Extraction, Identification and Antioxidant Activity of 3-Deoxyanthocyanidins from Sorghum bicolor L. Moench Cultivated in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization of the Extract of 3-DAs from Sorghum Grains
2.2.1. Pretreatment of Sorghum Grains
2.2.2. Extraction of 3-DAs from Sorghum Grains
2.2.3. Determination of 3-DAs in the Crude Extracts
2.3. Identification of the Extracts
2.3.1. Establishment of Standard Curves
2.3.2. Identification of the Compositions of Extracted 3-DAs
2.4. Antioxidant Activity Assays
2.5. Statistical Analyses
3. Results and Discussion
3.1. Optimization of the Extraction of 3-DAs from Sorghum
3.1.1. Single-Factor Analysis
3.1.2. Response Surface Analysis
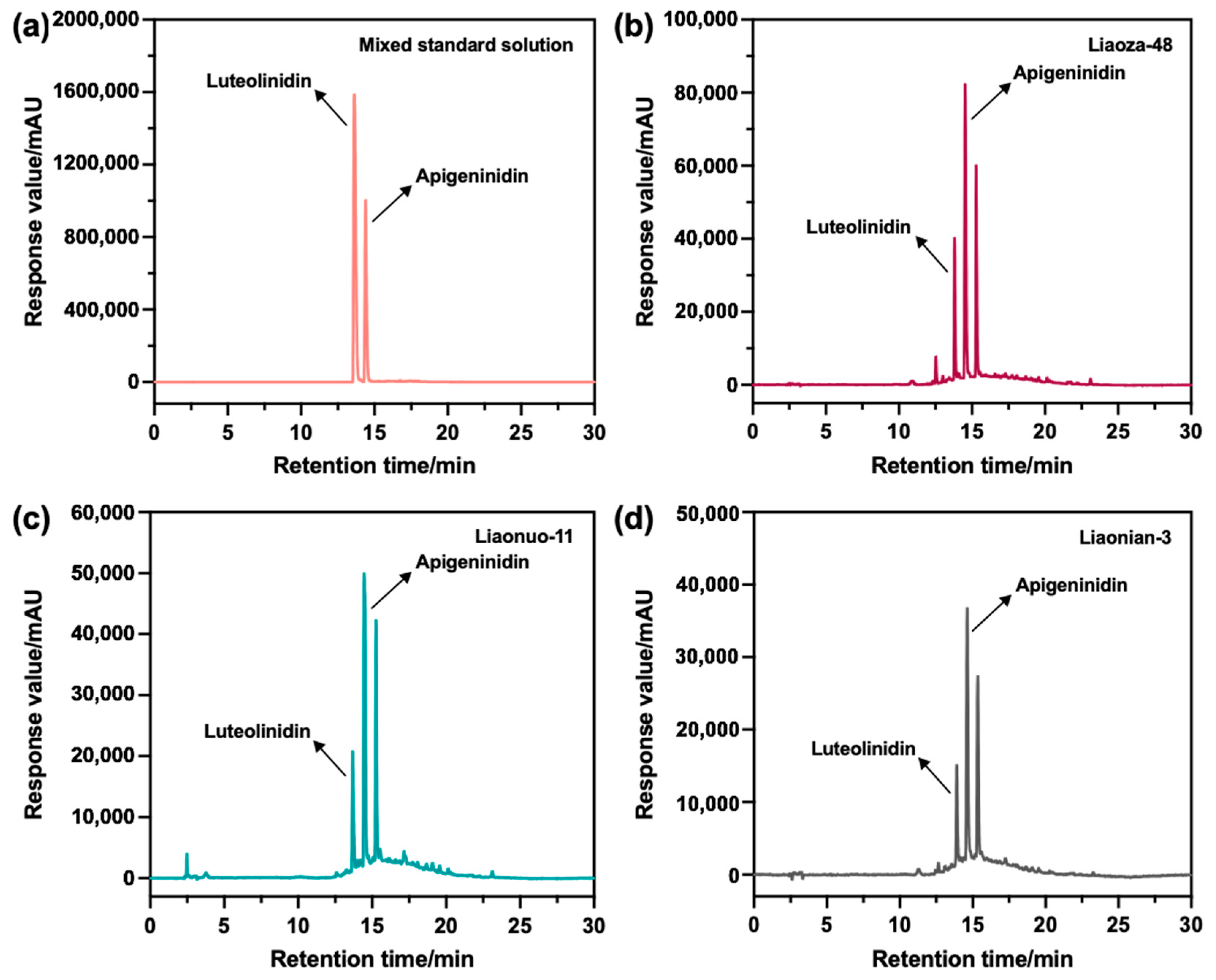
3.2. Identification of the Extracts
3.2.1. Identification of the Main Compounds in the Extracts
3.2.2. The Content of the Main Compounds in the Extracts
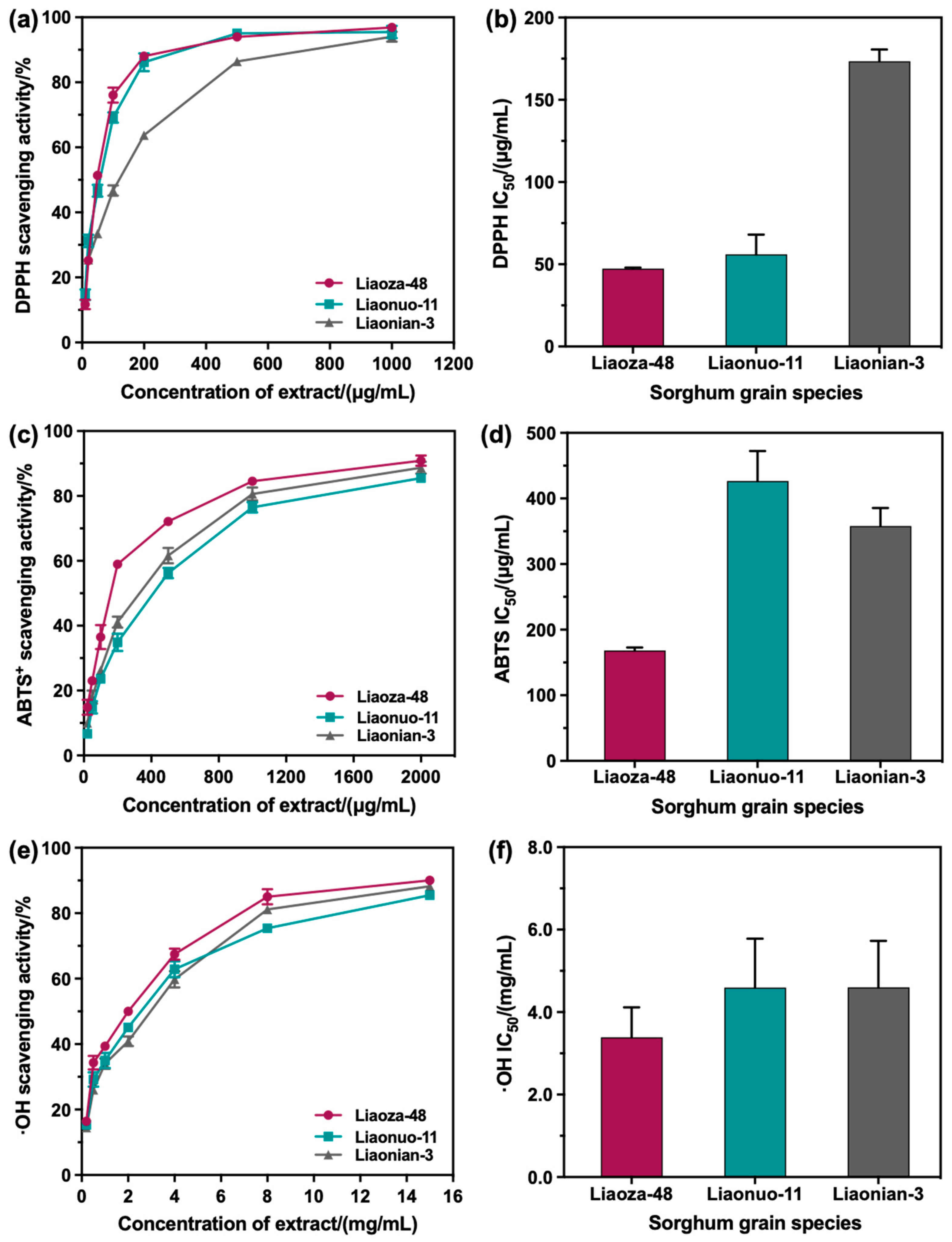
3.3. Antioxidant Activity of the Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Bakari, H.; Ruben, Z.F.; Roger, D.D.; Cedric, D.; Guillaume, P.; Pascal, D.; Philippe, M.; Gwendoline, C. Sorghum (Sorghum bicolor L. Moench) and Its Main Parts (By-Products) as Promising Sustainable Sources of Value-Added Ingredients. Waste Biomass Valorization 2022, 1–22. [Google Scholar] [CrossRef]
- Maunder, A.B. Sorghum worldwide. Sorghum Millet Dis. 2002, 11, 17. [Google Scholar]
- Dahlberg, J.; Berenji, J.; Sikora, V.; Latkovic, D. Assessing sorghum [Sorghum bicolor (L) Moench] germplasm for new traits: Food, fuels & unique uses. Maydica 2011, 56, 165. [Google Scholar]
- Vanamala, J.K.P.; Massey, A.R.; Pinnamaneni, S.R.; Reddivari, L.; Reardon, K.F. Grain and sweet sorghum (Sorghum bicolor L. Moench) serves as a novel source of bioactive compounds for human health. Crit. Rev. Food Sci. Nutr. 2018, 58, 2867–2881. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthocyanins from black sorghum and their antioxidant properties. Food Chem. 2005, 90, 293–301. [Google Scholar] [CrossRef]
- Carbonneau, M.-A.; Cisse, M.; Mora-Soumille, N.; Dairi, S.; Rosa, M.; Michel, F.; Lauret, C.; Cristol, J.-P.; Dangles, O. Antioxidant properties of 3-deoxyanthocyanidins and polyphenolic extracts from Côte d’Ivoire’s red and white sorghums assessed by ORAC and in vitro LDL oxidisability tests. Food Chem. 2014, 145, 701–709. [Google Scholar] [CrossRef]
- Awika, J.M.; McDonough, C.M.; Rooney, L.W. Decorticating sorghum to concentrate healthy phytochemicals. J. Agric. Food Chem. 2005, 53, 6230–6234. [Google Scholar] [CrossRef]
- Akogou, F.U.G.; Kayodé, A.P.P.; den Besten, H.M.W.; Linnemann, A.R.; Fogliano, V. Effects of processing and storage on the stability of the red biocolorant apigeninidin from sorghum. LWT 2018, 90, 592–597. [Google Scholar] [CrossRef]
- Smith, C.J. Tansley Review No. 86 Accumulation of phytoalexins: Defence mechanism and stimulus response system. New Phytol. 1996, 132, 1–45. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Kollipara, S.S.; Vincent, J.R.; Lyons, P.C.; Cadena-Gomez, G. Phytoalexin synthesis by the sorghum mesocotyl in response to infection by pathogenic and nonpathogenic fungi. Proc. Natl. Acad. Sci. USA 1987, 84, 5520–5524. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L. Flavonoid Composition And Antioxidant Activity of Pigmented Sorghums of Varying Genotypes. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2010. [Google Scholar]
- Taleon, V.; Dykes, L.; Rooney, W.L.; Rooney, L.W. Environmental effect on flavonoid concentrations and profiles of red and lemon-yellow sorghum grains. J. Food Compos. Anal. 2014, 34, 178–185. [Google Scholar] [CrossRef]
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Liu, J.; Zheng, W.; Zhang, Y.; Xu, T.; Gao, B.; Yu, L. Chemical composition profiling and biological activities of phenolic compounds in eleven red sorghums. J. Agric. Food Chem. 2021, 69, 9407–9418. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Properties of 3-deoxyanthocyanins from sorghum. J. Agric. Food Chem. 2004, 52, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Abugri, D.A.; Witola, W.H.; Jaynes, J.M.; Toufic, N. In vitro activity of Sorghum bicolor extracts, 3-deoxyanthocyanidins, against Toxoplasma gondii. Exp. Parasitol. 2016, 164, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Makanjuola, S.B.L.; Ogundaini, A.O.; Ajonuma, L.C.; Dosunmu, A. Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-E2 blockade. Int. J. Rheum. Dis. 2018, 21, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabadi, G.J.; Gilbert, R.G.; Gidley, M.J. Effect of particle size on kinetics of starch digestion in milled barley and sorghum grains by porcine alpha-amylase. J. Cereal Sci. 2009, 50, 198–204. [Google Scholar] [CrossRef]
- Choi, S.C.; Kim, J.M.; Lee, Y.G.; Kim, C. Antioxidant activity and contents of total phenolic compounds and anthocyanins according to grain colour in several varieties of Sorghum bicolor (L.) Moench. Cereal Res. Commun. 2019, 47, 228–238. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agric. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A novel, green environment-friendly cloud point extraction of polyphenols from pomegranate peels: A comparative assessment with ultrasound and microwave-assisted extraction. Sep. Sci. Technol. 2021, 56, 1014–1025. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Alaiz, M.; Vioque, J.; Girón-Calle, J.; Fernández-Bolaños, J. Polyphenols associated to pectic polysaccharides account for most of the antiproliferative and antioxidant activities in olive extracts. J. Funct. Foods 2019, 62, 103530. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Unusual anthocyanin reaction with acetone leading to pyranoanthocyanin formation. Tetrahedron Lett. 2001, 42, 1371–1373. [Google Scholar] [CrossRef]
- Barros, F.; Dykes, L.; Awika, J.M.; Rooney, L.W. Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci. 2013, 58, 305–312. [Google Scholar] [CrossRef]
- Geera, B.; Ojwang, L.O.; Awika, J.M. New highly stable dimeric 3-deoxyanthocyanidin pigments from Sorghum bicolor leaf sheath. J. Food Sci. 2012, 77, C566–C572. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Yang, L.; Dykes, L.; Awika, J.M. Thermal stability of 3-deoxyanthocyanidin pigments. Food Chem. 2014, 160, 246–254. [Google Scholar] [CrossRef]
- Devi, P.S.; Saravanakumar, M.; Mohandas, S. The effects of temperature and pH on stability of anthocyanins from red sorghum (Sorghum bicolor) bran. Afr. J. Food Sci. 2012, 24, 567–573. [Google Scholar]
- Benchikh, Y.; Aissaoui, A.; Allouch, R.; Mohellebi, N. Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J. Food Sci. Technol. 2021, 58, 1987–1995. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. 3-Deoxyanthocyanidin colorant: Nature, health, synthesis, and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1533–1549. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.K.; Umakanth, A.V.; Narsaiah, T.B.; Uma, A. Exploring anthocyanins, antioxidant capacity and α-glucosidase inhibition in bran and flour extracts of selected sorghum genotypes. Food Biosci. 2021, 41, 100979. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, Z.; Yao, Y.; Hao, Y.; Ren, G. Antioxidant and anti-cancer activities of proanthocyanidins-rich extracts from three varieties of sorghum (Sorghum bicolor) bran. Food Agric. Immunol. 2017, 28, 1530–1543. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Yeon, S.J.; Ham, H.J.; Kim, J.H.; Han, S.I.; Oh, D.H. Flavonoids in decorticated sorghum grains exert antioxidant, antidiabetic and antiobesity activities. Molecules 2020, 25, 2854. [Google Scholar] [CrossRef]


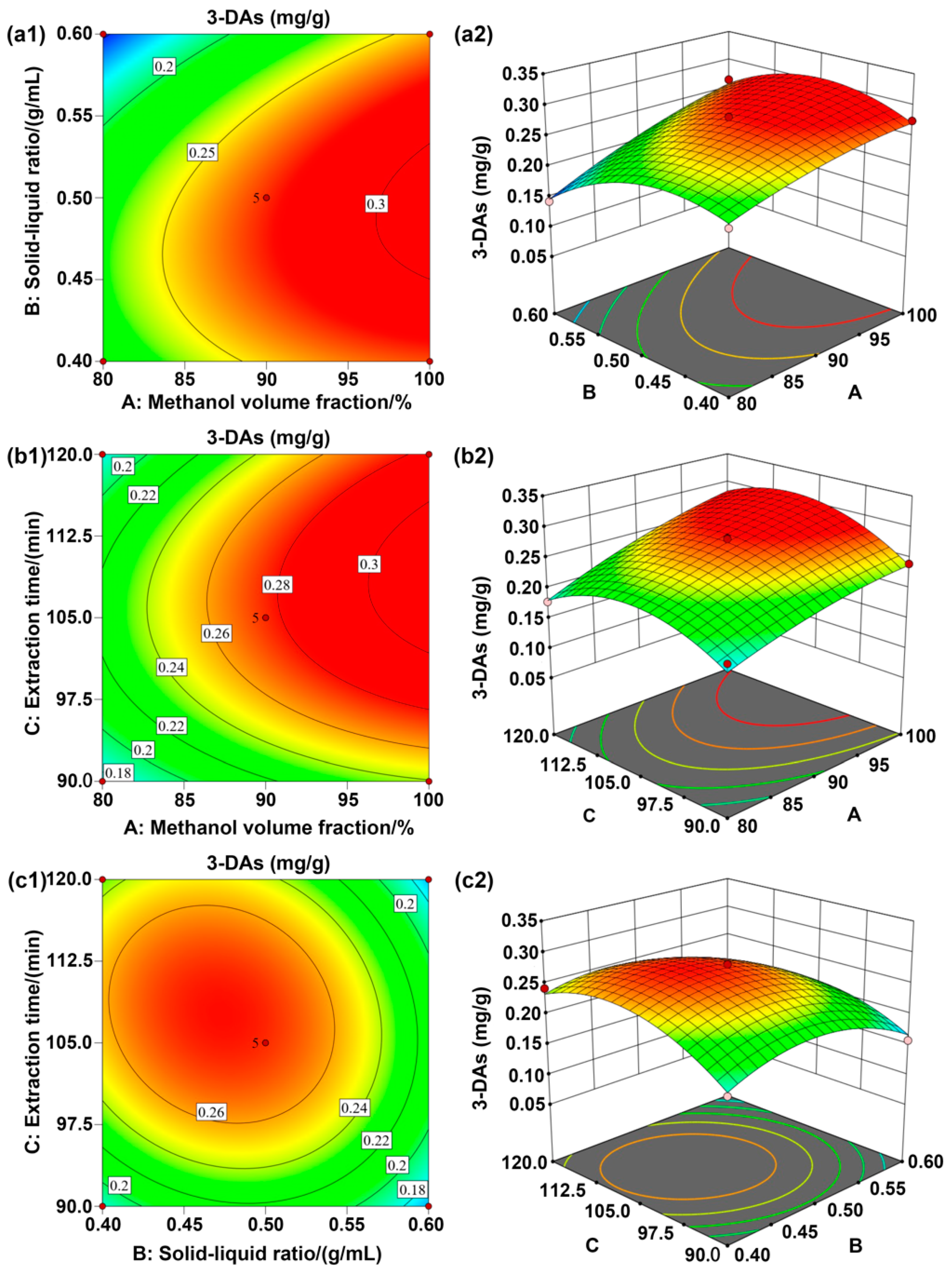


| Level | Factors | ||
|---|---|---|---|
| A: Methanol/(v/v, %) | B: Solid-Liquid Ratio/(g/mL) | C: Extraction Time /min | |
| −1 | 80 | 1:15 | 90 |
| 0 | 90 | 1:20 | 120 |
| 1 | 100 | 1:25 | 150 |
| No. | A | B | C | Y: 3-DAs Amount/(mg/g) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0.2082 |
| 2 | 0 | 0 | 0 | 0.2823 |
| 3 | 1 | 0 | 1 | 0.2712 |
| 4 | 0 | 1 | −1 | 0.1576 |
| 5 | −1 | 0 | −1 | 0.1874 |
| 6 | −1 | 0 | 1 | 0.1785 |
| 7 | 0 | 0 | 0 | 0.2728 |
| 8 | 0 | 0 | 0 | 0.2760 |
| 9 | −1 | 1 | 0 | 0.1432 |
| 10 | 1 | 1 | 0 | 0.2648 |
| 11 | 0 | −1 | −1 | 0.1784 |
| 12 | 1 | −1 | 0 | 0.2753 |
| 13 | 0 | 0 | 0 | 0.2813 |
| 14 | 0 | −1 | 1 | 0.2427 |
| 15 | 0 | 1 | 1 | 0.1742 |
| 16 | 1 | 0 | −1 | 0.2411 |
| 17 | 0 | 0 | 0 | 0.2677 |
| Variance Source | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 0.0386 | 0.0043 | 35.78 | <0.0001 ** |
| A | 1 | 0.0140 | 0.0140 | 116.99 | <0.0001 ** |
| B | 1 | 0.0034 | 0.0034 | 28.29 | 0.0011 ** |
| C | 1 | 0.0013 | 0.0013 | 10.86 | 0.0132 * |
| AB | 1 | 0.0007 | 0.0007 | 6.19 | 0.0417 * |
| AC | 1 | 0.0004 | 0.0004 | 3.17 | 0.1183 |
| BC | 1 | 0.0006 | 0.0006 | 4.74 | 0.0659 |
| A2 | 1 | 0.0005 | 0.0005 | 4.18 | 0.0803 |
| B2 | 1 | 0.0076 | 0.0076 | 62.60 | <0.0001 ** |
| C2 | 1 | 0.0087 | 0.0087 | 72.84 | <0.0001 ** |
| Residual | 7 | 0.0008 | 0.0001 | ||
| Lack of fit | 3 | 0.0007 | 0.0002 | 6.29 | 0.0539 |
| Pure error | 4 | 0.0001 | 0.0000 | ||
| Cor total | 16 | 0.0395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Y.; Liu, Z.; Wang, J. Extraction, Identification and Antioxidant Activity of 3-Deoxyanthocyanidins from Sorghum bicolor L. Moench Cultivated in China. Antioxidants 2023, 12, 468. https://doi.org/10.3390/antiox12020468
Wu Y, Wang Y, Liu Z, Wang J. Extraction, Identification and Antioxidant Activity of 3-Deoxyanthocyanidins from Sorghum bicolor L. Moench Cultivated in China. Antioxidants. 2023; 12(2):468. https://doi.org/10.3390/antiox12020468
Chicago/Turabian StyleWu, Yanbei, Yali Wang, Zhengyan Liu, and Jing Wang. 2023. "Extraction, Identification and Antioxidant Activity of 3-Deoxyanthocyanidins from Sorghum bicolor L. Moench Cultivated in China" Antioxidants 12, no. 2: 468. https://doi.org/10.3390/antiox12020468







