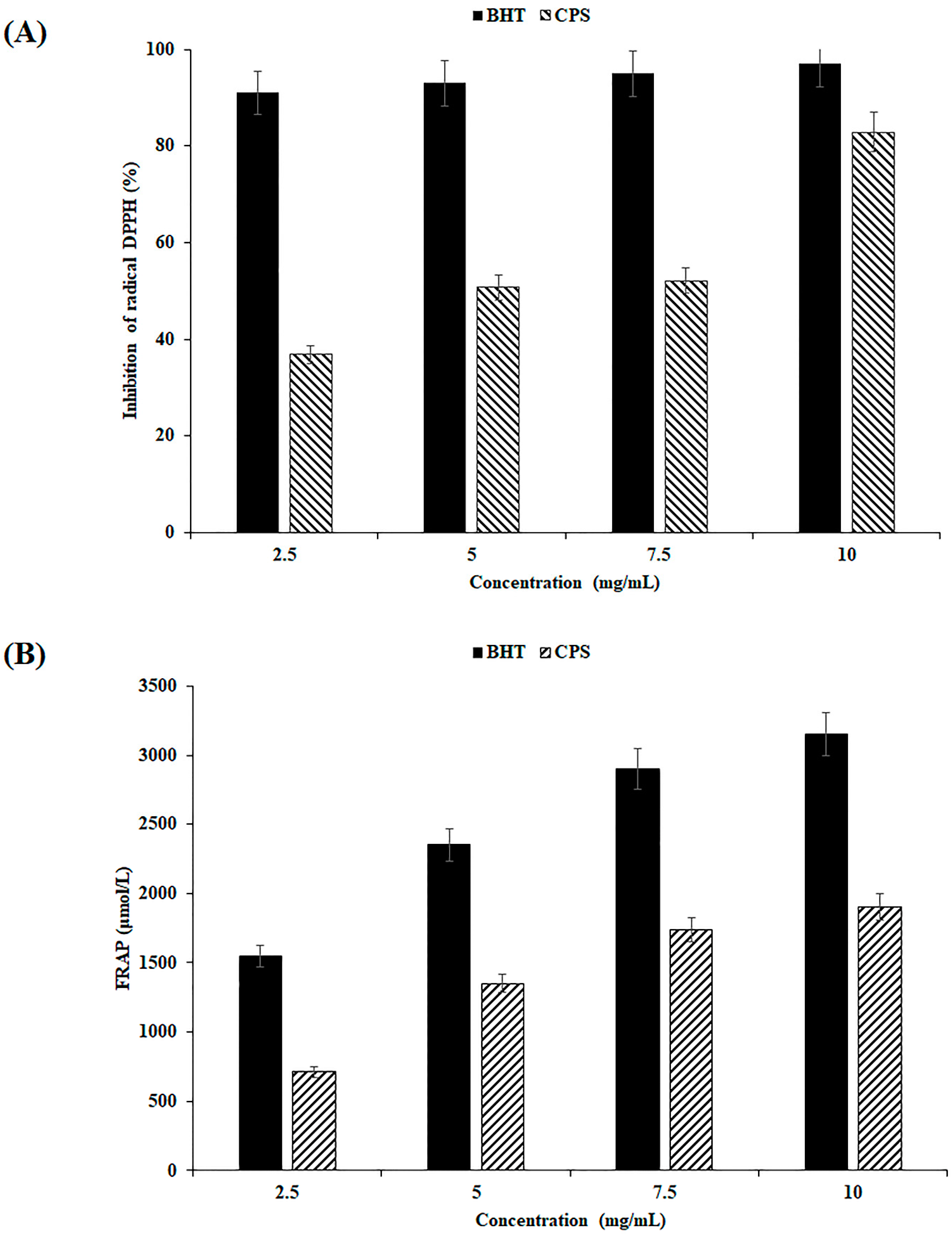
2.5. Antioxidant Activity Assays
2.5.1. DPPH Test
2.5.2. FRAP Assay
2.6. In Vivo Hepatotoxicity and Cytoprotective Assays
2.6.1. Animals and Experimental Design
- -
- Group. 1; (control group) receiving 1 mL of physiological saline (37 °C) daily;
- -
- Group. 2; receiving 250 mg kg−1 b.w. dose of CPS daily;
- -
- Group. 3; receiving 1 mL of 4 mg kg−1 b.w. dose of cadmium on the last day;
- -
- Group. 4; receiving 250 mg kg−1 b.w. dose of CPS daily and 1 mL of 4 mg kg−1 b.w. dose of cadmium on the last day.
2.6.2. Determination of Catalase CAT Assay
2.6.3. SOD Assay
2.6.4. Lipid Peroxidation (MDA)
2.6.5. Total Protein Content
2.7. Statistical Analysis
3. Results and Discussion
3.1. CPS Extraction and Identification
3.2. Antioxidant Capacities
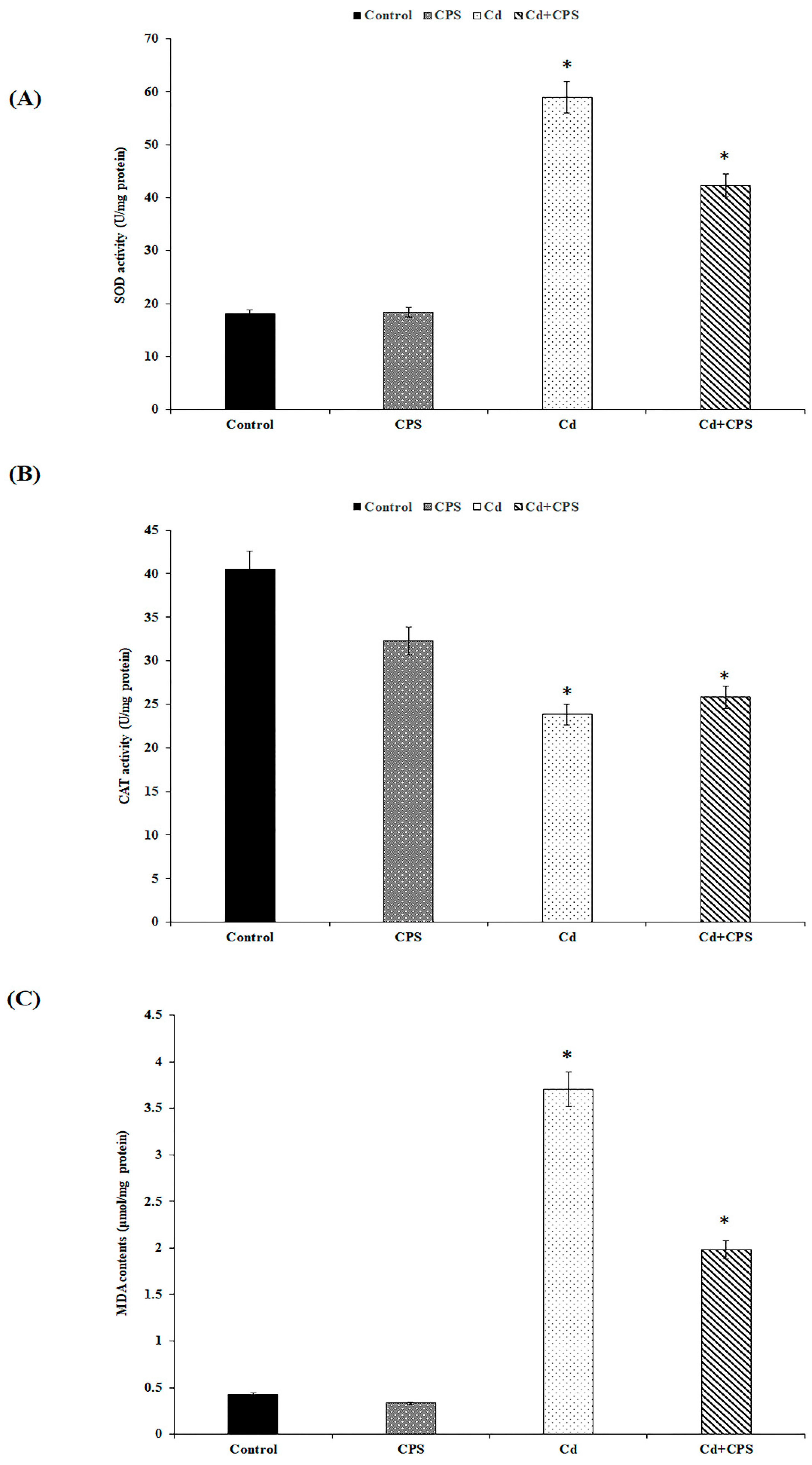
3.3. Effects of Coriandrum sativum Seed on CdCl2-Induced Hepatotoxicity
3.3.1. Lipid Peroxidation (MDA)
3.3.2. CAT Activity
3.3.3. MDA Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium and cadmium in vivo effects in teleost cardiac muscle: Metal accumulation and oxidative stress markers. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 147, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Souid, G.; Sfar, M.; Timoumi, R.; Romdhane, M.H.; Essefi, S.A.; Majdoub, H. Protective effect assessment of Moringa oleifera against cadmium-induced toxicity in HCT116 and HEK293 cell lines. Environ. Sci. Pollut. Res. 2020, 27, 23783–23792. [Google Scholar] [CrossRef] [PubMed]
- Teka, N.; Alminderej, F.M.; Souid, G.; El-Ghoul, Y.; Le Cerf, D.; Majdoub, H. Characterization of Polysaccharides Sequentially Extracted from Allium roseum Leaves and Their Hepatoprotective Effects against Cadmium Induced Toxicity in Mouse Liver. Antioxidants 2022, 11, 1866. [Google Scholar] [CrossRef]
- Yang, L.; Kang, Y.; Dai, H.; Wang, X.; Xie, M.; Liu, J.; Gao, C.; Sun, H.; Ao, T.; Chen, W. Differential responses of polysaccharides and antioxidant enzymes in alleviating cadmium toxicity of tuber traditional Chinese medicinal materials. Environ. Sci. Pollut. Res. 2022, 29, 60832–60842. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, characterization and microbiological performance of new biocompatible cellulosic dressing grafted chitosan and Suaeda fruticosa polysaccharide extract. Cellulose 2021, 28, 9821–9835. [Google Scholar] [CrossRef]
- Hamden, Z.; El-Ghoul, Y.; Alminderej, F.M.; Saleh, S.M.; Majdoub, H. High-Quality Bioethanol and Vinegar Production from Saudi Arabia Dates: Characterization and Evaluation of Their Value and Antioxidant Efficiency. Antioxidants 2022, 11, 1155. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Amaghnouje, A.; Boukhira, S.; Alotaibi, A.A.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Conte, R.; Amal, E.H.E.Y. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules 2021, 26, 487. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Zahra Jawhari, F.; Bari, A.; Cerruti, P.; Avella, M.; Grafov, A.; Bousta, D. Medicinal plants used to treat acute digestive system problems in the region of Fez-Meknes in Morocco: An ethnopharmacological survey. Ethnobot. Res. Appl. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Nadeem, M.; Anjum, F.M.; Khan, M.I.; Tehseen, S.; El-Ghorab, A.; Sultan, J.I. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.): A review. Br. Food J. 2013, 115, 743–755. [Google Scholar] [CrossRef]
- Coşkuner, Y.; Karababa, E. Physical properties of coriander seeds (Coriandrum sativum L.). J. Food Eng. 2007, 80, 408–416. [Google Scholar] [CrossRef]
- Rajeshwari, C.; Andallu, B. Isolation and simultaneous detection of flavonoids in the methanolic and ethanolic extracts of Coriandrum sativum L. seeds by RP-HPLC. Pak. J. Food Sci. 2011, 21, 13–21. [Google Scholar]
- Gupta, K.; Thakral, K.; Arora, S.; Wagle, D. Studies on growth, structural carbohydrate and phytate in coriander (Coriandrum sativum) during seed development. J. Sci. Food Agric. 1991, 54, 43–46. [Google Scholar] [CrossRef]
- Muthusamy, S.; Udayakumar, G.P.; Narala, V.R. Recent advances in the extraction and characterization of seed polysaccharides, and their bioactivities: A review. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100276. [Google Scholar] [CrossRef]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Oh, J.-I.; Lee, S.J.; Park, G. The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicology 2014, 41, 102–111. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Chakroun, I.; Hamida, S.B.; Rihouey, C.; Mansour, H.B.; Le Cerf, D.; Majdoub, H. Ozone treatment of polysaccharides from Arthrocnemum indicum: Physico-chemical characterization and antiproliferative activity. Int. J. Biol. Macromol. 2017, 105, 1315–1323. [Google Scholar] [CrossRef]
- Salem, Y.B.; Amri, S.; Hammi, K.M.; Abdelhamid, A.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Physico-chemical characterization and pharmacological activities of sulfated polysaccharide from sea urchin, Paracentrotus lividus. Int. J. Biol. Macromol. 2017, 97, 8–15. [Google Scholar] [CrossRef]
- Ali, G.; Rihouey, C.; Larreta-Garde, V.; Le Cerf, D.; Picton, L. Molecular size characterization and kinetics studies on hydrolysis of pullulan by pullulanase in an entangled alginate medium. Biomacromolecules 2013, 14, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-N.; Sung, T.-J.; Chou, C.-H.; Liu, K.-L.; Hsieh, L.-P.; Hsieh, C.-W. Characterization and antioxidant activities of yellow strain Flammulina velutipes (Jinhua mushroom) polysaccharides and their effects on ROS content in L929 cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fu, M.-X.; Wu, D.-T.; Zhao, Y.-X.; Li, H.; Li, H.-B.; Gan, R.-Y. Structural characteristics of crude polysaccharides from 12 selected Chinese teas, and their antioxidant and anti-diabetic activities. Antioxidants 2021, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Clairbone, A. Catalase Activity in: RA Greenwald; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Kallel, R.; Krichen, F.; Hakim, A.; Zeghal, K.; Boudawara, T.; Bougatef, A.; Sahnoun, Z. Polysaccharide from Pimpinella anisum seeds: Structural characterization, anti-inflammatory and laser burn wound healing in mice. Int. J. Biol. Macromol. 2020, 156, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Trigui, I.; Yaich, H.; Sila, A.; Cheikh-Rouhou, S.; Bougatef, A.; Blecker, C.; Attia, H.; Ayadi, M. Physicochemical properties of water-soluble polysaccharides from black cumin seeds. Int. J. Biol. Macromol. 2018, 117, 937–946. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Majdoub, H. Pectic polysaccharides from edible halophytes: Insight on extraction processes, structural characterizations and immunomodulatory potentials. Int. J. Biol. Macromol. 2021, 173, 554–579. [Google Scholar] [CrossRef]
- Hajji, M.; Falcimaigne-Gordin, A.; Ksouda, G.; Merlier, F.; Thomasset, B.; Nasri, M. A water-soluble polysaccharide from Anethum graveolens seeds: Structural characterization, antioxidant activity and potential use as meat preservative. Int. J. Biol. Macromol. 2021, 167, 516–527. [Google Scholar] [CrossRef]
- Ben Salem, Y.; Abdelhamid, A.; Mkadmini Hammi, K.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Microwave-assisted extraction and pharmacological evaluation of polysaccharides from Posidonia oceanica. Biosci. Biotechnol. Biochem. 2017, 81, 1917–1925. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Yang, Y.; Luo, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Extraction and structural characterisation of rhamnogalacturonan I-type pectic polysaccharides from potato cell wall. Food Chem. 2013, 139, 617–623. [Google Scholar] [CrossRef]
- Cheng, H.; Neiss, T.G. Solution NMR spectroscopy of food polysaccharides. Polym. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Pu, X.; Ma, X.; Liu, L.; Ren, J.; Li, H.; Li, X.; Yu, S.; Zhang, W.; Fan, W. Structural characterization and antioxidant activity in vitro of polysaccharides from angelica and astragalus. Carbohydr. Polym. 2016, 137, 154–164. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of ultrasound-assisted extraction and structural characterization of the polysaccharide from pumpkin (Cucurbita moschata) seeds. Molecules 2018, 23, 1207. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013, 98, 8–16. [Google Scholar] [CrossRef]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yin, J.; Lin, H.; Li, J.; Wang, Y.; Cui, S.W.; Nie, S.; Xie, M. Structural characterization of a highly branched polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2012, 87, 2416–2424. [Google Scholar] [CrossRef]
- Dammak, M.I.; Salem, Y.B.; Belaid, A.; Mansour, H.B.; Hammami, S.; Le Cerf, D.; Majdoub, H. Partial characterization and antitumor activity of a polysaccharide isolated from watermelon rinds. Int. J. Biol. Macromol. 2019, 136, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, F.; Liu, X.; Ange, K.S.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef]
- Jain, K.S.; Kathiravan, M.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorganic Med. Chem. 2007, 15, 4674–4699. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The aging process: Major risk factor for disease and death. Proc. Natl. Acad. Sci. USA 1991, 88, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. [Google Scholar] [CrossRef]

| Neutral Sugar (%) | Uronic Acid (%) | Protein (%) | Monosaccharide Composition (%) a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Xyl | Man | Fru | Gal | Glc | ||||
| CPS | 62.2 | 9.8 | - | 6.2 | 3.6 | 8.8 | 17.7 | 5.2 | 32.9 | 25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfar, M.; Souid, G.; Alminderej, F.M.; Mzoughi, Z.; El-Ghoul, Y.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Structural Characterization of Polysaccharides from Coriandrum sativum Seeds: Hepatoprotective Effect against Cadmium Toxicity In Vivo. Antioxidants 2023, 12, 455. https://doi.org/10.3390/antiox12020455
Sfar M, Souid G, Alminderej FM, Mzoughi Z, El-Ghoul Y, Rihouey C, Le Cerf D, Majdoub H. Structural Characterization of Polysaccharides from Coriandrum sativum Seeds: Hepatoprotective Effect against Cadmium Toxicity In Vivo. Antioxidants. 2023; 12(2):455. https://doi.org/10.3390/antiox12020455
Chicago/Turabian StyleSfar, Manel, Ghada Souid, Fahad M. Alminderej, Zeineb Mzoughi, Yassine El-Ghoul, Christophe Rihouey, Didier Le Cerf, and Hatem Majdoub. 2023. "Structural Characterization of Polysaccharides from Coriandrum sativum Seeds: Hepatoprotective Effect against Cadmium Toxicity In Vivo" Antioxidants 12, no. 2: 455. https://doi.org/10.3390/antiox12020455
APA StyleSfar, M., Souid, G., Alminderej, F. M., Mzoughi, Z., El-Ghoul, Y., Rihouey, C., Le Cerf, D., & Majdoub, H. (2023). Structural Characterization of Polysaccharides from Coriandrum sativum Seeds: Hepatoprotective Effect against Cadmium Toxicity In Vivo. Antioxidants, 12(2), 455. https://doi.org/10.3390/antiox12020455