Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Culture of Salmonella enterica Serovar Enteritidis (S. Enteritidis)
2.3. Ethanolic Extracted Cranberry Pomace
2.4. Determination of Minimum Inhibitory Concentrations (MICs) of Acidic and Neutral Cranberry Pomaces (CPOHs) against S. Enteritidis
2.5. Cell Viability Assay
2.6. Adhesion Assay
2.7. Invasion Assay (Gentamicin Protection Assay)
2.8. RNA Isolation, cDNA Synthesis, and Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
3.1. Antibacterial Effect of Neutral Cranberry Pomace (CPOH) on the Growth of S. Enteritidis
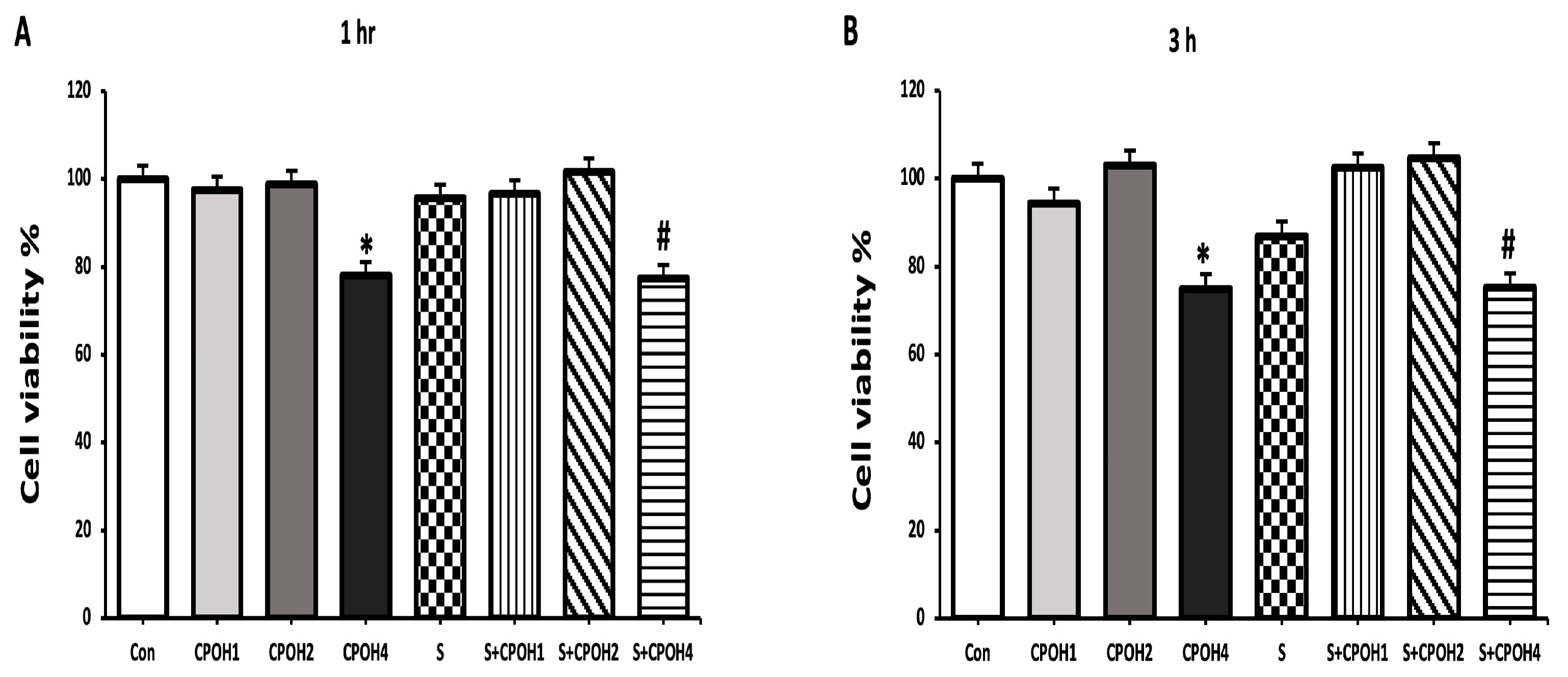
3.2. Effects of Neutral CPOH and S. Enteritidis on LMH Cell Viability
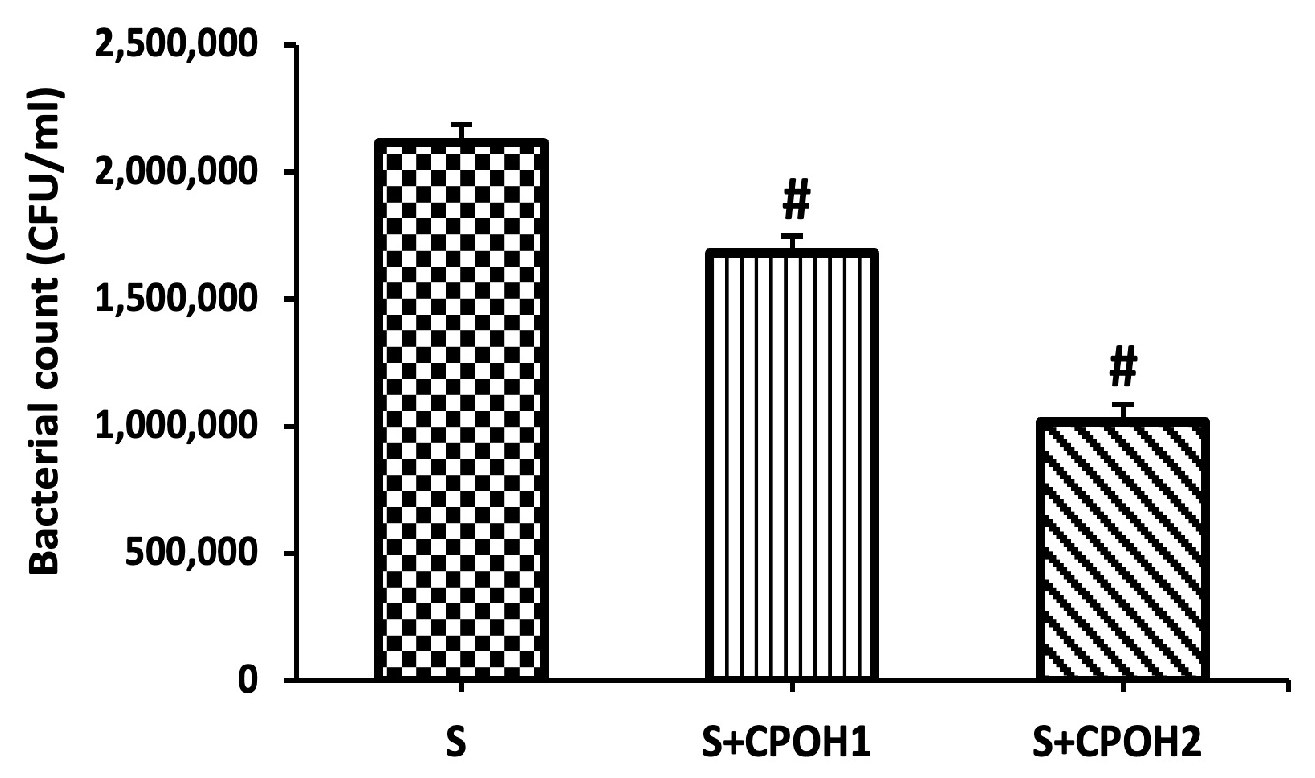
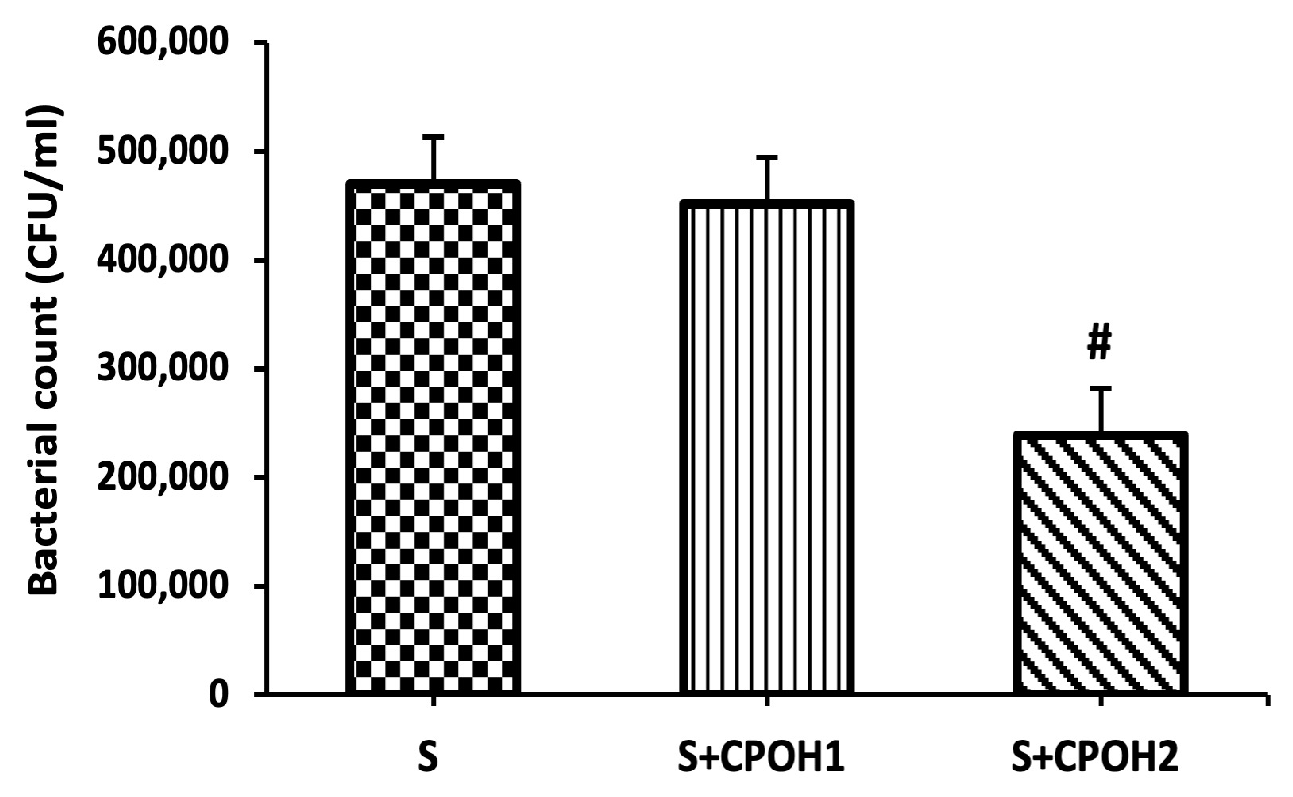
3.3. Effects of Neutral CPOH on S. Enteritidis Adhesion and Invasion to LMH Cells
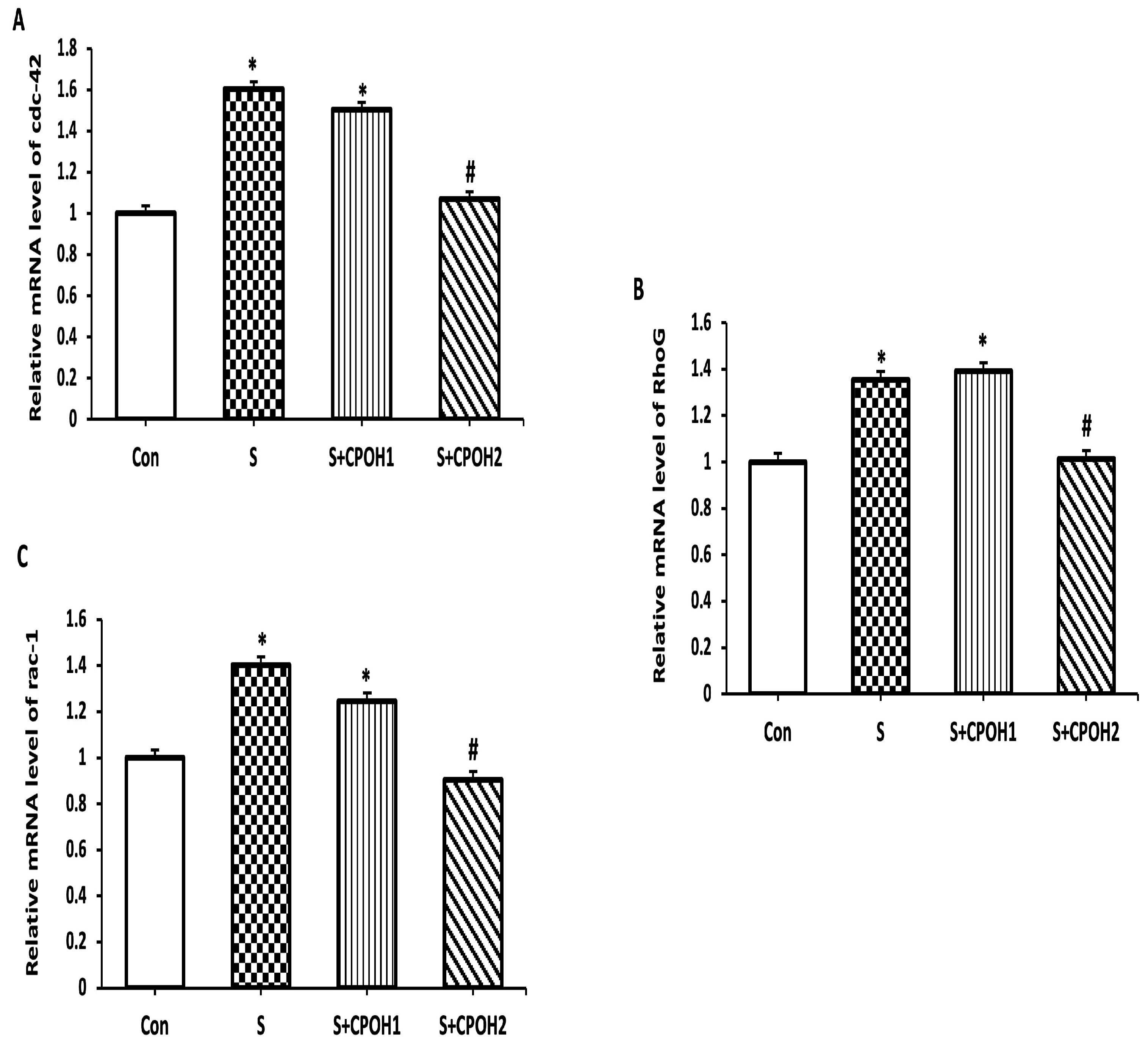
3.4. Impacts of Neutral CPOH on Expression of Invasion-Related Host Cell Protein Genes
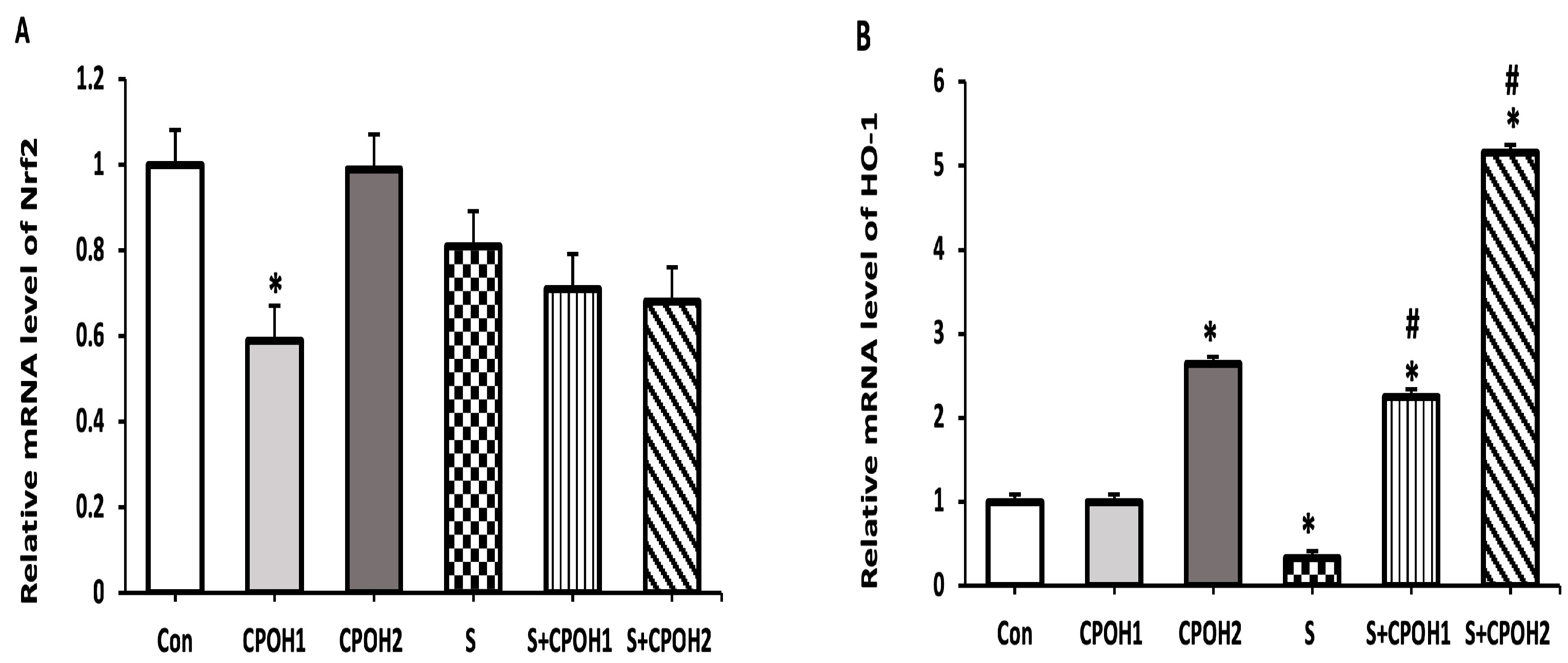
3.5. Effects of Neutral CPOH on Expression of Antioxidant-Related Genes in LMH Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar]
- El-Hack, M.E.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yu, C.-B.; Yan, D.; Huang, J.-R.; Li, L.-J. Effects of Salmonella infection on hepatic damage following acute liver injury in rats. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 399–405. [Google Scholar] [CrossRef]
- Hernández-Ramírez, J.O.; Nava-Ramírez, M.J.; Merino-Guzmán, R.; Téllez-Isaías, G.; Vázquez-Durán, A.; Méndez-Albores, A. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. 2019, 36, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Geissler, A.L.; Carrillo, F.B.; Swanson, K.; Patrick, M.E.; Fullerton, K.E.; Bennett, C.; Barrett, K.; Mahon, B.E. Increasing Campylobacter Infections, Outbreaks, and Antimicrobial Resistance in the United States, 2004–2012. Clin. Infect. Dis. 2017, 65, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention [CDC]. Chapter 4: Travel-Related Infectious Diseases: Salmonellosis (Nontyphoidal). Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/salmonellosis-nontyphoidal (accessed on 24 June 2019).
- Lanier, W.A.; Hale, K.R.; Geissler, A.L.; Dewey-Mattia, D. Chicken Liver–Associated Outbreaks of Campylobacteriosis and Salmonellosis, United States, 2000–2016: Identifying Opportunities for Prevention. Foodborne Pathog. Dis. 2018, 15, 726–733. [Google Scholar] [CrossRef]
- Gast, R.K.; Guraya, R.; Jones, D.R.; Guard, J.; Anderson, K.E.; Karcher, D.M. Colonization of internal organs by Salmonella serovars Heidelberg and Typhimurium in experimentally infected laying hens housed in enriched colony cages at different stocking densities. Poult. Sci. 2017, 96, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008, 6, 53–66. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Deng, Q.; Sun, L.; Dong, K.; Li, Y.; Wu, S.; Huang, R. Salmonella effector SpvB interferes with intracellular iron homeostasis via regulation of transcription factor NRF2. FASEB J. 2019, 33, 13450–13464. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Bayard, J.; Ramos-Morales, F. Salmonella Type III Secretion Effector SlrP is an E3 Ubiquitin Ligase for Mammalian Thioredoxin. J. Biol. Chem. 2009, 284, 27587–27595. [Google Scholar] [CrossRef]
- Salaheen, S.; Jaiswal, E.; Joo, J.; Peng, M.; Ho, R.; Oconnor, D.; Adlerz, K.; Aranda-Espinoza, J.H.; Biswas, D. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella Typhimurium. Int. J. Food Microbiol. 2016, 237, 128–135. [Google Scholar] [CrossRef]
- Shin, B.; Park, W. Zoonotic Diseases and Phytochemical Medicines for Microbial Infections in Veterinary Science: Current State and Future Perspective. Front. Veter-Sci. 2018, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Diarra, M.S.; Ibarra-Alvarado, C.; Oomah, B.D. Bioactivities of Pilot-Scale Extracted Cranberry Juice and Pomace. J. Food Process. Preserv. 2012, 37, 356–365. [Google Scholar] [CrossRef]
- Ross, K.A.; Ehret, D.; Godfrey, D.; Fukumoto, L.; Diarra, M. Characterization of Pilot Scale Processed Canadian Organic Cranberry (Vaccinium macrocarpon) and Blueberry (Vaccinium angustifolium) Juice Pressing Residues and Phenolic-Enriched Extractives. Int. J. Fruit Sci. 2016, 17, 202–232. [Google Scholar] [CrossRef]
- Colletti, A.; Sangiorgio, L.; Martelli, A.; Testai, L.; Cicero, A.; Cravotto, G. Highly Active Cranberry’s Polyphenolic Fraction: New Advances in Processing and Clinical Applications. Nutrients 2021, 13, 2546. [Google Scholar] [CrossRef]
- Das, Q.; Lepp, D.; Yin, X.; Ross, K.; McCallum, J.L.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Transcriptional profiling of Salmonella enterica serovar Enteritidis exposed to ethanolic extract of organic cranberry pomace. PLoS ONE 2019, 14, e0219163. [Google Scholar] [CrossRef] [PubMed]
- Glisan, S.L.; Ryan, C.; Neilson, A.P.; Lambert, J.D. Cranberry extract attenuates hepatic inflammation in high-fat-fed obese mice. J. Nutr. Biochem. 2016, 37, 60–66. [Google Scholar] [CrossRef]
- Han, L.; Wang, K.; Ma, L.; Delaquis, P.; Bach, S.; Feng, J.; Lu, X. Viable but Nonculturable Escherichia coli O157:H7 and Salmonella enterica in Fresh Produce: Rapid Determination by Loop-Mediated Isothermal Amplification Coupled with a Propidium Monoazide Treatment. Appl. Environ. Microbiol. 2020, 86, e02566-19. [Google Scholar] [CrossRef]
- Chang, S.-S.; Han, A.; Reyes-De-Corcuera, J.; Powers, J.; Kang, D.-H. Evaluation of steam pasteurization in controlling Salmonella serotype Enteritidis on raw almond surfaces. Lett. Appl. Microbiol. 2010, 50, 393–398. [Google Scholar] [CrossRef]
- Vu, K.D.; Carlettini, H.; Bouvet, J.; Côté, J.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Effect of different cranberry extracts and juices during cranberry juice processing on the antiproliferative activity against two colon cancer cell lines. Food Chem. 2012, 132, 959–967. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED32:2022&sbssok=CLSI%20M100%20ED32:2022%20SECTION%20COMMITTEE%20MEMBERSHIP%20[PREV] (accessed on 1 February 2022).
- Mechesso, A.F.; Quah, Y.; Park, S.-C. Ginsenoside Rg3 reduces the adhesion, invasion, and intracellular survival of Salmonella enterica serovar Typhimurium. J. Ginseng Res. 2019, 45, 75–85. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, R.; Li, S.; Jia, H.; Si, X.; Lei, Y.; Lyu, J.; Dai, Z.; Wu, Z. Protective Effects of Cinnamaldehyde on the Inflammatory Response, Oxidative Stress, and Apoptosis in Liver of Salmonella typhimurium-Challenged Mice. Molecules 2021, 26, 2309. [Google Scholar] [CrossRef]
- Roche, S.M.; Holbert, S.; Trotereau, J.; Schaeffer, S.; Georgeault, S.; Virlogeux-Payant, I.; Velge, P. Salmonella Typhimurium Invalidated for the Three Currently Known Invasion Factors Keeps its Ability to Invade Several Cell Models. Front. Cell. Infect. Microbiol. 2018, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Xia, J.; Wang, X.; Yin, K.; Hu, Y.; Yin, C.; Liu, Z.; Jiao, X. Virulence of Salmonella entericaserovar Pullorum isolates compared using cell-based and chicken embryo infection models. Poult. Sci. 2019, 98, 1488–1493. [Google Scholar] [CrossRef]
- Froebel, L.; Froebel, L.E.; Duong, T. Refined functional carbohydrates reduce adhesion of Salmonella and Campylobacter to poultry epithelial cells in vitro. Poult. Sci. 2020, 99, 7027–7034. [Google Scholar] [CrossRef]
- Wiedemann, A.; Virlogeux-Payant, I.; Chaussã, A.-M.; Schikora, A.; Evelge, P. Interactions of Salmonella with animals and plants. Front. Microbiol. 2015, 5, 791. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Das, Q.; Tang, J.; Yin, X.; Ross, K.; Warriner, K.; Marcone, M.F.; Diarra, M.S. Organic cranberry pomace and its ethanolic extractives as feed supplement in broiler: Impacts on serum Ig titers, liver and bursal immunity. Poult. Sci. 2020, 100, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Das, Q.; Islam, R.; Marcone, M.F.; Warriner, K.; Diarra, M.S. Potential of berry extracts to control foodborne pathogens. Food Control 2016, 73, 650–662. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Okshevsky, M.; Déziel, E.; Tufenkji, N. Proanthocyanidin Interferes with Intrinsic Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Adv. Sci. 2019, 6, 1802333. [Google Scholar] [CrossRef]
- Harmidy, K.; Tufenkji, N.; Gruenheid, S. Perturbation of Host Cell Cytoskeleton by Cranberry Proanthocyanidins and Their Effect on Enteric Infections. PLoS ONE 2011, 6, e27267. [Google Scholar] [CrossRef]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef]
- Pellerin, G.; Bazinet, L.; Grenier, D. Effect of cranberry juice deacidification on its antibacterial activity against periodontal pathogens and its anti-inflammatory properties in an oral epithelial cell model. Food Funct. 2021, 12, 10470–10483. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.-X.; Li, Y.-N.; Liu, N.; Yuan, L.-X.; Chen, S.-Y.; Zhu, Y.-H.; Wang, J.-F. Salmonella Infantis Delays the Death of Infected Epithelial Cells to Aggravate Bacterial Load by Intermittent Phosphorylation of Akt with SopB. Front. Immunol. 2021, 12, 4633. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. The protective effect of icariin and phosphorylated icariin against LPS-induced intestinal epithelial cells injury. Biomed. Pharmacother. 2019, 118, 109246–109254. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.D.P.M.; Ruviaro, A.R.; Martins, I.M.; Macedo, J.A.; LaPointe, G.; Macedo, G.A. Enzyme-assisted extraction of flavanones from citrus pomace: Obtention of natural compounds with anti-virulence and anti-adhesive effect against Salmonella enterica subsp. enterica serovar Typhimurium. Food Control 2021, 120, 107525. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type Proanthocyanidin Trimers from Cranberry that Inhibit Adherence of Uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Barbut, S.; Ross, K.; Diarra, M.S.; Balamurugan, S. The effect of cranberry pomace ethanol extract on the growth of meat starter cultures, Escherichia coli O157:H7, Salmonella enterica serovar Enteritidis and Listeria monocytogenes. LWT 2019, 115, 108452. [Google Scholar] [CrossRef]
- Patel, J.C.; Galán, J.E. Differential activation and function of Rho GTPases during Salmonella–host cell interactions. J. Cell Biol. 2006, 175, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kabirifar, R.; Ghoreshi, Z.-A.; Safari, F.; Karimollah, A.; Moradi, A.; Eskandari-Nasab, E. Quercetin protects liver injury induced by bile duct ligation via attenuation of Rac1 and NADPH oxidase1 expression in rats. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, C.U.I.; Ye, J.L.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z.Y. Protection of Porcine Intestinal-Epithelial Cells from Deoxynivalenol-Induced Damage by Resveratrol via the Nrf2 Signaling Pathway. J. Agric. Food Chem. 2018, 67, 1726–1735. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Schaefer, R.E.; Colgan, S.P. A Central Role for Heme Oxygenase-1 in the Control of Intestinal Epithelial Chemokine Expression. J. Innate Immun. 2018, 10, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Antimicrobial Actions of Reactive Oxygen Species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef]



| Gene | Primer Sequence | |
|---|---|---|
| cdc-42 | 5-TGGTGGTGCATCTCCCTATG-3 5-ATGGTGCCATGCTGAACACT-3 | Forward Reverse |
| RhoG | 5-TGCAGAGCATCAAATGCGTG-3 5-GGCGATGGAGAAGCAGATGA-3 | Forward Reverse |
| rac-1 | 5-ACCCCCAAACAGATGTCTTCTTA-3 5-TGCAACCAAGCCCTTACCAA-3 | Forward Reverse |
| Nrf2 | 5-CTGCTA GTG GATGGCGAGAC-3 5-CTC CGA GTT CTC CCC GAA AG-3 | Forward Reverse |
| HO-1 | 5-AGCTTCGCACAAGGAGTG TT-3 5-GGAGAGGTGGTCAGCATG TC-3 | Forward Reverse |
| Txn | 5-GTGCATGCCAACATTCCA GT-3 5-CTCCATGGCGGGAGATTAGAC-3 | Forward Reverse |
| Gclc | 5-GGA CGC TAT GGG GTT TGG AA-3 5-AGG CCA TCA CAA TGG GAC AG-3 | Forward Reverse |
| β-actin | 5-ATCTTTCTTGGGTATGGAGTC-3 5-GCCAGGGTACATTGTGG-3 | Forward Reverse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; El-Fateh, M.; Amer, M.S.; El-Shafei, R.A.; Bilal, M.; Diarra, M.S.; Zhao, X. Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells. Antioxidants 2023, 12, 460. https://doi.org/10.3390/antiox12020460
Ahmed N, El-Fateh M, Amer MS, El-Shafei RA, Bilal M, Diarra MS, Zhao X. Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells. Antioxidants. 2023; 12(2):460. https://doi.org/10.3390/antiox12020460
Chicago/Turabian StyleAhmed, Nada, Mohamed El-Fateh, Magdy S. Amer, Reham A. El-Shafei, Muhammad Bilal, Moussa S. Diarra, and Xin Zhao. 2023. "Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells" Antioxidants 12, no. 2: 460. https://doi.org/10.3390/antiox12020460
APA StyleAhmed, N., El-Fateh, M., Amer, M. S., El-Shafei, R. A., Bilal, M., Diarra, M. S., & Zhao, X. (2023). Antioxidative and Cytoprotective Efficacy of Ethanolic Extracted Cranberry Pomace against Salmonella Enteritidis Infection in Chicken Liver Cells. Antioxidants, 12(2), 460. https://doi.org/10.3390/antiox12020460







